Abstract
Objective
This study was designed to observe the effect of toll-like receptor 4 (TLR4)/nuclear factor kappa-B (NF-κB) pathway activity on sepsis-associated acute kidney injury (SA-AKI), thereby providing new considerations for the prevention and treatment of SA-AKI.
Methods
The rats were divided into Sham, cecal ligation and puncture (CLP), CLP + vehicle, and CLP + TAK-242 groups. Except the Sham group, a model of CLP-induced sepsis was established in other groups. After 24 h, the indicators related to kidney injury in blood samples were detected. The pathological changes in the kidneys were observed by hematoxylin-eosin staining, and tubular damage was scored. Oxidative stress-related factors, mitochondrial dysfunction-related indicators in each group were measured; the levels of inflammatory factors in serum and kidney tissue of rats were examined. Finally, the expression of proteins related to the TLR4/NF-κB signaling pathway was observed by western blot.
Results
Compared with the CLP + vehicle and CLP + TAK-242 groups, the CLP + TAK-242 group reduced blood urea nitrogen (BUN), creatinine (Cr), cystatin-C (Cys-C), reactive oxygen species (ROS), malondialdehyde (MDA), and inflammatory factors levels (p < 0.01), as well as increased superoxide dismutase (SOD) activity of CLP rats (p < 0.01). Additionally, TAK-242 treatment improved the condition of CLP rats that had glomerular and tubular injuries and mitochondrial disorders (p < 0.01). Further mechanism research revealed that TAK-242 can inhibit the TLR4/NF-κB signaling pathway activated by CLP (p < 0.01). Above indicators after TAK-242 treatment were close to those of the Sham group.
Conclusion
TAK-242 can improve oxidative stress, mitochondrial dysfunction, and inflammatory response by inhibiting the activity of TLR4/NF-κB signaling pathway, thereby preventing rats from SA-AKI.
Introduction
Sepsis has been regarded as a life-threatening syndrome for a long time. It is mainly characterized by multiple organ dysfunction (including kidney injury) due to the maladjustment of the body’s response to infection [Citation1]. It is worth noting that the kidney is one of the earliest organs affected by sepsis, and up to 60% patients with sepsis have acute kidney injury (AKI) [Citation2]. In addition, about two-thirds of patients suffer from AKI derived from sepsis. Therefore, some studies put forward that AKI should be listed as an early sign of sepsis [Citation3]. Possibly as a result of the differences in the diagnosis, there is a lack of epidemiological investigation on sepsis-associated AKI (SA-AKI) in recent years. In 2010, Adhikari et al. uncovered that there were 19 million new cases of SA-AKI in the world every year [Citation4]. A previous survey also pointed out that there was one SA-AKI patient in every 1000 people [Citation5]. However, the actual incidence of SA-AKI may be much higher. More terribly, SA-AKI is a common death-induced complication in hospitalized and critically ill patients. Studies have demonstrated that SA-AKI led to an increase in hospital mortality of 6–8 times, and moreover, and that the patients with SA-AKI had a three-fold higher death rate than those chronic kidney disease [Citation6]. However, there is still no clinically feasible way to stop SA-AKI. Early antibiotic administration and infection source control are preferred in the treatment of sepsis. However, the preventive effect of the above treatments is not satisfactory, and inappropriate antibiotic therapy may be detrimental to critically ill patients [Citation7,Citation8]. Collectively, the mechanism underlying sepsis-induced kidney injury is unclear, which limits the development of drugs for SA-AKI. Therefore, it is urgent to reveal the mechanism of SA-AKI and seek corresponding therapeutic drugs.
Sepsis can cause mitochondrial damage and immune dysfunction, and risk factors such as NO in the inflammatory response can induce mitochondrial dysfunction, oxidative stress, and apoptosis [Citation9–11]. Mitochondria, as the energy center of the organism, are involved in oxidative metabolic processes in eukaryotes and are where most reactive oxygen species (ROS) are produced [Citation12]. The leakage of mitochondrial DNA from damaged cells into the circulatory system when coupled with bacterial infection may lead to systemic inflammatory response syndrome [Citation13]. Toll-like receptor 4 (TLR4)/nuclear factor κB (NF-κB) signaling pathway is an important pathway involved in mediating inflammation, which is considered as the key factor for the pathogenesis of SA-AKI [Citation14,Citation15]. TLR4 is an important protein that participates in the inflammatory response and connects specific with nonspecific immunity in vivo. TLR4 is mainly distributed on the surface of immune cells such as monocytes, polymorphonuclear cells, T cells, B cells and natural killer cells [Citation16,Citation17]. NF-κB is present in almost all animal cells. When cells were stimulated by viruses, cytokines and other external factors, the activated NF-κB signaling pathway promotes the production of inflammatory responses and immune responses in the body, increases the level of inflammatory factors like interleukin (IL)-6 in vivo, and then exacerbates the systemic or local inflammatory responses [Citation18,Citation19]. The study conducted by Zhao et al. demonstrated that the levels of mRNA and protein expression related to the TLR4/NF-kB signaling pathway were enhanced in septic rats with kidney injury [Citation20]. Also, Zhu et al. proposed that the expression of TLR4/NF-kB signaling pathway in tubular cells was raised in SA-AKI tissues [Citation21].
Resatorvid (TAK-242) is an inhibitor of TLR4/NF-ΚB signaling pathway. Some studies have stated that TAK-242 plays a protective role in sepsis-induced kidney injury. For instance, Fenhammar et al. revealed that TAK-242 was able to reverse sepsis-induced kidney injury in sheep [Citation22]. Although TAK-242 has been reported to play a protective role in SA-AKI, its role has not been studied in cecal ligation and puncture (CLP)-induced SA-AKI. Whether TAK-242 have a preventive effect is also unknown. In this study, we assumed that TAK-242 improves the mitochondrial dysfunction in kidney tissues to prevent rats from CLP-induced SA-AKI by inhibiting TLR4/NF-κB signaling pathway. Briefly, this study was designed to explore the role of TAK-242 in preventing rats from SA-AKI and provide new thinking for the TAK-242 treatment in SA-AKI.
Materials and methods
In this study, twenty-four 8-week-old male Sprague Dawley (SD) rats were obtained from the institution of Beijing Vital River Laboratory Animal Technology Co., Ltd. The rats were randomly divided into four groups (6 rats per group) and named Sham group, CLP group, CLP + vehicle group, and CLP + TAK-242 group. To observe the effect of TLR4/NF-κB signaling pathway on sepsis, the same amount of normal saline or TLR4/NF-κB inhibitor (TAK-242, 10 mg/kg) [Citation23] was injected into the tail vein of rats in the CLP + vehicle and CLP + TAK-242 groups. Subsequently, CLP was used to induce the model of sepsis in the CLP, CLP + vehicle, and CLP + TAK-242 groups [Citation24]. Briefly, rats were completely anesthetized by sevoflurane, and then their ceca were exposed. Next, the ceca were punctured using a sterile tip and a small amount of content was squeezed out. Subsequently, the ceca was reset and the abdominal cavity was closed. Additionally, the anesthetized rats in the Sham group were subject to laparotomy, with the ceca exposed and subsequent the abdominal cavity closed. After 24 h of surgery, the serum of rats in each group was collected. Upon anesthesia, the kidney tissue was collected and the rats were euthanized. All experimental schemes related to rats in this study were approved by the Ethics Committee of Shanxi Bethune Hospital (SBQDL-2022-014) and in line with the welfare of experimental animals.
Detection of kidney injury level
The contents of blood urea nitrogen (BUN), creatinine (Cr), and cystatin-C (Cys-C) in the serum of rats in each group were measured by commercial kits. The commercial kits used in this assay included BUN detection kit (SH960W, G-CLONE, China), Cr detection kit (DICT, BioAssay Systems, Hayward, CA) and Cys-C ELISA kit. First, the collected blood samples were left to stand for 20 min. Next, the samples were centrifuged at 4 °C and 2000 rpm for 10 min, and then the supernatant (i.e., serum) was separated and stored in fresh EP tubes. Subsequently, the absorbance of each sample was measured using a high-throughput screening (HTS) enzyme labeling instrument (PHERAstar FSX, BMG Labtech, Germany) according to the instructions of corresponding kits. The detection wavelengths for BUN, Cr, and Cys-C were 630, 510, and 450 nm, respectively. Finally, the contents of BUN, Cr, and Cys-C in serum of rats in each group were calculated according to the standard curve.
Hematoxylin and eosin (H&E) staining
Some isolated kidneys were soaked in 4% paraformaldehyde for 48 h, dehydrated by ethanol solution with an increasing concentration gradient, embedded in paraffin, and then cut into 6 μm of sections. After dewaxing and hydration using ethanol solution with a decreasing concentration gradient, the sections were soaked in hematoxylin for 5 min and then washed with running water for 10 min. Next, the sections were differentiated with hydrochloric acid ethanol for 3 s, and then washed with running water for 10 min. Next, they were stained with eosin for 25 s, dehydrated using ethanol with an increasing concentration gradient, and finally sealed. After that, the histopathology of kidney in each group was observed under a fluorescent microscope (BX63 Evident, Olympus, Japan). Subsequently, tubular injury was scored and semi-quantitative analysis was performed. Kidney injury was defined as tubular degeneration, vacuolar degeneration, tubule formation, tubular necrosis and inflammatory infiltration. Tubular injury score was listed as follows: 0, normal; 1 point, focal necrosis; 2 points, area of tubular injury ≤25%; 3 points, area of tubular injury ranged from 25% to 50%; 4 points, area of tubular injury ranged from 21% to 75%; 5 points, area of tubular injury > 76% [Citation25].
Detection of oxidative stress levels
The main focus of this test was to investigate the levels of ROS and malondialdehyde (MDA), as well as the activity of superoxide dismutase (SOD), in the renal tissues of different groups of rats. Briefly, 150 mg of tissue samples were added to the lysis buffer and then thoroughly homogenized using a homogenizer (DY200, SCIENTZ, China). Next, the homogenate was centrifuged at 12,000 rpm, 4 °C for 5 min, and the supernatant was collected for further analysis. Subsequently, the levels of ROS in the tissue were determined using the BBoxiProbe® Tissue ROS Assay Kit (BB-470512, BestBio, China) on an HTS microplate reader (PHERAstar FSX, BMG Labtech, Germany). The signals of samples were measured at an excitation wavelength of 510 nm and an emission wavelength of 610 nm. Besides, the levels of MDA in tissues of each group were detected using the MDA Assay Kit (AK288, Bioss, China), and the absorbance was measured at a wavelength of 532 nm using the HTS microplate reader (PHERAstar FSX, BMG Labtech, Germany). Furthermore, the SOD activity in each group of samples was detected by SOD Assay Kit (AK060, Bioss, China), and the absorbance was assessed at a wavelength of 560 nm through the HTS microplate reader (PHERAstar FSX, BMG Labtech, Germany). According to the established standard curves, the concentration of MDA and the activity of SOD were calculated in each group of samples.
Detection of inflammatory factor levels
The levels of inflammatory factors, including tumor necrosis factor alpha (TNF-α), IL-1β and IL-6, in serum and kidney tissue of rats in each group were observed through the method similar to above. To be specific, the obtained blood samples were allowed to stand and centrifuged at a low temperature, then the supernatant was collected. Similarly, the supernatant after tissue homogenate was also gathered. Next, the ELISA kits of TNF-α (900-K73, Peprotech), IL-1β (900-K91, Peprotech, Rocky Hill, NJ, USA), and IL-6 (900-K86, Peprotech) were employed to measure the contents of corresponding indicators. Referring to the handbook, the absorbance at a wavelength of 450 nm was detected using the HTS microplate reader (PHERAstar FSX, BMG Labtech, Germany). Lastly, the levels of TNF-α, IL-1β, and IL-6 in the samples were calculated according to the established standard curves.
Detection of indicators related to mitochondrial damage
Adenosine triphosphate (ATP) level, myeloperoxidase (MPO) activity, and mitochondrial membrane potential (JC-1) were measured to assess mitochondrial damage in the kidney tissues of rats in each group. In brief, 100 mg of tissue samples were homogenized and centrifuged at a low temperature, and then the supernatant was collected. Next, PhosphoWorks™ Fluorimetric ATP Assay Kit was applied to detect the level of ATP. The signals of each group of samples were checked at an excitation wavelength of 540 nm and an emission wavelength of 590 nm through the HTS microplate reader (PHERAstar FSX, BMG Labtech, Germany). Besides, the MPO Assay Kit was adopted to check the activity of MPO, and the absorbance of each sample was measured at a wavelength of 470 nm on the HTS microplate reader (PHERAstar FSX, BMG Labtech, Germany). To observe the JC-1 of tissue samples, kidney tissues were first made into single cell suspensions by means of a cell suspension preparation kit (BB-4512, BestBio, China). Subsequently, the mitochondrial membrane potential in cells was determined using the Mitochondrial Membrane Potential Assay Kit (BB-4105, BestBio, China). The red fluorescent signal was detected through the HTS microplate reader (PHERAstar FSX, BMG Labtech, Germany) at an excitation wavelength of 488 nm and an emission wavelength of 590 nm. Then, the green fluorescence signal was checked at an excitation wavelength of 488 nm and an emission wavelength of 527 nm. Following that, changes in mitochondrial membrane potential were measured by the relative proportions of red and green fluorescence.
Western blot
Kidney tissues (50 mg) were added with 1 mL of RIPA (BB-3201, BestBio, China) and placed in a homogenizer for homogenization. The homogenate was centrifuged at a low temperature for the collection of the supernatant. Then, a BCA protein quantification kit (BB-3401, BestBio, China) was used to determine the protein concentration. Next, 25 μg of protein was mixed with 5× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sampling buffer (BB-3703, BestBio, China), heated in a boiling water bath for 10 min, and then fully denatured. After 10% SDS-PAGE, the protein was electrotransferred onto a polyvinylidene fluoride (PVDF) membrane and blocked for 2 h with 5% skimmed milk. Later, the membrane was incubated with TLR4 (1:1000, AF7017, Affinity, Changzhou, China), MyD88 (1:1000, AF5195, Affinity, Changzhou, China), p65 (1:1000, AF5006, Affinity, Changzhou, China), p-p65 (1:1000, AF2006, Affinity, Changzhou, China), and β-actin (1:1000, AF7018, Affinity, Changzhou, China) at a low temperature overnight. On the next day, the membrane was incubated with HRP conjugated goat anti-rabbit secondary antibody (1:5000, S0001, Affinity, Changzhou, China). Next, protein bands were visualized using an imaging system (ChemiDoc MP, Hercules, CA, Biored) after a brief incubation of the membrane with enhanced chemiluminescence (ECL) detection reagents (BB-3501, BestBio, China). At last, the density of bands was counted in Image J software, and the expression level of β-actin was used to normalize that of TLR4, MyD88, p65, and p-p65.
Data analysis
The data obtained were statistically analyzed in SPSS version 26.0 (SPSS Inc., Chicago, IL). The distribution of continuous data was checked for normality using Shapiro–Wilk test; one way analysis of variance (ANOVA) followed Tukey post hoc test was employed for comparisons among multiple groups. p < 0.05 indicates a significant difference. The graph was drawn in GraphPad version 8.0 (GraphPad Software, La Jolla, CA), and the data shown in the graph were presented as means ± standard deviation.
Results
TAK-242 alleviates sepsis-induced kidney injury
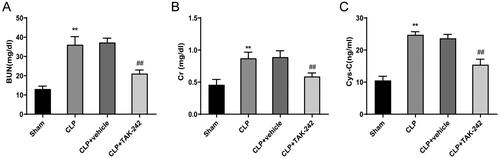
First, the effect of TAK-242 on SA-AKI was observed in CLP rats through measuring the levels of the kidney injury-related indicators (BUN, Cr, Cys-C) in serum of rats. The results showed that the levels of BUN (36.10 ± 4.23 vs. 13.08 ± 1.51), Cr (0.87 ± 0.09 vs. 0.46 ± 0.08), and Cys-C (24.77 ± 0.95 vs. 10.53 ± 1.32) were significantly higher in the CLP group than in the Sham group (p < 0.01), indicating the occurrence of kidney injury in the model rats. Notably, the serum levels of BUN (21.12 ± 1.84 vs. 37.26 ± 2.25), Cr (0.59 ± 0.05 vs. 0.89 ± 0.10), and Cys-C (15.44 ± 1.73 vs. 23.67 ± 1.23) were much lower in rats of the CLP + TAK-242 group than in rats of the CLP + vehicle group (p < 0.01) (). Collectively, TAK-242 may be able to prevent sepsis-induced kidney injury in rats.
Figure 1. TAK-242 Relieves sepsis-induced kidney injury. (A–C) Detection of BUN (A), Cr (B), and Cys-C (C) levels in serum of rats in the Sham group, CLP group, CLP + vehicle group, and CLP + TAK-242 group. **p < 0.01, vs. Sham group; ##p < 0.01, vs. CLP + vehicle group. BUN: blood urea nitrogen; Cr: creatinine; Cys-C: cystatin-C.
TAK-242 attenuates pathological damage of kidney tissues in septic rats
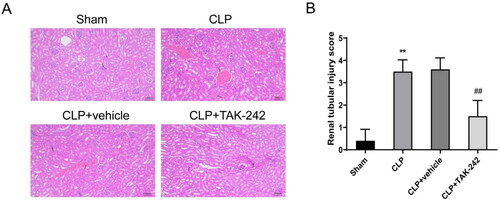
HE staining was performed to observe the pathological changes of kidney tissues in each group of rats. Unsurprisingly, rats in the CLP group and CLP + vehicle group occurred kidney injury symptoms, including renal tubular epithelial degeneration, edema and necrosis, renal tubular dilatation, massive inflammatory cell infiltration seen in the interstitium, and vascular congestion. Relative to rats in the CLP + vehicle group, rats in the CLP + TAK-242 group exhibited kidney injury improvement, relieved renal tubular epithelial degeneration and necrosis, and reduced inflammatory cell infiltration (). Further statistical results showed that the pathological damage scores (3.50 ± 0.53 vs. 0.40 ± 0.52) of rats in the CLP group were increased relative to those of rats in the Sham group (p < 0.01); while compared with the CLP + vehicle group, the pathological damage scores (3.60 ± 0.52 vs. 1.50 ± 0.71) in the CLP + TAK-242 group were decreased (p < 0.01) (). Overall, TAK-242 could attenuate kidney injury in septic rats.
Figure 2. TAK-242 attenuates pathological damage of kidney tissues in septic rats. (A) H&E staining to observe the pathological changes in the kidney tissues of rats in Sham group, CLP group, CLP + vehicle group, and CLP + TAK-242 group; (B) kidney tissue damage scores of rats in each group based on the results of . **p < 0.01, vs. Sham group; ##p < 0.01, vs. CLP + vehicle group.
TAK-242 reduces the level of oxidative stress in the kidney of septic rats
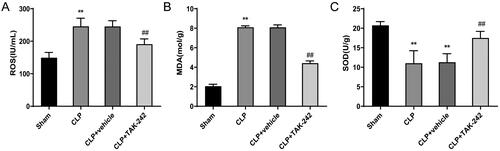
To explore the effect of TAK-242 on kidney tissues of CLP rats, the levels of ROS, MDA, and SOD were assessed. The assessment results revealed that, compared with rats in the Sham group, the levels of ROS (149.39 ± 16.27 vs. 245.92 ± 25.06) and MDA (2.06 ± 0.19 vs. 8.11 ± 0.14) were higher while the activity of SOD (20.74 ± 0.96 vs. 11.02 ± 3.22) was lower in the kidney tissues of rats in the CLP group (p < 0.01). However, compared with the CLP + vehicle group, the CLP + TAK-242 group had much lower levels of ROS (245.75 ± 17.46 vs. 191.09 ± 15.93) and MDA (8.09 ± 0.25 vs. 4.42 ± 0.24) while much higher activity of SOD (11.28 ± 2.19 vs. 17.51 ± 1.69) (p < 0.01) (). In view of these results, TAK-242 could lower the level of oxidative stress in kidney tissues induced by sepsis.
Figure 3. TAK-242 reduces the level of oxidative stress in kidney of septic rats. (A–C) Detection of ROS (A), MDA (B),SOD (C) levels in kidney tissues of rats in the Sham group, CLP group, CLP + vehicle group, and CLP + TAK-242 group. **p < 0.01, vs. Sham group; ##p < 0.01, vs. CLP + vehicle group. ROS: reactive oxygen species; MDA: malondialdehyde; SOD: superoxide dismutase.
TAK-242 improves mitochondrial function in kidney tissues of septic rats
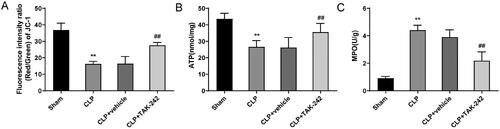
The results of the mitochondrial function in kidney tissues of rats in each group displayed that, relative to the Sham group, there was a reduction in the level of ATP (43.58 ± 3.48 vs. 26.61 ± 3.80) and the mitochondrial membrane potential (JC-1) (36.84 ± 4.15 vs. 16.26 ± 1.58), as well as a rise in the activity of MPO (0.91 ± 0.13 vs. 4.41 ± 0.35) in the CLP group (p < 0.01). However, compared with the CLP + vehicle group, the level of ATP (26.23 ± 6.07 vs. 35.55 ± 5.26) and the mitochondrial membrane potential (JC-1) (16.42 ± 4.30 vs. 27.58 ± 1.69) were much higher, and the activity of MPO (3.9 ± 0.53 vs. 2.19 ± 0.65) was lower in the CLP + TAK-242 group (p < 0.01) (). The above results suggested that TAK-242 could maintain the stability of mitochondrial function in kidney tissues of septic rats.
Figure 4. TAK-242 improves mitochondrial function in kidney tissues of septic rats. (A–C) Detection of mitochondrial membrane potential (JC-1, A), ATP content (B) and MPO activity (C) in kidney tissues of rats in the Sham group, CLP group, CLP + vehicle group, and CLP + TAK-242 group. **p < 0.01, vs. Sham group; ##p < 0.01, vs. CLP + vehicle group. ATP: adenosine triphosphate; MPO: myeloperoxidase.
TAK-242 reduces inflammation levels in septic rats
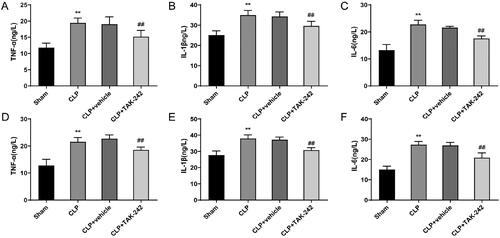
The levels of inflammatory factors (TNF-α, IL-1β, and IL-6) both in the serum and kidney tissues were examined. The results showed that, both in serum and kidney tissues, the TNF-α (19.47 ± 1.48 vs. 11.81 ± 1.37 and 21.56 ± 1.57 vs. 12.73 ± 2.38), IL-1β (34.96 ± 2.30 vs. 25.07 ± 2.19 and 38.00 ± 2.20 vs. 27.76 ± 2.53), and IL-6 (22.77 ± 1.55 vs. 13.25 ± 2.06 and 27.32 ± 1.58 vs. 15.02 ± 1.67) levels of rats in the CLP group were higher than those in the Sham group (p < 0.01). Nevertheless, the levels of TNF-α (15.18 ± 1.99 vs. 19.06 ± 2.24 and 18.58 ± 1.04 vs. 22.72 ± 1.39), IL-1β (29.61 ± 2.30 vs. 34.25 ± 2.27 and 30.84 ± 1.50 vs. 37.25 ± 1.61), and IL-6 (17.53 ± 0.97 vs. 21.60 ± 0.47 and 20.97 ± 2.31 vs. 26.94 ± 1.52) were lower in rats of the CLP + TAK-242 group than those in rats of the CLP + vehicle group (p < 0.01) (). It was evident that TAK-242 was able to reduce the excessive inflammatory response induced by sepsis.
Figure 5. TAK-242 reduces inflammation levels in septic rats. (A–F) ELISA to measure the levels of TNF-α, IL-1β, and IL-6 in the serum (A–C) and kidney tissues (D–F) of rats in the Sham group, CLP group, CLP + vehicle group, and CLP + TAK-242 group. **p < 0.01, vs. Sham group; ##p < 0.01, vs. CLP + vehicle group. TNF-α: tumor necrosis factor alpha; IL: interleukin.
TAK-242 inhibits activation of the TLR4/NF-κB signaling pathway in kidney tissues of septic rats
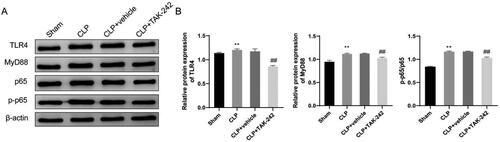
The expression of TLR4/NF-κB signaling pathway-related proteins in kidney tissues of rats was observed by western blot. According to the observation results, the TLR4 expression (1.20 ± 0.02 vs. 1.14 ± 0.02), MyD88 expression (1.11 ± 0.01 vs. 0.95 ± 0.03) and the ratio of p-p65/p65 (1.17 ± 0.02 vs. 0.84 ± 0.01) in kidney tissues of rats were elevated in the CLP group compared with those in the Sham group (p < 0.01). On the other hand, the TLR4 expression (0.86 ± 0.02 vs. 1.17 ± 0.05), MyD88 expression (1.03 ± 0.02 vs. 1.12 ± 0.01) and the ratio of p-p65/p65 (1.03 ± 0.02 vs. 1.17 ± 0.01) in the CLP + TAK-242 group were reduced compared with those in the CLP + vehicle group (p < 0.01) (). Taken together, the mechanism of TAK-242 in preventing sepsis-induced kidney injury may be achieved through inhibiting the TLR4/NF-κB signaling pathway activity.
Figure 6. TAK-242 inhibits activation of the TLR4/NF-κB signaling pathway in kidney tissues of septic rats. (A) Western blot to check the protein levels of TLR4, MyD88, p65, p-p65 in kidney tissues of rats in the Sham group, CLP group, CLP + vehicle group, CLP + TAK-242 group; (B) the protein expression levels of TLR4 and MyD88 and the ratio of p-p65/p65 in the tissues of each group analyzed based on the results of . **p < 0.01, vs. Sham group; ##p < 0.01, vs. CLP + vehicle group. TLR4: toll-like receptor 4.
Discussion
Sepsis has long been recognized as the most common cause of AKI, posing a serious threat to human life [Citation26]. However, clinical treatments are nonspecific and effective therapy is still lacked, which greatly limits the therapy for sepsis [Citation27]. TLR4/NF-κB has been identified as one of the signaling pathways involved in sepsis-induced kidney injury [Citation22]. TAK-242 acts as an inhibitor of the TLR4/NF-κB signaling pathway. The lipopolysaccharide-induced SA-AKI model has been used to explore the role of TAK-242 in SA-AKI; however, the CLP-based SA-AKI model is closer to the inflammatory characteristics of clinical sepsis. In this study, we successfully used CLP to induce SA-AKI and confirmed that TAK-242 improved the mitochondrial dysfunction in kidney tissues to prevent rats from CLP-induced SA-AKI by inhibiting TLR4/NF-κB signaling pathway, which provided effective data support to the treatment of SA-AKI. Notably, TAK-242 was administered as prophylaxis before model establishment in this study, and this is an innovation of our study.
First, we attempted to observe whether TAK-242 has a preventive effect on SA-AKI. Among the markers of kidney injury in the serum, BUN, Cr, and Cys-C showed significantly increased levels in CLP rats. The increased levels of BUN, Cr, and Cys-C are considered to be the gold standard for the diagnosis of AKI [Citation28]. The above findings further indicate that sepsis is prone to induce AKI. However, injection of TAK-242 in advance could significantly reduce the levels of these indicators in the serum of CLP rats. Moreover, the results of HE staining revealed that pre-injection of TAK-242 also significantly relieved the histological damage of kidney tissues caused by sepsis. Interestingly, a previous study proposed that TAK-242 reduced serum levels of kidney injury markers and attenuated histological changes in rats with crush injury-induced AKI [Citation29]. In addition, Mohammad et al. demonstrated that TAK-242 could improve the classical phenotype of AKI induced by ischemia/reperfusion and thus aid in protecting kidney [Citation23]. Furthermore, Salama et al. proved that TAK-242 also prevented the kidney failure [Citation30]. Therefore, it is clear that TAK-242 exerts functions in preventing SA-AKI.
Numerous ROS or nitric NOS are produced when innate immune cells are overactivated during sepsis, according to earlier research. These factors will induce uncontrolled oxidative stress, damage deoxyribonucleic acid (DNA) and ultimately promote cell death [Citation31–33]. ROS has been recognized as a key factor to induce symptoms of kidney injury such as hypertrophy of mesangial cells, podocyte apoptosis, glomerular sclerosis, and endothelial dysfunction. In addition, ROS is an important medium for the production of albuminuria and the destruction of glomerular hemodynamics. Accordingly, oxidative stress is believed to be one of the necessary conditions for SA-AKI [Citation34]. Besides, there are some studies demonstrating that inhibiting the oxidative stress of septic model animals can attenuate kidney injury [Citation35]. In this study, TAK-242 was discovered to effectively reduce the level of ROS in kidney tissues of CLP rats, down-regulate the content of MDA and increase the activity of SOD. Overall, TAK-242 can reduce oxidative stress in CLP rats and then perform renal protective actions. Previous studies have stated that mitochondrial dysfunction is not only a key contributor to oxidative stress imbalance in cells [Citation36], but also a part in the mechanism of sepsis that leads to various organ injuries [Citation37]. In sepsis, due to limited oxygen supply, incomplete oxidation reaction and hypoxia, the production of free radicals increases significantly; and the damaged mechanism of antioxidant system further results in mitochondrial dysfunction [Citation38]. Interestingly, prior studies have proved that TAK-242 can promote mitochondrial biogenesis [Citation39], control the quality of mitochondria [Citation40], and improve mitochondrial dysfunction [Citation41]. Here, we observed that there was mitochondrial dysfunction in the kidney of CLP rats, which could be improved by TAK-242. All in all, TAK-242 can maintain and protect the normal function of mitochondria in the kidney tissues with SA-AKI.
Inflammatory imbalance is the basis of the pathogenesis of sepsis and runs through the whole process of sepsis [Citation42]. Hence, the role of inflammatory response in SA-AKI cannot be ignored. The most typical phenomenon mediated by sepsis is to activate the innate immune system of the body, secrete inflammatory factors continuously, and finally form inflammatory factor storm [Citation43]. These inflammatory mediators are transported to various organs of the body, including the kidney, with blood circulation, thereby inducing ROS production, mitochondrial damage, and kidney injury [Citation44]. It’s interesting to note that earlier research has demonstrated the inhibitory effect of TAK-242 on inflammatory factors. For example, Wei et al. claimed that TAK-242 could effectively suppress lipopolysaccharide-induced inflammation in human coronary artery endothelial cells [Citation45]. Zhu et al. discovered that TAK-242 could inhibit the level of inflammatory factors in hippocampus of rats induced by hypoxia and ischemia [Citation46]. This study also proved that TAK-242 was able to lower the levels of inflammatory factors in serum and kidney of CLP rats. Therefore, we concluded that TAK-242 could also inhibit the release of excessive inflammatory factors caused by sepsis and regulate the inflammatory response in the kidney.
Subsequently, the results of western blot confirmed that TAK-242 may exert its biological function by inhibiting the activity of TLR4/NF-κB pathway. Furthermore, previous studies have verified that TLR4/NF-κB is closely related to oxidative stress and inflammatory responses of cells [Citation47,Citation48]. Moreover, it is believed that inhibiting the activity of TLR4/NF-κB signaling pathway can reduce the production of oxidative stress and pro-inflammatory factors [Citation49,Citation50]. Mitochondrial dysfunction is associated with oxidative stress and inflammatory responses, which has been considered to be a key factor in SA-AKI [Citation51], and the TLR4/NF-κB signaling pathway is responsible for initiating mitochondrial dysfunction [Citation52]. More importantly, studies have reported that TLR4/NF-κB is closely associated with the occurrence of AKI, and activation of this pathway may cause symptoms of SA-AKI [Citation53]. Therefore, TAK-242 may reduce oxidative stress and mitochondrial dysfunction by inhibiting the TLR4/NF-κB signaling pathway.
There are still some limitations in this study. First, transgenic mice with TAK-242 knockout were not used in this study to further corroborate the role of TAK-242. Second, this study was performed only on SD rats and did not re-validate the specific mechanism of action of TAK-242 at the cellular experimental level. Third, due to the limitations of experimental conditions, this study lacks direct observation of morphological changes of mitochondria, and there was no intuitive evidence to support the mitochondrial biogenesis. What is more, we did not treat CLP rats through combing TAK-242 with TLR4/NF-κB activator to further determine the relationship between TLR4/NF-κB and SA-AKI. Further clinical research is necessary to confirm the preventive effect of inhibiting the activity of TLR4/NF-κB pathway on SA-AKI.
Conclusion
TAK-242 can reduce the inflammatory responses of CLP rats by inhibiting the activity of TLR4/NF-κB signaling pathway. In addition, TAK-242 can also improve the mitochondrial dysfunction in kidney tissues, reduce the level of oxidative stress, and then ameliorate SA-AKI. Therefore, inhibiting the activity of TLR4/NF-κB signaling pathway may be an effective strategy to prevent SA-AKI, which deserves further clinical verification.
Supplemental Material
Download PDF (107.7 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The datasets generated and or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Poston JT, Koyner JL. Sepsis associated acute kidney injury. BMJ. 2019;364:1. doi: 10.1136/bmj.k4891.
- Peerapornratana S, Manrique-Caballero CL, Gómez H, et al. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019;96(5):1083–10. doi: 10.1016/j.kint.2019.05.026.
- Kellum JA, Chawla LS, Keener C, et al. The effects of alternative resuscitation strategies on acute kidney injury in patients with septic shock. Am J Respir Crit Care Med. 2016;193(3):281–287. doi: 10.1164/rccm.201505-0995OC.
- Adhikari NKJ, Fowler RA, Bhagwanjee S, et al. Critical care and the global burden of critical illness in adults. Lancet. 2010;376(9749):1339–1346. doi: 10.1016/S0140-6736(10)60446-1.
- Murugan R, Karajala-Subramanyam V, Lee M, et al. Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int. 2010;77(6):527–535. doi: 10.1038/ki.2009.502.
- Manrique-Caballero CL, Del Rio-Pertuz G, Gomez H. Sepsis-Associated acute kidney injury. Crit Care Clin. 2021;37(2):279–301. doi: 10.1016/j.ccc.2020.11.010.
- Pickkers P, Mehta RL, Murray PT, et al. Effect of human recombinant alkaline phosphatase on 7-Day creatinine clearance in patients with sepsis-associated acute kidney injury: a randomized clinical trial. JAMA. 2018;320(19):1998–2009. doi: 10.1001/jama.2018.14283.
- Cantaluppi V, Medica D, Quercia AD, et al. Perfluorocarbon solutions limit tubular epithelial cell injury and promote CD133+ kidney progenitor differentiation: potential use in renal assist devices for sepsis-associated acute kidney injury and multiple organ failure. Nephrol Dial Transplant. 2018;33(7):1110–1121. doi: 10.1093/ndt/gfx328.
- van der Slikke EC, Star BS, van Meurs M, et al. Sepsis is associated with mitochondrial DNA damage and a reduced mitochondrial mass in the kidney of patients with sepsis-AKI. Crit Care. 2021;25(1):36. doi: 10.1186/s13054-020-03424-1.
- Jiang T, Wang Q, Lv J, et al. Mitochondria-endoplasmic reticulum contacts in sepsis-induced myocardial dysfunction. Front Cell Dev Biol. 2022;10:1036225. doi: 10.3389/fcell.2022.1036225.
- Zhang H, Feng YW, Yao YM. Potential therapy strategy: targeting mitochondrial dysfunction in sepsis. Mil Med Res. 2018;5(1):41. doi: 10.1186/s40779-018-0187-0.
- Bhatti JS, Bhatti GK, Reddy PH. Mitochondrial dysfunction and oxidative stress in metabolic disorders - A step towards mitochondria based therapeutic strategies. Biochim Biophys Acta Mol Basis Dis. 2017;1863(5):1066–1077. doi: 10.1016/j.bbadis.2016.11.010.
- Marchi S, Guilbaud E, Tait SWG, et al. Mitochondrial control of inflammation. Nat Rev Immunol. 2023;23(3):159–173. doi: 10.1038/s41577-022-00760-x.
- Wang HQ, Wang SS, Chiufai K, et al. Umbelliferone ameliorates renal function in diabetic nephropathy rats through regulating inflammation and TLR/NF-kappaB pathway. Chin J Nat Med. 2019;17(5):346–354. doi: 10.1016/S1875-5364(19)30040-8.
- Shi M, Zeng X, Guo F, et al. Anti-inflammatory pyranochalcone derivative attenuates LPS-Induced acute kidney injury via inhibiting TLR4/NF-kappaB pathway. Molecules. 2017;22(10):1683. doi: 10.3390/molecules22101683.
- Lin C, Wang H, Zhang M, et al. TLR4 biased small molecule modulators. Pharmacol Ther. 2021;228:107918. doi: 10.1016/j.pharmthera.2021.107918.
- Kuzmich NN, Sivak KV, Chubarev VN, et al. TLR4 signaling pathway modulators as potential therapeutics in inflammation and sepsis. Vaccines (Basel). 2017;5(4):34. doi: 10.3390/vaccines5040034.
- Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651–a001651. doi: 10.1101/cshperspect.a001651.
- Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nat Immunol. 2011;12(8):695–708. doi: 10.1038/ni.2065.
- Zhao H, Zheng Q, Hu X, et al. Betulin attenuates kidney injury in septic rats through inhibiting TLR4/NF-kappaB signaling pathway. Life Sci. 2016;144:185–193. doi: 10.1016/j.lfs.2015.12.003.
- Zhu J, Zhang Y, Shi L, et al. RP105 protects against ischemic and septic acute kidney injury via suppressing TLR4/NF-kappaB signaling pathways. Int Immunopharmacol. 2022;109:108904. doi: 10.1016/j.intimp.2022.108904.
- Fenhammar J, Rundgren M, Hultenby K, et al. Renal effects of treatment with a TLR4 inhibitor in conscious septic sheep. Crit Care. 2014;18(5):488. doi: 10.1186/PREACCEPT-1770070480124389.
- Mohammad BI, Raheem AK, Hadi NR, et al. Reno-protective effects of TAK-242 on acute kidney injury in a rat model. Biochem Biophys Res Commun. 2018;503(1):304–308. doi: 10.1016/j.bbrc.2018.06.020.
- Shi X, Li J, Han Y, et al. The alpha7 nicotinic acetylcholine receptor agonist PNU-282987 ameliorates sepsis-induced acute kidney injury through CD4 + CD25+ regulatory T cells in rats. Bosn J Basic Med Sci. 2022;22(6):882–893. doi: 10.17305/bjbms.2022.7111.
- Zhang Y, Huang H, Liu W, et al. Endothelial progenitor cells-derived exosomal microRNA-21-5p alleviates sepsis-induced acute kidney injury by inhibiting RUNX1 expression. Cell Death Dis. 2021;12(4):335. doi: 10.1038/s41419-021-03578-y.
- Ma S, Evans RG, Iguchi N, et al. Sepsis-induced acute kidney injury: a disease of the microcirculation. Microcirculation. 2019;26(2):e12483. doi: 10.1111/micc.12483.
- Wei S, Gao Y, Dai X, et al. SIRT1-mediated HMGB1 deacetylation suppresses sepsis-associated acute kidney injury. Am J Physiol Renal Physiol. 2019;316(1):F20–F31. doi: 10.1152/ajprenal.00119.2018.
- Wen Y, Parikh CR. Current concepts and advances in biomarkers of acute kidney injury. Crit Rev Clin Lab Sci. 2021;58(5):354–368. doi: 10.1080/10408363.2021.1879000.
- Wang J, Chen Z, Hou S, et al. TAK-242 attenuates crush injury induced acute kidney injury through inhibiting TLR4/NF-kappaB signaling pathways in rats. Prehosp Disaster Med. 2020;35(6):619–628. doi: 10.1017/S1049023X20001132.
- Salama M, Elgamal M, Abdelaziz A, et al. Toll-like receptor 4 blocker as potential therapy for acetaminophen-induced organ failure in mice. Exp Ther Med. 2015;10(1):241–246. doi: 10.3892/etm.2015.2442.
- Su LJ, Zhang JH, Gomez H, et al. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid Med Cell Longev. 2019;2019:5080843–5080813.
- Zou Z, Liu B, Zeng L, et al. Cx43 inhibition attenuates sepsis-induced intestinal injury via downregulating ROS transfer and the activation of the JNK1/Sirt1/FoxO3a signaling pathway. Mediators Inflamm. 2019;2019:7854389–7854313. doi: 10.1155/2019/7854389.
- Oliveira FRMB, Assreuy J, Sordi R. The role of nitric oxide in sepsis-associated kidney injury. Biosci Rep. 2022;42(7):BSR20220093. doi: 10.1042/BSR20220093.
- Jha JC, Banal C, Chow BSM, et al. Diabetes and kidney disease: role of oxidative stress. Antioxid Redox Signal. 2016;25(12):657–684. doi: 10.1089/ars.2016.6664.
- Sun J, Ge X, Wang Y, et al. USF2 knockdown downregulates THBS1 to inhibit the TGF-beta signaling pathway and reduce pyroptosis in sepsis-induced acute kidney injury. Pharmacol Res. 2022;176:105962. doi: 10.1016/j.phrs.2021.105962.
- Zhang J, Bao Y, Zhou X, et al. Polycystic ovary syndrome and mitochondrial dysfunction. Reprod Biol Endocrinol. 2019;17(1):67. doi: 10.1186/s12958-019-0509-4.
- Yang H, Zhang Z. Sepsis-induced myocardial dysfunction: the role of mitochondrial dysfunction. Inflamm Res. 2021;70(4):379–387. doi: 10.1007/s00011-021-01447-0.
- Rocha M, Herance R, Rovira S, et al. Mitochondrial dysfunction and antioxidant therapy in sepsis. Infect Disord Drug Targets. 2012;12(2):161–178. doi: 10.2174/187152612800100189.
- Pan LF, Yu L, Wang LM, et al. The toll-like receptor 4 antagonist transforming growth factor-beta-activated kinase(TAK)-242 attenuates taurocholate-induced oxidative stress through regulating mitochondrial function in mice pancreatic acinar cells. J Surg Res. 2016;206(2):298–306. doi: 10.1016/j.jss.2016.08.011.
- Park JS, Choi HS, Yim SY, et al. Heme oxygenase-1 protects the liver from septic injury by modulating TLR4-Mediated mitochondrial quality control in mice. Shock. 2018;50(2):209–218. doi: 10.1097/SHK.0000000000001020.
- Yang J, Zhang R, Jiang X, et al. Toll-like receptor 4-induced ryanodine receptor 2 oxidation and sarcoplasmic reticulum Ca(2+) leakage promote cardiac contractile dysfunction in sepsis. J Biol Chem. 2018;293(3):794–807. doi: 10.1074/jbc.M117.812289.
- Hwang JS, Kim KH, Park J, et al. Glucosamine improves survival in a mouse model of sepsis and attenuates sepsis-induced lung injury and inflammation. J Biol Chem. 2019;294(2):608–622. doi: 10.1074/jbc.RA118.004638.
- Huang M, Cai S, Su J. The pathogenesis of sepsis and potential therapeutic targets. Int J Mol Sci. 2019;20(21):5376.
- Chang YM, Chou YT, Kan WC, et al. Sepsis and acute kidney injury: a review focusing on the bidirectional interplay. Int J Mol Sci. 2022;23(16):9159.
- Wei Z, Sun X, Xu Q, et al. TAK-242 suppresses lipopolysaccharide-induced inflammation in human coronary artery endothelial cells. Pharmazie. 2016;71(10):583–587.
- Zhu K, Zhu X, Sun S, et al. Inhibition of TLR4 prevents hippocampal hypoxic-ischemic injury by regulating ferroptosis in neonatal rats. Exp Neurol. 2021;345:113828. doi: 10.1016/j.expneurol.2021.113828.
- Zusso M, Lunardi V, Franceschini D, et al. Ciprofloxacin and levofloxacin attenuate microglia inflammatory response via TLR4/NF-kB pathway. J Neuroinflammation. 2019;16(1):148. doi: 10.1186/s12974-019-1538-9.
- Wu Y, Zhao Y, Yang HZ, et al. HMGB1 regulates ferroptosis through Nrf2 pathway in mesangial cells in response to high glucose. Biosci Rep. 2021;41(2):BSR20202924.
- Zhang J, Zheng Y, Luo Y, et al. Curcumin inhibits LPS-induced neuroinflammation by promoting microglial M2 polarization via TREM2/TLR4/NF-kappaB pathways in BV2 cells. Mol Immunol. 2019;116:29–37. doi: 10.1016/j.molimm.2019.09.020.
- Dhlamini Q, Wang W, Feng G, et al. FGF1 alleviates LPS-induced acute lung injury via suppression of inflammation and oxidative stress. Mol Med. 2022;28(1):73. doi: 10.1186/s10020-022-00502-8.
- Wang L, Li J, Liao R, et al. Resolvin D1 attenuates sepsis induced acute kidney injury targeting mitochondria and NF-kappaB signaling pathway. Heliyon. 2022;8(12):e12269. doi: 10.1016/j.heliyon.2022.e12269.
- Huang Q, Gao W, Mu H, et al. HSP60 regulates monosodium urate Crystal-Induced inflammation by activating the TLR4-NF-kappaB-MyD88 signaling pathway and disrupting mitochondrial function. Oxid Med Cell Longev. 2020;2020:8706898–8706816. doi: 10.1155/2020/8706898.
- Jin YH, Li ZT, Chen H, et al. Effect of dexmedetomidine on kidney injury in sepsis rats through TLR4/MyD88/NF-kappaB/iNOS signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(11):5020–5025.

