Abstract
Background
The clinical significance of mesangial immunoglobulin (Ig) M deposition in IgA nephropathy (IgAN) has been less explored and remains a topic of debate. Therefore, our study aimed to investigate the prognostic value of mesangial IgM deposition in a long-term follow-up cohort of IgAN patients.
Methods
A unicentric retrospective study was conducted on 93 consecutive IgAN patients (median age 41 years, 68% male, eGFR 48.7 mL/min, proteinuria 1.1 g/g) from 2010 to 2015. They were followed until end-stage kidney disease (ESKD), death, or until the end of the study in January 2021, with a median follow-up of 7 years. An independent pathologist evaluated the IgM immunofluorescence pattern, Oxford MEST-C score, and transmission electron microscopy (TEM) lesions following a comprehensive protocol.
Results
In our cohort, 70% had mesangial IgM-positive deposits, while 30% were IgM-negative. Both groups were similar in age, sex, prevalence of arterial hypertension, Charlson comorbidity scores, kidney function (eGFR and proteinuria), pathology findings (Oxford MEST-C score, IgG and C3 immune deposition), and TEM analysis. Treatment with RASI and immunosuppression, and death rates were also comparable. However, 37% of IgM-positive patients progressed to ESKD, significantly higher than the 11% in the IgM-negative group. Univariate and multivariate Cox proportional hazards regression analyses identified lower eGFR, higher Oxford MEST-C score, and mesangial IgM deposits as independent factors associated with shorter kidney survival.
Conclusions
Our study highlights mesangial IgM deposition as a potential risk factor for ESKD in patients with advanced IgAN, laying a foundation for further research in this area.
Introduction
Immunoglobulin A nephropathy (IgAN) stands as the most frequent primary glomerular disease identified through kidney biopsy and is a significant contributor to end-stage kidney disease (ESKD) [Citation1,Citation2]. A key challenge in managing IgAN is its diverse clinical and pathological characteristics at diagnosis, complicating the prediction of patient outcomes and the efficacy of therapeutic interventions [Citation3,Citation4].
Despite being fundamental for the diagnosis, the immuno-staining pattern and intensity are not included in the Oxford MEST-C classification [Citation5]. Staining for IgA is almost always accompanied by C3 deposits, and often with co-deposition of IgG and/or IgM.
While the presence of C3 and IgG deposits has been extensively studied and shown to be associated with prognosis to some extent, the clinical significance of mesangial IgM deposition has been less explored and remains a subject of debate [Citation6,Citation7].
Liu et al.’s study on IgAN patients showed increased mesangial IgM deposition over time (id est at second kidney biopsy), indicating it as a secondary phenomenon linked to poorer prognosis [Citation8]. In contrast, pediatric IgAN cases with IgM didn’t show worsened outcomes [Citation9]. Heybeli et al. noted a higher prevalence of IgM deposition in patients with nephrotic-range proteinuria, also associated with C4d-positive staining, a poor renal survival indicator [Citation10,Citation11]. However, Moriyama et al. reported that mesangial IgM deposition correlated with certain chronic glomerular changes but did not affect kidney survival in the short term [Citation12].
Therefore, we aimed to study the relationship between mesangial IgM deposits assessed by immunofluorescence at IgAN diagnosis with disease activity and outcomes in a monocentric cohort of Caucasian patients.
Methods
Study design and population
In this retrospective, observational study at “Dr. Carol Davila” Teaching Hospital of Nephrology, we enrolled adult patients diagnosed with IgAN via kidney biopsy between 2010 and 2015. Patients were followed until ESKD, defined as dialysis initiation or kidney transplantation, death or end of study (January 2021), whichever came first. Exclusion criteria were: patients under 18 years old, those with biopsy specimens having fewer than 8 scorable glomeruli, insufficient clinical data, less than 3 months of follow-up, or absence of a functional glomerulus in the fragment for IgM immunofluorescence analysis. Additionally, we excluded patients with secondary IgAN related to liver diseases (e.g. alcoholic cirrhosis), viral infections (hepatitis B and C), autoimmune disorders (including autoimmune thyroiditis, ankylosing spondylitis, psoriasis, rheumatoid arthritis), or concurrent glomerular diseases like ANCA vasculitis, membranous nephropathy, or minimal change disease [Citation13,Citation14].
Covariates and treatment
Demographic data, comorbidities (including Charlson comorbidity score – CCS, diabetes mellitus, arterial hypertension), and laboratory parameters (such as serum creatinine, estimated glomerular filtration rate – eGFR, 24-h proteinuria, and hematuria) were extracted from electronic medical records. The decision regarding supportive and/or immunosuppressive treatment was made based on the judgment of the attending nephrologist.
Pathology review of kidney biopsy specimens
Routine processes of light microscopy, immunofluorescence, and transmission electron microscopy (TEM) were applied to each kidney biopsy sample. The diagnosis of IgAN was established through light microscopy and immunofluorescence, characterized by dominant IgA presence in the mesangium, and confirmed by TEM, which identified para-mesangial electron-dense deposits.
The deposition of immune complexes was assessed semi-quantitatively, graded from negative trace through to 3(+), increasing in intensity. A grade of at least 1(+) was considered indicative of deposition presence (). In our study, patients were classified as mesangial IgM positive based on the presence of granular deposits predominantly located in the mesangial area of the glomerulus. IgM was found exclusively in segmental sclerosis lesions in 5 patients, in both mesangial and segmental sclerosis lesions (predominantly mesangial) in 10 patients, and solely in the mesangial area in 55 patients.
Figure 1. Immunofluorescence findings in IgAN; A: Immunofluorescence microscopy image showing very bright (3+) granular deposits for IgA located in the mesangium of a glomerulus; B: Immunofluorescence microscopy image showing positive granular deposits (2+) for IgM predominantly located in the mesangium of a glomerulus.
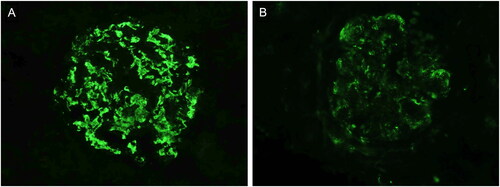
One pathologist (GTB) reviewed the slides without knowledge of the original biopsy interpretation and assessed the immunofluorescence (the evaluation was conducted retrospectively using archived images from the initial immunofluorescence assessments), Oxford MEST-C score, and TEM lesions of podocytes, endothelial cells, mesangium and glomerular basement membrane (GBM), according to the Mayo Clinic/Renal Pathology Society Consensus [Citation15]. The correlation coefficient for mesangial IgM deposition by IF was 0.89 between the initial assessment and the reassessment. Discrepancies identified post-reassessment were subsequently discussed and resolved.
The histopathological classification adhered to the MEST-C score based on the Oxford criteria [Citation5]. TEM was performed routinely, and the lesions were classified as previously described by our group (see Supplementary Table 1) [Citation16,Citation17].
Statistical analyses
Descriptive statistics are summarized as mean ± standard deviation or median (quartile 1, quartile 3) for continuous variables, and frequency distribution as percentages for categorical variables. Group comparisons were performed with Student’s t-test, χ2 test, and Mann–Whitney U test, as appropriate.
Survival analyses were conducted with the Kaplan–Meier method, and the log rank test was used for comparison. Univariate and multivariate Cox proportional hazard (CPH) analyses were performed to identify independent predictors of ESKD. The multivariable model incorporated only those characteristics significant to p < 0.1 in the univariate model. Of note, each component of the Oxford MEST-C score demonstrated statistical significance in univariate analysis (Supplementary Table 2); consequently, the total Oxford MEST-C score was incorporated as a singular continuous variable in the multivariate Cox regression model, adhering to the rule of including one variable per five events [Citation18]. Results were expressed as hazard ratio (HR) and 95% confidence interval (CI).
All statistical tests were two-sided, and a p < 0.05 was considered significant. Statistical analyses were performed using the SPSS program (SPSS version 26, Chicago, IL).
Ethics approval
The study was conducted in accordance with the provisions of the Declaration of Helsinki and the protocol was approved by the local ethics committee (“Dr. Carol Davila” Teaching Hospital of Nephrology, Bucharest, Romania, approval number 2021-012). Since all data were anonymized, informed consent was not obtained from individual patients.
Results
Baseline clinical characteristics
Within the study timeframe, we reviewed 137 IgAN cases from our electronic records. We excluded 44 patients due to inadequate kidney biopsies (including absence of immunofluorescence specimen for IgM, n = 19), insufficient follow-up (n = 7), or secondary IgAN (n = 18), resulting in 93 subjects for our study. At renal biopsy, the median patient age was 41 years, eGFR was 48.7 mL/min, and proteinuria was 1.1 g/g. The majority had arterial hypertension (68%), while 7% had type 2 diabetes mellitus ().
Table 1. Clinical and histopathological characteristics of all patients and a comparative analysis according to the presence or absence of IgM deposition in kidney biopsy.
Treatment-wise, close to two-thirds were on a renin-angiotensin system inhibitor (RASI), and a third received immunosuppressants – 50% on steroids, 40% on steroids plus cyclophosphamide, and 10% on steroids with other agents (). The median follow-up of the entire cohort was 7 years.
IgM-positive versus IgM-negative comparison
In our population, the intensity of mesangial IgM as measured by IF was observed as follows: 0 in 30% of patients, 1 in 48%, 2 in 19%, and 3 in 2% of cases. Therefore, 65 participants (70%) had mesangial IgM-positive deposits in immunofluorescence, while 28 participants (30%) were IgM-negative.
The age and sex distribution were similar across both groups. In terms of comorbid conditions, the prevalence of type 2 diabetes mellitus and arterial hypertension was found to be comparable between the two groups, and the CCS were similar. Kidney function, as measured by eGFR and urine proteinuria, showed no significant difference between the groups ().
The pathology examination, which involved assessing the Oxford MEST-C score and the extent of immune deposition in immunofluorescence (IgA, IgG, C3), revealed similar findings across both groups. Moreover, there was no relationship between the intensity of mesangial IgM deposits and Oxford MEST-C classes (Supplementary Table 3). TEM analysis also showed no significant differences in podocyte and endothelial cell lesions, TEM deposit locations, and GBM abnormalities ().
Regarding treatment and outcomes, the use of RASI and immunosuppression treatments were comparable in both groups (p = 0.4, p = 0.9). The death rate during the follow-up period was 10% in the IgM-positive group and 7% in the IgM-negative group, with no significant difference observed (p = 0.5) (). The main causes of death were cardiovascular diseases (60%), followed by infectious (19%), gastroenterological (12%), and neoplasia (9%) diseases.
Kidney survival according to mesangial IgM deposits
Out of the total patients studied, 27 (29%) reached ESKD. Median kidney survival time for the entire cohort was 86.0 (95%CI 78.5, 93.4) months; kidney survival at 1, 3 and 5 years were 92%, 85%, and 82%, respectively.
In the IgM-positive group, 24 patients (37%) progressed to ESKD, compared to just 3 patients (11%) in the IgM-negative group, with this difference being statistically significant (p = 0.01). Moreover, the Kaplan-Meier analysis revealed that IgM-positive patients had a significantly shorter kidney survival time as compared to those without IgM deposits ().
Figure 2. Patients with mesangial IgM positivity at immunofluorescence had a shorter mean renal survival time: 90.3 (95%CI 78.8, 101.7) versus 116.1 (95%CI 103.3, 128.8) months.
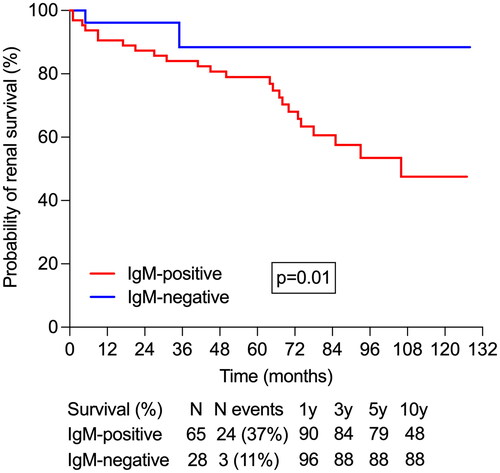
In univariate Cox proportional hazards regression, the presence of arterial hypertension, lower eGFR, higher proteinuria, increased Oxford MEST-C score, and the presence of mesangial IgM deposits were associated with shorter kidney survival (). However, in the multivariate CPH regression, only lower eGFR, higher Oxford MEST-C score, and the presence of mesangial IgM deposits were independently associated with ESKD ().
Table 2. Prediction of end stage kidney disease (ESKD) according to the IgM status on immunofluorescence and known risk factors of progression (univariate and multivariate Cox regression analysis).
Discussion
In this study, we have demonstrated that mesangial IgM deposition is an independent risk factor for ESKD in patients with IgAN. Mesangial IgM deposition has been reported in varying frequencies, ranging from 22 to 70% in previous studies, and similarly, in our cohort, IgM was present in 70% of the patients [Citation10–12,Citation19]. Our study indicates that despite the usual association of IgM deposition in segmental sclerosis with a higher grade of chronicity in the kidney, we did not observe a relationship between the presence or intensity of mesangial IgM deposits and the Oxford MEST-C classes.
Previous studies conducted by Moriyama et al. and Heybeli et al. on IgAN patients showed that IgM deposition was associated with chronic lesions like tuft adhesion, glomerular obsolescence and segmental sclerosis [Citation10–12]. These findings were attributed to IgM molecules being trapped in sclerotic areas due to their increased size and to the IgM's physiological role of healing damaged tissues [Citation10]. Interestingly, in our cohort, characterized by more severe IgAN, we observed no relationship between IgM deposition and the Oxford MEST-C classes. Additionally, IgM deposition showed no association with the lesions identified by TEM. This lack of correlation, particularly in a cohort with more severe IgAN than those in previous studies reporting on IgM deposition in IgAN, could be attributed to the advanced chronicity stage in our population, or it might suggest that IgM deposition is an epiphenomenon with potential prognostic implications.
In line, in a study on 30 Chinese primary IgAN patients, there was a significant increase in IgM deposition at the second kidney biopsy, from 11/30 to 21/30 cases, while IgA, IgG, and complement deposits remained constant [Citation8]. The authors hypothesized that this phenomenon of secondary IgM “deposition” and not “co-deposition” (like C3 or IgG) could be responsible for IgAN progression since patients who developed IgM deposits showed a higher incidence of nephrosis, glomerulosclerosis, severe tubular-interstitial lesions, and renal insufficiency [Citation8].
In the study by Heybeli et al. a correlation between mesangial IgM deposition and C4d staining was observed, suggesting the activation of the lectin complement pathway, a known independent risk factor for ESKD progression in IgAN patients [Citation11,Citation20]. This implies that the adverse outcomes in patients with IgM deposition might be due to lectin complement system activation. However, it remains to be explored if the complement-activating potential of IgM, evident in mouse ischemia-reperfusion injury models, extends to human primary glomerulonephritis [Citation21]. In ischemic/reperfusion injuries, IgM initiates lectin pathway activation by binding to antigens, which exposes specific carbohydrate patterns. These patterns then bind to mannose-binding lectin (MBL), leading to the activation of MBL-associated serine proteases and triggering the lectin pathway [Citation22].
The phenomenon of IgM deposition is not unique to IgAN. Thus, in Focal Segmental Glomerulosclerosis (FSGS), IgM is frequently found in low or moderate amounts in the mesangium. Thurman et al. suggest that in cases of nonimmune injury, newly exposed mesangial neoepitopes may attract IgM [Citation23]. This binding of IgM can lead to complement deposition and subsequent damage to the glomeruli [Citation23]. In line, research conducted by Zhang et al. revealed that the concurrent deposition of IgM and C3 in the glomeruli of patients with primary FSGS was independently associated with poorer responses to treatment and worse renal outcomes [Citation24].
These insights into the role of IgM deposition in both IgAN and FSGS underscore the broader implication of IgM in the pathology of diverse glomerular diseases, highlighting a possible common mechanistic thread that links these distinct conditions.
It is important to acknowledge certain limitations in our study. A notable constraint is that our patient sample was derived from a single center in Europe, suggesting that our findings need to be validated across different populations to confirm their generalizability. Moreover, due to the small cohort size, we were not able to perform a detailed subanalysis of IgM deposition patterns in mesangial versus segmental sclerosis lesions. Additionally, the retrospective nature of our study represents a significant limitation, as it can introduce inherent biases and limitations in data collection and analysis, including restricted access to comprehensive pre-biopsy data such as duration of eGFR decline, proteinuria, and RASI treatment history.
Our study’s strengths lie in its extended follow-up period and the employment of a definitive endpoint, such as ESKD. Additionally, the strength is bolstered by the blinded and thorough pathological evaluations conducted. The high correlation (0.88) between initial IF reports and reassessment, conducted independently, underscores the robustness of our findings.
In conclusion, our study identifies mesangial IgM deposition as a key risk factor for ESKD in IgAN, particularly in advanced stages of the disease. Given the study’s limitations, further research is essential to deepen our understanding of IgM's role in IgAN. However, the insights gained, supported by extensive follow-up and comprehensive pathology, provide a valuable foundation for future investigations.
Authors’ contributions
G.T.B., G.S. responsible for conceptualization, methodology and original draft preparation. S.S. and A.Z. carried out data bibliographic research, data recording and coordinated the study. G.S. performed the statistical analysis. All authors contributed to clinical care of patients, commented on previous versions of the manuscript, and read and approved the final manuscript.
Ethical approval
The study was conducted with the provisions of the Declaration of Helsinki and the protocol was approved by the local ethics committee (“Dr Carol Davila” Teaching Hospital of Nephrology, Bucharest, Romania, approval number 2021-012). Since all data were anonymized, informed consent was not obtained from individual patients.
Supplemental Material
Download PDF (153.9 KB)Acknowledgments
The publication of this paper was supported by the University of Medicine and Pharmacy “Carol Davila,” through the institutional program “Publish not Perish.”
Disclosure statement
The authors declare no conflicts of interest or competing interests. The results presented in this paper have not been published previously in whole or part, except in abstract format.
Data availability statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Additional information
Funding
References
- McGrogan A, Franssen CF, de Vries CS. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant. 2011;26(2):1–7. doi:10.1093/ndt/gfq665.
- O'Shaughnessy MM, Hogan SL, Thompson BD, et al. Glomerular disease frequencies by race, sex and region: results from the international kidney biopsy survey. Nephrol Dial Transplant. 2018;33(4):661–669. doi:10.1093/ndt/gfx189.
- Maillard N, Mariat C. The oxford classification for immunoglobulin a nephropathy: a common language blurred by dissonant voices. Nephrol Dial Transplant. 2019;34(10):1617–1618. doi:10.1093/ndt/gfz009.
- Ștefan G, Stancu S, Zugravu A, et al. Immunosuppressive therapy versus supportive care in IgA nephropathy patients with stage 3 and 4 chronic kidney disease. Medicine (Baltimore). 2022;101(36):e30422. doi:10.1097/MD.0000000000030422.
- Trimarchi H, Barratt J, Cattran DC, et al. Oxford classification of IgA nephropathy 2016: an update from the IgA nephropathy classification working group. Kidney Int. 2017;91(5):1014–1021. doi:10.1016/j.kint.2017.02.003.
- Ştefan G, Ismail G, Stancu S, et al. Validation study of oxford classification of IgA nephropathy: the significance of extracapillary hypercellularity and mesangial IgG immunostaining. Pathol Int. 2016;66(8):453–459. doi:10.1111/pin.12442.
- Shin DH, Lim BJ, Han IM, et al. Glomerular IgG deposition predicts renal outcome in patients with IgA nephropathy. Mod Pathol. 2016;29(7):743–752. doi:10.1038/modpathol.2016.77.
- Liu Z, Li L. [Significance of IgM deposition in IgA nephropathy: an appraisal based on renal re-biopsy]. Zhonghua Yi Xue Za Zhi. 1990;70(6):324–326, 24.
- Welch TR, McAdams J. Immunoglobulin M and C1q mesangial labeling in IgA nephropathy. Am J Kidney Dis. 1998;32(4):589–592. doi:10.1016/s0272-6386(98)70021-6.
- Heybeli C, Oktan MA, Yıldız S, et al. Clinical significance of mesangial IgM deposition in patients with IgA nephropathy. Clin Exp Nephrol. 2019;23(3):371–379. doi:10.1007/s10157-018-1651-6.
- Heybeli C, Unlu M, Yildiz S, et al. IgA nephropathy: association of C4d with clinical and histopathological findings and possible role of IgM. Ren Fail. 2015;37(9):1464–1469. doi:10.3109/0886022X.2015.1077319.
- Moriyama T, Shimizu A, Takei T, et al. Characteristics of immunoglobulin a nephropathy with mesangial immunoglobulin G and immunoglobulin M deposition. Nephrology (Carlton). 2010;15(8):747–754. doi:10.1111/j.1440-1797.2010.01296.x.
- Obrișcă B, Ștefan G, Gherghiceanu M, et al. “Associated” or “secondary” IgA nephropathy? An outcome analysis. PLoS One. 2019;14(8):e0221014. doi:10.1371/journal.pone.0221014.
- Ștefan G, Terinte-Balcan G, Stancu S, et al. IgA nephropathy with serum ANCA positivity: case series and literature review. Rheumatol Int. 2021;41(7):1347–1355. doi:10.1007/s00296-021-04888-2.
- Sethi S, Haas M, Markowitz GS, et al. Mayo clinic/renal pathology society consensus report on pathologic classification, diagnosis, and reporting of GN. J Am Soc Nephrol. 2016;27(5):1278–1287. May doi:10.1681/ASN.2015060612.
- Terinte-Balcan G, Stancu S, Zugravu A, et al. Prognostic role of glomerular electron microscopy lesions in IgA nephropathy: “the devil is in the details”. J Nephrol. 2023;36(8):2233–2243. doi:10.1007/s40620-023-01744-3.
- Terinte-Balcan G, Stefan G. A closer look: ultrastructural evaluation of high-risk progression IgA nephropathy. Ultrastruct Pathol. 2023;47(6):461–469.
- Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165(6):710–718. doi:10.1093/aje/kwk052.
- Xiong L, Liu L, Tao Y, et al. Clinical significance of IgM and C3 deposition in children with primary immunoglobulin a nephropathy. J Nephrol. 2023;36(8):2213–2222. doi:10.1007/s40620-023-01724-7.
- Barratt J, Lafayette RA, Zhang H, et al. IgA nephropathy: the lectin pathway and implications for targeted therapy. Kidney Int. 2023;104(2):254–264. doi:10.1016/j.kint.2023.04.029.
- Zhang M, Takahashi K, Alicot EM, et al. Activation of the lectin pathway by natural IgM in a model of ischemia/reperfusion injury. J Immunol. 2006;177(7):4727–4734. doi:10.4049/jimmunol.177.7.4727.
- Auriti C, Prencipe G, Moriondo M, et al. Mannose-binding lectin: biologic characteristics and role in the susceptibility to infections and ischemia-reperfusion related injury in critically ill neonates. J Immunol Res. 2017;2017:7045630–7045611. doi:10.1155/2017/7045630.
- Strassheim D, Renner B, Panzer S, et al. IgM contributes to glomerular injury in FSGS. J Am Soc Nephrol. 2013;24(3):393–406. doi:10.1681/ASN.2012020187.
- Zhang YM, Gu QH, Huang J, et al. Clinical significance of IgM and C3 glomerular deposition in primary focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2016;11(9):1582–1589. doi:10.2215/CJN.01190216.

