Abstract
Acute kidney injury (AKI) is a prevalent and serious condition in the intensive care unit (ICU), associated with significant morbidity and mortality. Septic acute kidney injury (SAKI) contributes substantially to AKI cases in the ICU. However, current diagnostic methods have limitations, necessitating the exploration of novel biomarkers. In this study, we investigated the potential of plasma and urine CCL2 levels as diagnostic markers for AKI and SAKI in 216 ICU patients. Our findings revealed significant differences in plasma (p < 0.01) and urine CCL2 (p < 0.0001) levels between AKI and non-AKI patients in the ICU. Notably, urine CCL2 demonstrated promising predictive value for AKI, exhibiting high specificity and sensitivity (AUC = 0.8976; p < 0.0001). Furthermore, we observed higher urine CCL2 levels in SAKI compared to non-septic AKI (p < 0.001) and urine CCL2 could also differentiate SAKI from non-septic AKI (AUC = 0.7597; p < 0.0001). These results suggest that urine CCL2 levels hold promise as early biomarkers for AKI and SAKI, offering valuable insights for timely intervention and improved management of ICU patients.
Introduction
Acute kidney injury (AKI) is a prevalent condition among critically ill patients in the intensive care unit (ICU), and it is associated with substantial morbidity and mortality rates [Citation1]. The occurrence rate of AKI varies depending on the studied population and definition used, estimated to affect approximately 20–50% of ICU patients [Citation2]. Septic acute kidney injury (SAKI), a subset caused by sepsis, contributes significantly to the overall cases of AKI in the ICU, accounting for approximately 50% of instances [Citation3].
The current diagnostic methods for AKI primarily rely on measuring serum creatinine (SCr) levels and urine output [Citation4]. However, these methods have clear limitations. SCr levels are influenced by factors, such as muscle mass, age, gender, and fluid status, impacting their accuracy as diagnostic markers [Citation5,Citation6]. Moreover, SCr levels may not rise until substantial kidney damage has already occurred, leading to delayed diagnosis and potential compromise of patient outcomes [Citation7]. Urine output as a diagnostic criterion may also be unreliable in critically ill patients due to factors like diuretic use, fluid resuscitation, or renal replacement therapy [Citation8].
Early diagnosis of AKI and SAKI holds great importance in the ICU setting as it enables timely interventions that can potentially halt or mitigate the progression of kidney injury [Citation9]. Prompt recognition allows for interventions, such as fluid resuscitation, optimization of hemodynamics, and targeted antimicrobial therapy, which can attenuate the severity of AKI and SAKI, reduce organ dysfunction, and improve patient outcomes [Citation10]. Furthermore, identifying AKI and SAKI at an early stage allows clinicians to implement renal protective strategies and tailor treatment plans to individual patients, thereby optimizing the management of these conditions [Citation10].
The search for novel biomarkers enabling early and accurate diagnosis of AKI and SAKI is an area of active research. Among the promising biomarkers, urine biomarkers have gained attention due to their noninvasiveness, ease of collection, and direct association with renal injury [Citation11]. Chemokine ligand 2 (CCL2), also known as monocyte chemoattractant protein-1 (MCP-1), is a pro-inflammatory chemokine involved in the recruitment and activation of monocytes and macrophages, playing a crucial role in the inflammatory response [Citation12,Citation13]. A previous has provided crucial insights into the role of CCL2 in the pathogenesis of sepsis-associated kidney dysfunction. The study demonstrated that CCL2 produced by proximal tubular epithelial cells triggers the recruitment of macrophages and subsequent inflammatory injury, contributing to the development of sepsis-induced AKI [Citation14]. These findings underscore the critical involvement of CCL2 in the pathophysiology of kidney dysfunction associated with sepsis. Considering the specific role of CCL2 in AKI, we in our current study aimed to investigate the potential of urine CCL2 as a diagnostic biomarker for early diagnosis of AKI and SAKI.
Patients and methods
Ethical statement
This study was approved by the Independent Ethics Committee (IEC) at the First People’s Hospital of Kunshan (Approval No. 2021-03-014-H01-K01). All protocols involving patient information and human samples were reviewed by the IEC and performed in accordance with the Declaration of Helsinki and relevant national and institutional guidelines. Written consent was obtained from all participants or their legal representatives prior to their inclusion in this study. Participants were clearly informed that this study was independent of their treatment, and they were also given ample opportunity to ask questions, seek clarifications, and were assured of their right to withdraw their consent at any point during the study, without any adverse consequences.
Patients and sample collection
This was a prospective study, and patient enrollment started from March 2021 to December 2022, with the aim of recruiting around 25 patients in the Control group, and around 60–65 patients in each study group. When the patient number reached the aimed target in a group, the enrollment for that group was ended. As a result, the percentage of AKI occurrence in this cohort was not a true reflection of AKI occurrence frequency in the ICU. A total of 216 patients admitted to the ICU of the First People’s Hospital of Kunshan were included in this study. These patients were divided into four groups: Control (non-sepsis and non-AKI; n = 27), Sepsis (sepsis and non-AKI; n = 60), non-septic AKI (n = 66), and Septic AKI (n = 63). Inclusion criteria: age > = 18 years; no known previous renal disease; no AKI before ICU admission. Exclusion criteria: chronic kidney disease (CKD; defined as an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 for at least 3 months, according to the kidney disease: Improving Global Outcomes (KDIGO) criteria [Citation15]); urinary tract infection before enrollment; AKI before ICU admission; the use of nephrotoxic drugs before ICU admission; incomplete data (2 cases were excluded due to lack of plasma and urine samples upon hospital admission). The patients were enrolled upon ICU admission based on the inclusion and exclusion criteria, but they were only assigned to one of the four study groups later in the ICU when they developed certain symptoms. For example, if an enrolled patient developed AKI without sepsis during their stay in ICU, this patient was then assigned to the non-septic AKI group. The clinical characteristics of the patients are summarized in .
Table 1. Patients’ clinical characteristics.
Blood samples were collected upon hospital admission, ICU admission, and every other day during the ICU stay. Urine samples were collected upon hospital admission, ICU admission, and daily throughout the ICU stay. The mean interval time between hospital admission and ICU admission for this cohort was 16.4 d. Plasma and serum were obtained from coagulated and uncoagulated blood samples, respectively. The collected plasma, serum, and urine samples were aliquoted and stored at −80 °C until further use.
Definitions
AKI was determined in accordance with the Kidney Disease Improving Global Outcomes (KDIGO) criteria [Citation15]. In brief, an increase in SCr of ≥50% above the baseline within 7 d, or by 0.3 mg/dL (26.5 µmol/L) in 48 h was considered indicative of AKI. The following guidelines were adhered to for establishing the baseline SCr level. For patients with available medical records from the preceding 1–2 years, the lowest recorded SCr level within that timeframe was considered as the baseline. In cases where no such records were available, the lower value between the SCr level at admission or the value estimated by the Modification of Diet in Renal Disease equation was used as the baseline [Citation16]. AKI severity was classified into 3 stages, according to the KDIGO criteria [Citation15]. In detail, Stage 1 was defined by the increase of SCr by 0.3 mg/dL (26.5 µmol/L) in 48 h or ≥50% above the baseline within 7 d. Stage 2 was defined by the increase of SCr by 2.0–2.9 times above baseline. Stage 3 was defined by the increase of SCr by more than 3.0 folds above baseline, or reach 4.0 mg/dL (353.6 µmol/L), or initiation of renal replacement therapy. The definition of sepsis followed the criteria outlined in the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3 definition) [Citation17].
CCL2 measurement
The CCL2 levels in plasma and urine were measured using a commercial human CCL2 ELISA kit (R&D Systems, Bio-Techne, Minneapolis, USA) following the manufacturer’s instructions. Briefly, plasma and urine samples, and standards were diluted with Assay Diluent and added to the pre-coated ELISA plate. The plate was then incubated for 2 h at room temperature. After washing, anti-CCL2 Conjugate was added and incubated for 1 h (urine samples) and 2 h (plasma samples) at room temperature, respectively. Following another round of washes, a colorimetric reaction was initiated by adding the Substrate Solution and incubating for 30 min. The color development was halted by adding the Stop Solution, and the optical density (OD) values of the samples and standards were recorded using a microplate reader. The testing wavelength was set at OD450, and the reference wavelength was set at OD570. The CCL2 concentration of the tested samples was then calculated using the standard curve generated from the same ELISA plate. All samples were tested in duplicates. Urine CCL2 was calculated using urine creatinine as a reference to correct for urine dilution, and the final CCL2 level in urine was expressed as ng/mmol creatinine [Citation18].
Statistical analysis
All statistical analyses were conducted using GraphPad Prism version 9 (GraphPad Software Inc., La Jolla, CA). The unpaired Mann-Whitney test was employed to compare differences between two groups, while the Kruskal–Wallis test followed by Dunn’s multiple comparisons test was utilized to compare differences among three or more groups. The predictive value of CCL2 as a biomarker for AKI and SAKI was assessed using receiver operating characteristic (ROC) curve analysis. Area under the curve (AUC) values, along with corresponding prediction sensitivity and specificity, were calculated. Youden’s index was used in our current study to select the sensitivity and specificity. Statistical significance was defined as a two-sided p value of less than 0.05.
Results
Study population characteristics
A total number of 216 patients were enrolled in our study, according to the inclusion and exclusion criteria listed in the Patients and Methods section above. The clinical characteristics of the cohort are summarized in . The age of the study group was 67 ± 7 years, and 107 of them were male (50%). The main underlying conditions for this cohort were hypertension (53%) and cardiac diseases (44%), while the main reasons for hospitalization were pulmonary diseases (59%), cardiac diseases (25%) and cancer (11%). These patients were divided into four groups: Control (non-sepsis and non-AKI; n = 27), Sepsis (sepsis and non-AKI; n = 60), Non-septic AKI (n = 66), and Septic AKI (n = 63). Most of the clinical characteristics were similar among the four groups, except for white blood cell counts (WBC) and neutrophil-to-lymphocyte ratio (NLR). Both WBC and NLR were higher in the sepsis and SAKI groups than the other 2 groups ().
Plasma and urine CCL2 levels between AKI and non-AKI patients in ICU
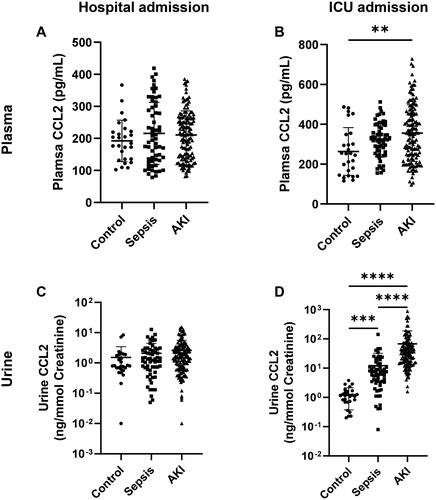
To investigate whether there are differences in plasma and urine CCL2 levels between AKI and non-AKI patients in the ICU, we combined SAKI and non-septic AKI patients and compared them with the Control and Sepsis groups. Upon hospital admission, there were no apparent differences in CCL2 levels in both plasma and urine among these three groups (). However, significant differences were observed among the groups by the time patients were transferred to the ICU (). Specifically, the plasma CCL2 level was significantly increased in the AKI group compared to the Control group (). In urine, the differences were even more pronounced. The control group exhibited the lowest urine CCL2 levels, while the Sepsis group showed significantly elevated levels compared to the control group. Furthermore, the AKI group demonstrated further enhanced levels of this cytokine in the urine (). In comparison, SCr levels were comparable between AKI and non-AKI patients at both hospital admission and ICU admission (Figure S1). It seemed that SCr changed suddenly when AKI occurred (Figure S2). These findings indicate that CCL2 levels in both plasma and urine of AKI patients undergo changes as early as their admission to the ICU.
Urine CCL2 is a good predictive marker for AKI in ICU
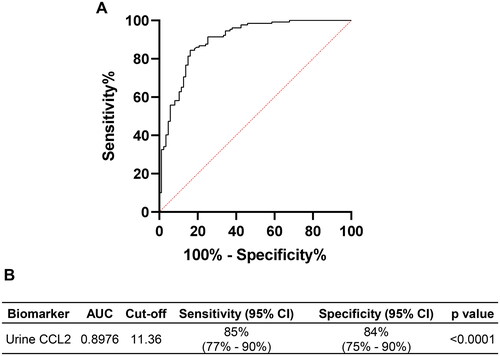
Given the significant differences in urine CCL2 levels between AKI patients and the control and sepsis groups at ICU admission, we assessed whether urine CCL2 levels at this time point could serve as an indicator of AKI. ROC analysis was performed to evaluate the predictive value of urine CCL2 for AKI in the ICU setting. As shown in , urine CCL2 could be a potential predictive marker to differentiate AKI from non-AKI patients in the ICU, with an AUC of 0.8976 (p < 0.0001). At a cutoff value of 11.36 ng/mmol Creatinine, urine CCL2 demonstrated a specificity of 85% (95% CI: 77–90%) and a sensitivity of 84% (95% CI: 75– 90%) in predicting AKI.
Plasma and urine CCL2 levels between septic and non-septic AKI patients in ICU
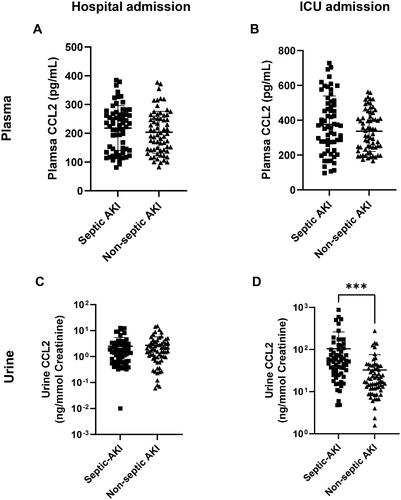
While both SAKI and non-septic AKI involve AKI, the cause of AKI is different. The underlying infection in SAKI can have a significant impact on the management and treatment strategies compared to non-septic AKI [Citation19,Citation20]. Given these distinctions, we further examined whether there were differences in plasma and urine CCL2 levels between these two groups at hospital admission and ICU admission. Our data indicated that CCL2 levels in both plasma and urine did not exhibit apparent differences between SAKI and non-septic AKI upon hospital admission (). However, at ICU admission, while plasma CCL2 levels remained comparable between the two groups, urine CCL2 levels were significantly higher in SAKI compared to non-septic AKI ().
Urine CCL2 is a good predictive marker for SAKI in ICU
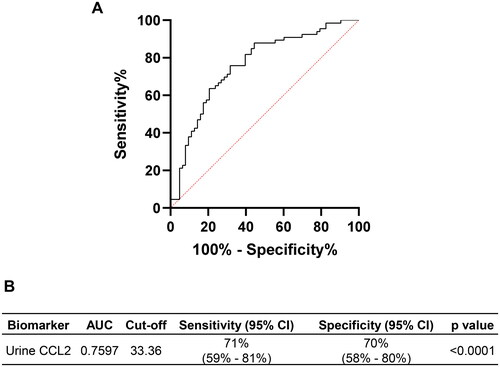
Considering the observed differences in urine CCL2 levels between SAKI and non-septic AKI, we further assessed its value in differentiating between these two conditions using ROC analysis. Our data demonstrated that urine CCL2 could predict SAKI from non-septic AKI in the ICU setting, with an AUC of 0.7597 (). At a cutoff value of 33.36 ng/mmol Creatinine, the sensitivity and specificity for predicting SAKI were 71% (95% CI: 59–81%) and 70% (95% CI: 58–80%), respectively ().
Discussion
Early diagnosis of AKI and SAKI is of great importance in the clinical management of critically ill patients within the ICU. Timely recognition enables interventions that can potentially mitigate the progression of kidney injury, attenuate organ dysfunction, and improve patient outcomes [Citation21]. Prompt identification allows for the implementation of strategies, such as fluid resuscitation, optimization of hemodynamics, and targeted antimicrobial therapy, which can significantly impact the course of AKI and SAKI [Citation22,Citation23]. Furthermore, early detection facilitates the implementation of renal protective measures and personalized treatment plans tailored to individual patients, optimizing the management of these conditions [Citation22,Citation23]. In our study, we investigated the potential of plasma and urine levels of CCL2 as biomarkers for differentiating AKI patients from non-AKI patients in the ICU, as well as distinguishing SAKI from non-septic AKI. Our findings indicate that urine CCL2 levels hold greater promise as an early marker for AKI and SAKI compared to plasma CCL2 levels, as an elevation of urine CCL2 could be detected as early as ICU admission and before SCr level increase. In our study, patients showed significantly elevated CCL2 in urine 1–3 d before AKI occurrence. By contrast, the SCr and eGFR did not show significant change at the time of ICU admission compared to hospital admission. It seemed that both SCr and eGFR changed suddenly when AKI occurred (Figure S2).
The increase of plasma CCL2 in sepsis patients has been previously described; however, there are quite some major differences between these previous publications and this study [Citation24–26]. First, these previous publications have focused on plasma CCL2 and sepsis, while our study has focused on CCL2 (from both plasma and urine) and AKI (including both non-septic and septic AKIs). Second, of the 3 publications, only 1 has studied the predictive value of plasma CCL2 for sepsis in trauma patients [Citation26], while the other 2 has concentrated on the role of CCL2 in sepsis [Citation24,Citation25]. Our study has however focused on the evaluation of CCL2 as an early diagnostic marker in predicting AKI at early time points. Third, all previous studies have only focused on plasma CCL2, while our study has studied CCL2 of both plasma and urine origin, and our results have shown that urine CCL2 is a better diagnostic biomarker for AKI than plasma CCL2.
The mechanism underlying the elevation of urine CCL2 in AKI and SAKI may be attributed to the role of CCL2 in the inflammatory response and renal injury. Previous studies have provided crucial insights into the involvement of CCL2 in the pathogenesis of sepsis-associated kidney dysfunction [Citation14, Citation27]. A study by Jia et al. demonstrated that CCL2 produced by proximal tubular epithelial cells triggers the recruitment of macrophages and subsequent inflammatory injury, contributing to the development of sepsis-induced AKI [Citation14]. This suggests that CCL2 could be induced locally in the kidney during sepsis and SAKI and this chemokine may play a critical role in the pathophysiology of kidney dysfunction associated with sepsis.
Urine CCL2 as an AKI and SAKI marker has several key advantages. First, urine CCL2 level rises at a much earlier stage than conventional serum SCr, which may not increase until significant kidney damage has already occurred. Second, Urine output, another diagnostic criterion, can be unreliable in critically ill patients due to factors like diuretic use, fluid resuscitation, or renal replacement therapy. Third, the noninvasive nature of urine collection makes urine CCL2 a convenient and easily accessible marker in clinical practice. While our study highlights the potential of urine CCL2 as a diagnostic marker for early AKI and SAKI, further research is needed to translate these findings into clinical practice. Validation studies involving larger patient cohorts and multi-center trials are essential to confirm the utility and reliability of urine CCL2 as a diagnostic biomarker. In these validation studies, the inclusion of multivariate analysis could further confirm the association of CCL2 with AKI in a logistic regression model. Additionally, the development of standardized assays and cutoff values for urine CCL2 measurement would facilitate its incorporation into routine clinical practice. We have also compared serum and urine CCL2 levels among AKI patients at different disease stages, however, a difference of statistical significance was not observed (data not shown). It would also be beneficial to confirm this finding in a larger scale study.
The discovery of urine CCL2 as a marker for AKI and SAKI suggests that other cytokines and chemokines may also hold potential as diagnostic markers for these diseases. Numerous studies have investigated the roles of various chemokines and cytokines in inflammation, immune response, and tissue injury [Citation28–30]. This growing body of research indicates that similar to CCL2, other molecules of the same class may serve as valuable markers for AKI and SAKI. For instance, a study by Al-Saegh et al. demonstrated the diagnostic and prognostic value of urinary interleukin-18 (IL-18) as an early marker for AKI and as a predictor of ICU mortality [Citation31]. Additionally, research has explored the diagnostic utility of chemokines, such as CXCL1, CXCL2, and CCL5 in AKI [Citation32–34]. To advance our understanding and improve diagnostic accuracy, future investigations should assess the performance of these chemokines and cytokines, either individually or in combination with CCL2. We in our current study did not check the levels of other inflammatory cytokines and chemokines, but given the positive results of CCL2, we in our future studies plan to extend the investigation to a broader range of inflammatory biomarkers. By exploring the potential of these molecules, we can further unravel the complex pathophysiology of AKI and SAKI, and potentially identify a panel of markers that offer comparable or even superior diagnostic capabilities.
Conclusions
Our findings suggest that plasma and urine CCL2 levels have potential as early biomarkers for AKI and SAKI in the ICU, aiding in timely intervention and improved patient management.
Ethics approval and consent to participate
This study was approved by the Independent Ethics Committee (IEC) at the First People’s Hospital of Kunshan (Approval No. 2021-03-014-H01-K01). All protocols involving patient information and human samples were reviewed by the IEC and performed in accordance with the Declaration of Helsinki and relevant national and institutional guidelines. Written consent was obtained from all participants or their legal representatives prior to their inclusion in this study.
Consent for publication
Not applicable.
Authors’ contributions
YP, QW, and QQ designed the experiment. YP, QW, and FJ conducted the experiment. YP, QW, FJ, and TT analyzed the data. YP and QQ wrote the article, and all authors approved the final version.
Supplemental Material
Download PDF (153.7 KB)Acknowledgments
None.
Disclosure statement
The authors declare that they have no competing interests.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Kellum JA, Romagnani P, Ashuntantang G, et al. Acute kidney injury. Nat Rev Dis Primers. 2021;7(1):1. doi: 10.1038/s41572-021-00284-z.
- Case J, Khan S, Khalid R, et al. Epidemiology of acute kidney injury in the intensive care unit. Crit Care Res Pract. 2013;2013:479730–9. doi: 10.1155/2013/479730.
- Wan L, Bagshaw SM, Langenberg C, et al. Pathophysiology of septic acute kidney injury: what do we really know? Crit Care Med. 2008;36(4):S198–S203. doi: 10.1097/CCM.0b013e318168ccd5.
- Ostermann M, Bellomo R, Burdmann EA, et al. Controversies in acute kidney injury: conclusions from a kidney disease: improving global outcomes (KDIGO) conference. Kidney Int. 2020;98(2):294–309. doi: 10.1016/j.kint.2020.04.020.
- Moledina DG, Parikh CR. Phenotyping of acute kidney injury: beyond serum creatinine. Semin Nephrol. 2018;38(1):3–11. doi: 10.1016/j.semnephrol.2017.09.002.
- Delanaye P, Cavalier E, Stehlé T, et al. Serum creatinine: not so simple. Nephron. 2017;136(4):302–308. doi: 10.1159/000536243.
- Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365(9466):1231–1238. doi: 10.1016/S0140-6736(05)74811-X.
- Bagshaw SM, Darmon M, Ostermann M, et al. Current state of the art for renal replacement therapy in critically ill patients with acute kidney injury. Intensive Care Med. 2017;43(6):841–854. doi: 10.1007/s00134-017-4762-8.
- Liu H, Hou S, Tian X. Risk factors of sepsis associated acute kidney injury in patients with sepsis: a meta-analysis. Intensive Care Res. 2023;3:1–8.
- Athavale AM, Fu C-Y, Bokhari F, et al. Incidence of, risk factors for, and mortality associated with severe acute kidney injury after gunshot wound. JAMA Netw Open. 2019;2(12):e1917254-e1917254. doi: 10.1001/jamanetworkopen.2019.17254.
- Jing J, Gao Y. Urine biomarkers in the early stages of diseases: current status and perspective. Discov Med. 2018;25(136):57–65.
- Cerri C, Genovesi S, Allegra M, et al. The chemokine CCL2 mediates the seizure-enhancing effects of systemic inflammation. J Neurosci. 2016;36(13):3777–3788. doi: 10.1523/JNEUROSCI.0451-15.2016.
- Maus UA, Waelsch K, Kuziel WA, et al. Monocytes are potent facilitators of alveolar neutrophil emigration during lung inflammation: role of the CCL2-CCR2 axis. J Immunol. 2003;170(6):3273–3278. doi: 10.4049/jimmunol.170.6.3273.
- Jia P, Xu S, Wang X, et al. Chemokine CCL2 from proximal tubular epithelial cells contributes to sepsis-induced acute kidney injury. Am J Physiol Renal Physiol. 2022;323(2):F107–F119. doi: 10.1152/ajprenal.00037.2022.
- Kellum JA, Lameire N, Aspelin P, et al. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):1–138.
- Pickering JW, Endre ZH. Back-calculating baseline creatinine with MDRD misclassifies acute kidney injury in the intensive care unit. Clin J Am Soc Nephrol. 2010;5(7):1165–1173. doi: 10.2215/CJN.08531109.
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287.
- Gniewkiewicz MS, Czerwińska M, Gozdowska J, et al. Urinary levels of CCL2 and CXCL10 chemokines as potential biomarkers of ongoing pathological processes in kidney allograft: an association with BK virus nephropathy. Pol Arch Intern Med. 2019;129(9):592–597. doi: 10.20452/pamw.14926.
- Pinheiro KHE, Azêdo FA, Areco KCN, et al. Risk factors and mortality in patients with sepsis, septic and non septic acute kidney injury in ICU. J Bras Nefrol. 2019;41(4):462–471. doi: 10.1590/2175-8239-JBN-2018-0240.
- Samsu N, Marzuki MJ, Pratiwi IC, et al. Predictors in-hospital mortality of septic vs non-septic acute kidney injury patients: an observational cohort study. F1000Res. 2022;10:1184. doi: 10.12688/f1000research.74540.2.
- Westenfelder C. Earlier diagnosis of acute kidney injury awaits effective therapy. Kidney Int. 2011;79(11):1159–1161. doi: 10.1038/ki.2011.19.
- Dobilienė D, Masalskienė J, Rudaitis Š, et al. Early diagnosis and prognostic value of acute kidney injury in critically ill patients. Medicina. 2019;55(8):506. doi: 10.3390/medicina55080506.
- Abarca Rozas B, Mestas Rodríguez M, Widerström Isea J, et al. A current view on the early diagnosis and treatment of acute kidney failure. Medwave. 2020;20(5):e7928–e7928. doi: 10.5867/medwave.2020.05.7928.
- Bossink AW, Paemen L, Jansen PM, et al. Plasma levels of the chemokines monocyte chemotactic proteins-1 and -2 are elevated in human sepsis. Blood. 1995;86(10):3841–3847. doi: 10.1182/blood.V86.10.3841.bloodjournal86103841.
- He J, Chen Y, Lin Y, et al. Association study of MCP-1 promoter polymorphisms with the susceptibility and progression of sepsis. PLoS One. 2017;12(5):e0176781. doi: 10.1371/journal.pone.0176781.
- Wang Y, Liu Q, Liu T, et al. Early plasma monocyte chemoattractant protein 1 predicts the development of sepsis in trauma patients: a prospective observational study. Medicine (Baltimore). 2018;97(14):e0356. doi: 10.1097/MD.0000000000010356.
- Hüsing AM, Wulfmeyer VC, Gaedcke S, et al. Myeloid CCR2 promotes atherosclerosis after AKI. J Am Soc Nephrol. 2022;33(8):1487–1500. doi: 10.1681/ASN.2022010048.
- Leon LR, Blaha MD, DuBose DA. Time course of cytokine, corticosterone, and tissue injury responses in mice during heat strain recovery. J Appl Physiol (1985). 2006;100(4):1400–1409. doi: 10.1152/japplphysiol.01040.2005.
- Song P, Li W, Xie J, et al. Cytokine storm induced by SARS-CoV-2. Clin Chim Acta. 2020;509:280–287. doi: 10.1016/j.cca.2020.06.017.
- Tweedie D, Karnati HK, Mullins R, et al. Time-dependent cytokine and chemokine changes in mouse cerebral cortex following a mild traumatic brain injury. Elife. 2020;9:e55827. doi: 10.7554/eLife.55827.
- Al-Saegh RM, Mohanad M, Khudhair NJ, et al. Using urinary interleukin-18 as a potential marker for early detection of acute kidney injury in intensive care unit. Saudi J Kidney Dis Transpl. 2021;32(2):341–347. doi: 10.4103/1319-2442.335445.
- Liu P, Li X, Lv W, et al. Inhibition of CXCL1-CXCR2 axis ameliorates cisplatin-induced acute kidney injury by mediating inflammatory response. Biomed Pharmacother. 2020;122:109693. doi: 10.1016/j.biopha.2019.109693.
- Nagarjun Batchu S, Ganesh Yerra V, Hong Y, et al. 397-P: epigenetic regulation of the chemokine CXCL2 in acute kidney injury in diabetes. Diabetes. 2023;72(1):397. doi: 10.2337/db23-397-P.
- Ma K, Meng Y, Zheng Z. WCN23-1031 CCL5 blockade reduces leukocyte accumulation and renal tubule injury in obese mice with acute kidney injury. Kidney Int Rep. 2023;8(3):S37–S38. doi: 10.1016/j.ekir.2023.02.085.