Abstract
Background
This study aimed to evaluate the patient survival rates based on the use of angiotensin-converting enzyme inhibitor (ACEI) or angiotensin II receptor blocker (ARB) in a large cohort of patients undergoing maintenance hemodialysis (HD).
Methods
Data from a national HD quality assessment program were used in this retrospective study. The patients were classified into four groups based on the use of renin-angiotensin system blockers (RASBs) as follows: No group, patients without a prescription of any anti-hypertensive drugs including RASBs; Other group, patients with a prescription of anti-hypertensive drugs excluding RASBs; ACEI group, patients with a prescription of an ACEI; and ARB group, patients with a prescription of an ARB.
Results
The 5-year survival rates in the no, other, ACEI, and ARB groups were 68.6%, 67.8%, 70.6%, and 69.2%, respectively. The ACEI group had the best patient survival trend among the four groups. In multivariable Cox regression analyses, no differences were observed between the ACEI and ARB groups. Among young patients and patients without diabetes or heart disease, the ACEI group had the best patient survival among the four groups. However, among patients with DM or heart disease, the ARB group had the best patient survival.
Conclusions
Our study found that patients receiving ACEI and ARB had comparable survival. However, patients receiving ARB had better survival in the subgroups of patients with DM or heart disease, and patients receiving ACEI had better survival in the subgroup of young patients or patients without diabetes or heart disease.
Introduction
Hemodialysis (HD) is the most common renal replacement therapy used to treat end-stage renal disease [Citation1,Citation2]. Patients undergoing HD have a lower chance of survival than those not undergoing dialysis [Citation3]. Therefore, various interventions are recommended to decrease the high mortality in HD, such as dialysis adequacy, glucose control, blood pressure control, and prevention of chronic kidney disease-mineral bone disease. Cardiovascular disease is the leading cause of death in patients undergoing HD and many researchers are focusing on ways to effectively decrease cardiovascular disease events or deaths in the context of HD [Citation1]. Activation of the renin-angiotensin system (RAS) is associated with poor outcomes in patients with essential hypertension and various heart diseases [Citation4–7]. Angiotensin-converting enzyme inhibitors (ACEI) and angiotensin II receptor blockers (ARB), which down-regulate the RAS, are among the most commonly used anti-hypertensive drugs. Furthermore, ACEI and ARB are the first choice of anti-hypertensive drugs in patients with non-dialysis chronic kidney disease, especially in those with diabetes mellitus (DM) and proteinuria [Citation8].
It is well known that RAS inhibition improves patient survival and cardiovascular events in various populations [Citation4–7]. However, results regarding the benefits of RAS inhibitors on survival in patients undergoing HD are inconsistent [Citation9]. Moreover, most studies on the association between RAS inhibitors and patient survival have been performed without distinguishing between ACEI and ARB therapy. ACEI and ARB have differences in their dialyzability characteristics and effects beyond blood pressure-lowering, and these differences may be more pronounced in patients undergoing HD. Therefore, analyses after dividing RAS inhibitors into ACEI and ARB categories, rather than composites of the two drugs, would be useful for identifying the different effects of the two drugs or the real effect of RAS inhibitors in patients undergoing HD. This study aimed to evaluate the patient survival rates based on the use of ACEI or ARB in a large cohort of patients undergoing maintenance HD.
Methods
Data source and study population
Laboratory and clinical data from a national HD quality assessment program and claims data from the Health Insurance Review and Assessment (HIRA) of South Korea were used in this retrospective study [Citation10,Citation11]. The fourth, fifth, and sixth HD quality assessment programs were conducted over a period of five years. The fourth program was conducted between July 2013 and December 2013, the fifth program was conducted between July 2015 and December 2015, and the sixth program was conducted between March 2018 and August 2018. The programs included patients who had been undergoing maintenance HD for at least three months, were receiving HD at least twice a week, and were at least 18 years old. We analyzed the HD quality assessment and claims data of all patients who had undergone HD and HD quality assessment.
Initially, 88,678 patients were included in the study. However, 32,459 patients were excluded because of repeated participation (n = 32,440) or insufficient data (n = 19). Additionally, 1316 patients were excluded because they had undergone HD with a non-tunneled or tunneled catheter. Finally, we excluded 3442 patients who were prescribed two or more renin-angiotensin system blockers (RASBs) or those who were prescribed RASB for less than 30 d during the six months of each assessment. A total of 51,461 patients were included in this study. The study was approved by the institutional review board of Yeungnam University Medical Center (approval no. YUMC 2022-01-010). Patients did not provide informed consent because their records and information were anonymized and de-identified before the analysis.
Variables
Data on age, sex, underlying cause of end-stage renal disease, HD vintage (months), and type of vascular access were collected. Data on hemoglobin (g/dL) level; Kt/Vurea; serum albumin (g/dL), serum calcium (mg/dL), serum phosphorus (mg/dL), and serum creatinine (mg/dL) levels; pre-dialysis systolic blood pressure (SBP, mmHg); pre-dialysis diastolic blood pressure (DBP, mmHg); and ultrafiltration volume (L/session) were collected as part of the evaluation. These data were collected monthly and the averages of all monthly laboratory values were calculated. Kt/Vurea was calculated using the Daugirdas equation [Citation12].
Table S1 lists the medication codes used in this study. Usage of anti-hypertensive drugs including RASBs for ≥ 30 d during 6 months of each HD quality assessment period was considered as administration. The patients were classified into four groups based on the use of anti-hypertensive drugs and/or RASBs as follows: No group, patients without a prescription of any anti-hypertensive drugs including RASBs; Other group, patients with a prescription of anti-hypertensive drugs excluding RASBs; ACEI group, patients with a prescription of an ACEI; and ARB group, patients with a prescription of an ARB.
Medications, such as aspirin, clopidogrel, and statins, were also evaluated. If one or more prescriptions were identified for a year before the HD quality assessment program, it was defined as ‘use of the medication’. Comorbidities were assessed for one year prior to the HD quality assessment program. The Charlson Comorbidity Index (CCI), which includes 17 comorbidities, was used to define comorbidities [Citation13,Citation14]. All the patients in our study underwent HD and had renal disease. CCI scores were calculated for all patients.
We followed up the patients until April 2022. The end of the follow-up period was when the patients was transferred to peritoneal dialysis or kidney transplantation. The data were censored at this point. Clinical outcomes, except death, were defined using electronic data collected during the follow-up period. The codes for censoring were O7072, O7071, and O7061 for peritoneal dialysis and R3280 for kidney transplantation. Data on mortality status and the time of death were obtained from the HIRA claims data. In South Korea, the majority of the population is required to enroll in the National Health Insurance program and pay for insurance according to their income, regardless of whether they received medical care. Patients who receive medical care, except non-essential care such as cosmetic surgery, pay approximately 5–30% of the total costs to the hospital where they undergo the relevant procedures. Afterward, the hospital submits the claims data to the HIRA service, which reimburses the remaining cost. This claims data process typically occurs within one month after the medical treatment. Moreover, claims data include items related to the outcome of the treatment, and it is mandatory to specify this information during each claim. The options for these items include treatment continuation, transfer, conveyance, death, or discharge. Determining the mortality status is closely associated with the costs to the patient and the subsequent expenses for medical treatment. Therefore, it is relatively accurately calculated, and the mortality status is confirmed and applied within a few weeks.
Statistical analyses
The data were analyzed using two statistical software packages: the SAS Enterprise Guide version 7.1 (SAS Institute Inc, Cary, NC) or R version 3.5.1 (Vienna, Austria). Categorical variables were presented as frequencies and percentages, whereas continuous variables were presented as means and standard deviations or standard errors of the mean. The statistical significance of the differences between categorical variables was assessed using Pearson’s χ2 test or Fisher’s exact test. Differences between continuous variables were assessed using one-way analysis of variance, followed by Tukey’s post-hoc test. Cohort after weighting was compared using a survey-weighted generalized linear model among the groups.
There were significant differences in baseline characteristics and sample sizes among the four groups. We used propensity score weighting to balance these characteristics and to ensure that the results of our analyses were not biased. We created propensity score weights for the four groups using generalized boosted models for the following variables: age; sex; underlying cause of end-stage renal disease; CCI score; HD vintage; ultrafiltration volume; Kt/Vurea; hemoglobin, albumin, creatinine, phosphorus, and calcium serum levels; SBP; DBP; the presence of myocardial infarction (MI) or congestive heart failure (CHF); and administration of aspirin, clopidogrel, or statins. Propensity scores were calculated using a generalized boosted model and were used to further calculate the inverse probability treatment weights. Finally, we defined the cohort after weighting as the sample with weights assigned to each case. The sample sizes in the No, Other, ACEI, or ARB groups was 49,982, 47,939, 40,003, and 49,927, respectively, as shown in . We assessed three assumptions required to obtain valid estimates from the propensity score model. First, to evaluate the balance, we compared absolute standardized mean differences between the treatment groups on the observed characteristics, before and after weighting. Standardized differences < 0.2 for each covariate indicated the balance on their propensity score weighted distribution of covariates among different groups. Second, to identify non-zero probability of receiving each treatment, we evaluated the adequacy of propensity score model using boxplots to compare the distribution of propensity scores across groups to assess the overlap assumption. Third, we compared the distribution of all covariates among the groups using a survey-weighted generalized linear model.
Table 1. Clinical characteristics of the patients.
Additionally, we performed propensity score matching between two groups and estimated propensity scores using logistic regression models and the following variables: age; sex; underlying cause of end-stage renal disease; CCI score; HD vintage; ultrafiltration volume; Kt/Vurea; hemoglobin, albumin, creatinine, phosphorus, and calcium serum levels; SBP; DBP; the presence of MI or CHF; and administration of aspirin, clopidogrel, or statins. Participants in the small sample size group were matched with participants in the large sample size using 3:1 nearest neighbor matching without replacement and with a matching tolerance (caliper) of 0.2; the nearest neighborhood matching was based on propensity scores.
Survival curves were estimated using Kaplan–Meier curves. Hazard ratios (HRs) and confidence intervals (CIs) were calculated using the Cox regression analysis. p Values for comparison of survival curves were determined using the log-rank test. Multivariable Cox regression analyses were adjusted for age; sex; type of vascular access; underlying cause of end-stage renal disease; CCI score; HD vintage; ultrafiltration volume; Kt/Vurea; hemoglobin, albumin, creatinine, phosphorus, and calcium serum levels; SBP; DBP; the presence of MI or CHF; and administration of aspirin, clopidogrel, or statins. Multivariable Cox regression analyses were performed using the enter mode. Additionally, for the cohort after weighting, we performed weighted Cox regression to estimate HR and 95% CI among the groups. We performed competing risk analyses to decrease the effects of censored data. We defined kidney transplantation or peritoneal dialysis as a competing risk and applied the Fine and Gray competing risk model. Statistical significance was set at p < 0.05.
Results
Clinical characteristics
The numbers of patients in the No, Other, ACEI, and ARB groups were 19,863; 5,205; 790; and 25,603, respectively. There were significant differences in the baseline characteristics among the four groups (Table S2). Participants’ data after using propensity score weights are shown in . Subsequently, we compared the distribution of all covariates using survey-weighted generalized linear model and the differences in baseline characteristics among the four groups were attenuated. A histogram of propensity score is shown in Figure S1. The minimal propensity score was 0.005 in our analyses (Figure S1). A relatively large minimal propensity score would attenuate the possibility of over-weighting by extreme values of propensity scores. Figure S2 illustrates that the absolute standardized mean differences significantly decreased after weighting, which indicate that the propensity score model was well balanced. In addition, Figure S2 shows covariate balance before and after weighting for each comparison and all values were < 0.2. Figure S3 shows the empirical propensity score distributions, which reveal that the overlap assumption appears to be met in our model.
Survival analyses
The number of patients in the survivor, death, peritoneal dialysis, or kidney transplantation subgroups at the end-point of follow-up was 26,802 (53.6%), 19,477 (39.0%), 154 (0.3%), and 3,549 (7.1%) in the No group; 23,402 (49.4%), 19,945 (42.1%), 223 (0.5%), and 3,823 (8.1%) in the Other group; 21,797 (54.5%), 14,506 (36.3%), 155 (0.4%), and 3,545 (8.9%) in the ACEI group; and 26,962 (54.0%), 18,990 (38.0%), 175 (0.4%), and 3,800 (7.6%) in the ARB group, respectively (p < 0.001).
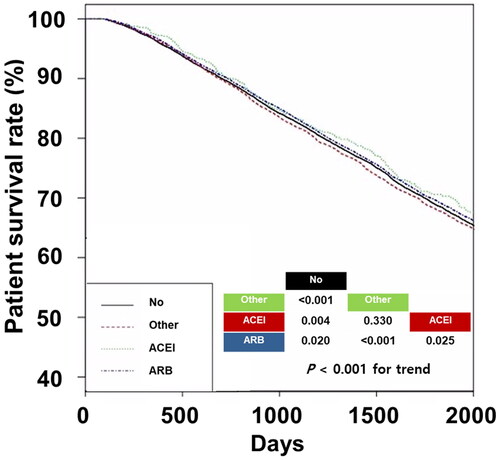
The 5-year survival rates in the No, Other, ACEI, and ARB groups were 68.6%, 67.8%, 70.6%, and 69.2%, respectively (, p < 0.001 for trend). The ACEI group had the best patient survival trend among the four groups. Univariate Cox regression analyses showed that the HRs were 1.05 (95% CI, 1.03–1.07) in the other group, 0.92 (95% CI, 0.91–0.95) in the ACEI group, and 0.98 (95% CI, 0.96–0.99) in the ARB group compared to that of the No group (). In addition, the ACEI and ARB groups had lower HRs than the other group, and the ARB group had higher HRs than the ACEI group. Multivariable Cox regression analyses showed similar trends, except in the comparison between the ACEI and ARB groups.
Table 2. Cox regression analyses for patient survival.
Additionally, we performed analyses using total cohort before propensity score weighting (). Multivariate Cox regression analyses using total cohort before weighting showed that the ARB group had better patient survival than no or other groups; however, there was no significant difference in patient survival between the ACE and ARB groups. Although not statistically significant, the ACEI group had better patient survival than no or other groups. presents the results using the competing risk model.
Table 3. Cox regression analyses using a competing risk model.
Subgroup analyses were performed based on sex, age, and presence of DM and heart disease (MI or CHF). Multivariable Cox regression analyses showed that in both sexes and in older patients, there was no significant difference in patient survival between the ACEI and ARB groups (Figure S4). Among male patients, the ACEI and ARB groups had better survival rates than the other and no groups. Among female and older patients, the ACEI and ARB groups had better patient survival than the other group. Among young patients and patients without DM or heart disease, the ACEI group had the best patient survival among the four groups. However, among patients with DM or heart disease, the ARB group had the best patient survival.
Sensitivity analyses
We compared the groups after propensity score matching and found that the differences between two groups were attenuated (Table S3). Cox regression analyses using the propensity score matched cohort showed that ACEI group had an HR of 1.06 (95% CI, 0.93–1.21, p = 0.360) in univariate and 1.00 (95% CI, 0.88–1.14, p = 0.965) in multivariate analyses compared to ARB group. No significant differences in patient survival were observed between the groups after propensity score matching.
To enhance the accuracy of subgroup analyses, we analyzed the ACEI and ARB groups within each subgroup (Table S4). In patients without DM or heart disease, the statistical significance was weak; however, the ACEI group showed better patient survival than the ARB group. Although in patients with DM or heart disease, the statistical significance was weak, the ARB group had lower HR compared to the ACEI group. Despite weaker overall statistical significances than results using the cohort after weighting, the trends were similar with those using cohort after weighting. Furthermore, we analyzed the subgroups of the cohort before propensity score weighting (Figure S5). After matching the subgroups by sex, the presence or absence of heart disease, or the presence of DM, the ARB group had better survival rates than those of the no or other groups. In young patients, or in patients without DM or heart disease, the statistical significance was weak; however, the ACEI group showed a better survival rate than that of the ARB group. Similarly, in patients with DM or heart disease, the ARB group had a lower HR than that of the ACEI group, although the difference was not significant. Although the overall statistical significance of the observed correlations was weaker than those observed after weighting, the trends were similar to those observed after weighting.
Furthermore, we performed subgroup analyses for MI and CHF, separately, using the original cohort, the cohort after weighting, or the cohort after propensity score matching for the ACEI and ARB groups (Table S5). In the original cohort, all four groups with/without MI or CHF in the ACEI and ARB groups showed similar survival rates; however, the ARB group had better survival rates than those of the no or other groups. In the cohort after weighting, the presence or absence of MI was not associated with differences in survival rates between the ACEI and ARB groups. However, the ACEI group had a better survival rate than that of the ARB group in the patients without CHF, whereas the ARB group had a better survival rate than that of the ACE group among those with CHF. The survival rates did not differ significantly between ACE and ARB groups in the matched cohort. Thus, the cohort after weighting exhibited more significant differences than those observed before weightings due to large sample size. Moreover, CHF may play a greater role than that of MI in the difference between the ACEI and ARB groups.
Discussion
Our study included 51,461 patients undergoing maintenance HD. In our study, there were significant differences in the number of patients and their characteristics between groups, and we performed analyses using propensity score weighting. When analyzed using the total population, the ACEI or ARB groups had better patient survival, and no differences were observed between the ACEI and ARB groups. Among young patients or patients without DM or heart disease, the ACEI group had the best patient survival among the four groups. Among patients with DM or heart disease, ARB had the best patient survival among the four groups. In both sexes and in older patients, the ACEI and ARB groups had similar survival rates.
Comparative studies between ACEI and ARB have shown inconsistent results according to the comorbidities of patients, but also within studies using patients with the same comorbidities. Shireman et al. included 4997 patients undergoing dialysis (3555 ACEI and 1442 ARB users) and compared all-cause mortality and cardiovascular events [Citation15]. In their study, ACEI use was associated with greater all-cause mortality and a trend toward an increased risk of cardiovascular events than ARB use. Kido et al. enrolled 3762 patients with secondary hyperparathyroidism and HD [Citation16]. They showed that patients with ARB use had better survival than non-users, and patients with ACEI use had similar survival rates compared to non-users, despite the non-significant difference between ACEI and ARB use. Chan et al. included 28,628 patients undergoing HD [Citation17]. Their study showed that ARB use was associated with better patient survival than ACEI use in the univariate analysis, but the difference was not significant after adjusting for other factors in the multivariate analysis. Subgroup analyses showed that patients with DM or coronary artery disease who received ARB had a slightly higher survival rate than those who received ACEI. However, this difference was not statistically significant. In contrast, other studies on patients undergoing peritoneal dialysis, with non-dialysis chronic kidney disease, vascular disease, or high-risk DM alone showed comparable patient survival with ACEI and ARB use [Citation18–20]. In addition, a meta-analysis of 20 studies on hypertensive patients showed better patient outcomes in ACEI users than in ARB users [Citation21]. These studies revealed that ARB may have a modest benefit in patient survival compared to ACEI in patients undergoing HD, especially in high-risk groups such as those with DM or heart disease. In patients with peritoneal dialysis, non-dialysis chronic kidney disease, or high cardiovascular risk alone, a comparable trend in patient survival was observed with ACEI and ARB. A better trend of patient survival in ACEI users was observed in patients with less severe comorbidities than in those on dialysis or with high cardiovascular risk.
Our study enrolled relatively stable HD patients without admission or transfusion during the six months of each HD quality assessment. Overall, the patients’ condition was better in our study than in other studies involving patients undergoing HD. In our study, the ACEI and ARB groups exhibited similar patient survival in the overall cohort. The ARB group was associated with better survival in subgroups of patients with DM or heart disease, whereas the ACEI group was associated with better survival in subgroups of young patients or patients without DM or heart disease. Our results showed trends similar to those reported in previous studies based on different comorbidities.
Some issues may be associated with the different trends in survival between ACEI and ARB in patients undergoing HD, with or without severe comorbidities. ACEI affect ARBs, increase angiotensin II levels through alternative pathways, and upregulate kinin synthesis [Citation22–24]. In addition, a previous study showed that ACEI had a better effect on endothelial dysfunction than ARB, whereas ARB had a better effect on inflammation than ACEI [Citation25]. The different effects of ACEI and ARB, beyond blood pressure-lowering, may contribute to the difference in the outcomes in patients with different comorbidities. However, the mechanism by which these complex effects manifest in various diseases remains unclear. Additionally, most ACEI, but not ARB, can be easily dialyzed during an HD session [Citation26,Citation27]. Therefore, removal of ACEI during HD sessions can lead to fluctuations in drug levels, which can lead to poorer patient survival with ACEI than with ARB.
Several randomized controlled trials have evaluated the effect of RAS blockades in HD patients. Iseki et al. enrolled 469 HD patients with hypertension and compared patients receiving olmesartan and other medications except RAS blockades [Citation28]. The study had a median follow up period of 3.5 years; however, statistically significant differences in major cardiovascular event or death were not obtained. They suggested that the statistical non-significance was associated with low event rate. Additionally, they revealed that tachycardia was associated with a high mortality and use of β-blockers in other medication groups may attenuate the efficacy of olmesartan. Two other studies evaluated the efficacy of different ACEI in HD patients [Citation29,Citation30]. Zannad et al. compared the patients with fosinopril and placebo in HD patients [Citation29]. They identified a favorable trend in fosinopril despite no statistical significance and suggested that the statistical significance might have occurred when the sample size exceeded 476 patients for each group. Agarwal et al. compared the patients with HD using lisinopril and β-blockers [Citation30]. However, their study was terminated early due to superiority of β-blockers in all-cause serious events, all-cause hospitalization, and hyperkalemia. They suggested that a large sample is requred to identify statistical significance. Furthermore, lisinopril is easily dialyzed, which would attenuate the efficacy of RAS inhibitors in HD patients [Citation31]. However, fosinopril is not dialyzed and demonstrates a relatively favorable outcome compared to using lisinopril. Similarly, ARB is generally not dialyzed compared to ACEI; thus, the favorable outcome of ARB in some subgroups compared to those of no or other groups may be associated with the non-dialyzability of ARB compared to ACEI.
Another issue is the comparison of efficacy between β-blockers compared to RAS blockades in HD patients. Agarwal et al. have shown the clinical benefit of β-blockers compared to the weak benefit of RAS blockades [Citation30]. Both activation of sympathetic nervous system and volume overload are well-known risk factors of hypertension and high mortality in patients with HD. They can suppress renin activity in HD patients who are at a high risk of volume overload. These effects may explain weak efficacy of RAS blockades in previous studies using HD patients. Conversely, the sympathetic nervous system is frequently activated in HD patients and β-blockers more effectively suppress the sympathetic nervous system than RAS blockade does. However, insufficient evidence is available in this aspect. A recent study evaluated the combination therapy of β-blockers and RAS blockades and showed clinical benefits compared to monotherapy [Citation32]. Nonetheless, further investigations are required to identify the effect of monotherapy or combination therapy in HD patients.
We performed sensitivity analyses using original cohort, cohort with propensity score matching between ACEI and ARB groups within each subgroup. Moreover, we performed Cox regression analyses considering kidney transplantation and peritoneal dialysis as competing risks. The cohort after weighting had very large sample sizes; however, the survival rates did not differ significantly between ACEI and ARB groups. Some subgroups alone had statistical significance. Furthermore, in our study, the overall statistical significance was weaker in analyses without very large sample sizes, such as the original cohort or propensity score matched cohort compared the findings observed after weighting. Cox regression analyses revealed no significant differences with or without considering competing risk. However, in this relation to this, one aspect that requires consideration is the requirement for a substantial number of patients to discern the clinical impact between ACEI and ARB groups. Moreover, the fact that a large sample size is required for statistical significance and that HR values are not markedly significant can be associated with weak clinical significance and the possibility of positive results by statistical chance. Nevertheless, the significance of this study lies in its comparison of ACEI and ARB groups with a large sample size. differences might have arisen in certain subgroups, suggesting the need for careful consideration when comparing these two. In this respect, identifying potential differences in specific subgroups may be deemed meaningful, positioning this as a pilot study for future research.
Our study had some limitations. First, it was retrospective, and the sample size and baseline characteristics of the four groups differed significantly. However, we attempted to attenuate the differences in sample size and baseline characteristics by using propensity score weighting. After weighting, the sample size and baseline characteristics were similar among the four groups. Second, comorbidities and the use of anti-hypertensive drugs were evaluated using claims data, which may have discrepancy between the actual administration and physician prescriptions. In addition, clinicians may use ICD-10 codes for various comorbidities despite the absence of disease. Additionally, our study lacked information on the indication for ACEI or ARB administration. The effects of ACEI or ARB on patient survival may differ between patients receiving medications for MI or CHF and those receiving medications to lower blood pressure. Third, our study did not include data on the cause of death or detailed data on heart function such as heart rate, left ventricular hypertrophy, cardiac mass, or ejection fraction. The benefits of ACEI and ARB are mainly related to cardiovascular diseases, and information on cardiovascular death and/or heart function would be useful in identifying differences beyond all-cause mortality.
In conclusion, our study found that patients receiving ACEI and ARB had comparable survival despite demonstrating a better survival than patients without a prescription of anti-hypertensive drugs or with a prescription of other anti-hypertensive drugs apart from ACEI or ARB. However, patients receiving ARB had better survival in the subgroups of patients with DM or heart disease, and patients receiving ACEI had better survival in the subgroup of young patients or patients without DM or heart disease. These findings reveal that the benefit of ACEI or ARB may differ according to the characteristics of patients undergoing HD. However, our findings cannot be used to conclude the superiority of any medication due to conflicting results in different cohorts. Considering the limitations of this study, the results should be interpreted with caution. Further, randomized prospective studies are required to determine whether ACEI and ARB have different effects on survival in patients undergoing HD.
Statement of ethics
This study was approved by the Institutional Review Board of Yeungnam University Medical Center (approval no. YUMC 2022-01-010). The research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The Institutional Review Boarders waived the requirement for informed consent because of the retrospective study design and the anonymization and de-identification of patient records and information before the analysis.
Author contributions
SH Kang conceptualized and designed the study, and performed the analysis. BY Kim, EJ Son, and GO Kim generated and collected the data. SH Kang, BY Kim, EJ Son, and GO Kim interpretation of data. SH Kang and JY Do wrote the manuscript. All authors approved the final version of the manuscript.
Supplemental Material
Download PDF (1.4 MB)Acknowledgments
The epidemiologic data used in this study were obtained from Periodic HD Quality Assessment by HIRA. The requirement for informed consent was waived due to the retrospective nature of the study. De-identifcation was performed, and data usage was permitted by the National Health Information Data Request Review Committee of HIRA.
Disclosure statement
The authors declare no competing interests.
Data availability statement
The raw data were generated at the HIRA Service. The database can be requested from the HIRA Service by sending a study proposal including the purpose of the study, study design, and duration of analysis through an e-mail ([email protected]) or at the web site (https://www.hira.or.kr). The authors cannot distribute the data without permission.
Additional information
Funding
References
- ESRD Registry Committee: Korean Society of Nephrology. Current renal replacement therapy in Korea, 2021. [assessed 14 June 2023]. Available from: https://ksn.or.kr/bbs/index.php?code=report
- US Renal Data System. USRDS 2020 annual data report: atlas of chronic kidney disease in the United States. Bethesda (MD): National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2020.
- Choi H, Kim M, Kim H, et al. Excess mortality among patients on dialysis: comparison with the general population in Korea. Kidney Res Clin Pract. 2014;33(2):1–10. doi: 10.1016/j.krcp.2014.04.001.
- Yusuf S, Pitt B, Davis CE, et al. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325(5):293–302. doi: 10.1056/NEJM199108013250501.
- Pfeffer MA, Braunwald E, Moyé LA, et al. The save investigators. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. N Engl J Med. 1992;327(10):669–677. doi: 10.1056/NEJM199209033271001.
- The Acute Infarction Ramipril Efficacy (AIRE) Study Investigators. Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure. Lancet. 1993;342:821–828.
- Allikmets K, Parik T, Viigimaa M. The renin-angiotensin system in essential hypertension: associations with cardiovascular risk. Blood Press. 1999;8(2):70–78. doi: 10.1080/080370599438239.
- Kidney Disease: improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2021;99(3S):S1–S87. doi: 10.1016/j.kint.2020.11.003.
- Georgianos PI, Tziatzios G, Roumeliotis S, et al. Effect of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers on cardiovascular outcomes in dialysis patients: a systematic review and meta-analysis. Nephrol Dial Transplant. 2023;38(1):203–211. doi: 10.1093/ndt/gfac253.
- Kang SH, Kim BY, Son EJ, et al. Comparison of patient survival according to Erythropoiesis-Stimulating agent type of treatment in maintenance hemodialysis patients. J Clin Med. 2023;12(2):625. doi: 10.3390/jcm12020625.
- Health Insurance Review & Assessment Service. 6th Hemodialysis quality assessment program. [assessed 14 June 2023]. Available from: https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA020002000100&brdScnBltNo=4&brdBltNo=6619#none
- Daugirdas JT. Second generation logarithmic estimates of single-Pool variable volume Kt/V: an analysis of error. J Am Soc Nephrol. 1993;4(5):1205–1213. doi: 10.1681/ASN.V451205.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8.
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83.
- Shireman TI, Mahnken JD, Phadnis MA, et al. Comparative effectiveness of renin-angiotensin system antagonists in maintenance dialysis patients. Kidney Blood Press Res. 2016;41(6):873–885. doi: 10.1159/000452590.
- Kido R, Akizawa T, Fukagawa M, et al. Interactive effectiveness of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers or their combination on survival of hemodialysis patients. Am J Nephrol. 2017;46(6):439–447. doi: 10.1159/000482013.
- Chan KE, Ikizler TA, Gamboa JL, et al. Combined angiotensin-converting enzyme inhibition and receptor blockade associate with increased risk of cardiovascular death in hemodialysis patients. Kidney Int. 2011;80(9):978–985. doi: 10.1038/ki.2011.228.
- Messerli FH, Bangalore S, Ram VS, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;359(4):426–427; author reply 427.
- Xie X, Liu Y, Perkovic V, et al. Renin-Angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: a bayesian network meta-analysis of randomized clinical trials. Am J Kidney Dis. 2016;67(5):728–741. doi: 10.1053/j.ajkd.2015.10.011.
- Shen JI, Saxena AB, Montez-Rath ME, et al. Comparative effectiveness of angiotensin receptor blockers vs. angiotensin-converting enzyme inhibitors on cardiovascular outcomes in patients initiating peritoneal dialysis. J Nephrol. 2017;30(2):281–288. doi: 10.1007/s40620-016-0340-3.
- van Vark LC, Bertrand M, Akkerhuis KM, et al. Angiotensin-converting enzyme inhibitors reduce mortality in hypertension: a meta-analysis of randomized clinical trials of renin-angiotensin-aldosterone system inhibitors involving 158,998 patients. Eur Heart J. 2012;33(16):2088–2097. doi: 10.1093/eurheartj/ehs075.
- Suzuki H. Therapeutic efficacy of renin-angiotensin blockade in patients receiving dialysis. Ther Adv Cardiovasc Dis. 2009;3(5):397–405. doi: 10.1177/1753944709338339.
- Wenzel UO, Krebs C, Benndorf R. The angiotensin II type 2 receptor in renal disease. J Renin Angiotensin Aldosterone Syst. 2010;11(1):37–41. doi: 10.1177/1470320309347787.
- Kovarik JJ, Antlanger M, Domenig O, et al. Molecular regulation of the renin-angiotensin system in haemodialysis patients. Nephrol Dial Transplant. 2015;30(1):115–123. doi: 10.1093/ndt/gfu265.
- Gamboa JL, Pretorius M, Todd-Tzanetos DR, et al. Comparative effects of angiotensin-converting enzyme inhibition and angiotensin-receptor blockade on inflammation during hemodialysis. J Am Soc Nephrol. 2012;23(2):334–342. doi: 10.1681/ASN.2011030287.
- Cice G, Di Benedetto A, D’Isa S, et al. Effects of telmisartan added to angiotensin-converting enzyme inhibitors on mortality and morbidity in hemodialysis patients with chronic heart failure a double-blind, placebo-controlled trial. J Am Coll Cardiol. 2010;56(21):1701–1708. doi: 10.1016/j.jacc.2010.03.105.
- Denker MG, Cohen DL. Antihypertensive medications in end-stage renal disease. Semin Dial. 2015;28(4):330–336. doi: 10.1111/sdi.12369.
- Iseki K, Arima H, Kohagura K, et al. Effects of angiotensin receptor blockade (ARB) on mortality and cardiovascular outcomes in patients with long-term haemodialysis: a randomized controlled trial. Nephrol Dial Transplant. 2013;28(6):1579–1589. doi: 10.1093/ndt/gfs590.
- Zannad F, Kessler M, Lehert P, et al. Prevention of cardiovascular events in end-stage renal disease: results of a randomized trial of fosinopril and implications for future studies. Kidney Int. 2006;70(7):1318–1324. doi: 10.1038/sj.ki.5001657.
- Agarwal R, Sinha AD, Pappas MK, et al. Hypertension in hemodialysis patients treated with atenolol or lisinopril: a randomized controlled trial. Nephrol Dial Transplant. 2014;29(3):672–681. doi: 10.1093/ndt/gft515.
- Weir MA, Fleet JL, Dixon SN, et al. Angiotensin converting enzyme inhibitor dialyzability and outcomes in older patients receiving hemodialysis. Blood Purif. 2015;40(3):232–242. doi: 10.1159/000438821.
- Luño J, Varas J, Ramos R, et al. The combination of beta blockers and renin-angiotensin system blockers improves survival in incident hemodialysis patients: a propensity-matched study. Kidney Int Rep. 2017;2(4):665–675. doi: 10.1016/j.ekir.2017.03.001.