Abstract
Background
This retrospective study aims to investigate the prevalence and immunopathologic characteristics of seropositive and seronegative hepatitis B virus-associated membranous nephropathy (HBV-MN).
Methods
Clinicopathologic and serologic records of 420 patients with histologically confirmed HBV-MN between January 2014 and July 2021 were examined to determine the prevalence of seropositive and seronegative HBV-MN. Serum anti-PLA2R antibody testing was conducted on 280 patients with HBV-associated membranous nephropathy (HBV-MN) from August 2018 to July 2021. Immunopathologic characteristics of HBV-MN patients and anti-PLA2R antibody positivity were analyzed.
Results
Among 420 pathologically confirmed HBV-MN patients, 230 (54.8%) were seropositive for HBV. The seropositive group exhibited higher blood creatinine values and incidence of liver function abnormalities than the seronegative group (p < .05). Serum anti-PLA2R antibody testing on 280 HBV-MN patients revealed a total positive rate of 44.6%, with the seronegative group showing a significantly higher rate (62.6%) compared to the seropositive group (32.1%) (p < .01). The anti-PLA2R antibody-positive group displayed higher levels of urine protein (p < .05), serum cholesterol (p < .01), and IgG4 subtypes (p < .05) compared to the negative group. Additionally, the positive group had significantly lower levels of serum albumin and IgG than the negative group (p < .01).
Conclusions
This comprehensive study reveals a significantly higher prevalence of seronegative HBV-MN than previously thought. The blood creatinine values and incidence of liver function abnormalities was higher in the serology-positive group than in the serology-negative group. Notably, the seronegative group displayed a higher positive rate of anti-PLA2R antibodies compared to the seropositive group, indicating distinctive clinical and immunopathologic features.
Introduction
The World Health Organization (WHO) estimates that approximately 2 billion people worldwide are infected with the hepatitis B virus (HBV), with 350 million of them having chronic infection [Citation1]. In China, HBV remains the most common cause of liver disease and liver failure, with over 93 million individuals estimated to have chronic HBV infection, and one in five of them having chronic hepatitis B [Citation2]. A recent seroepidemiological survey of approximately 150,000 individuals in China found a 7.70% (95% confidence interval [CI]: 7.57%–7.84%) positive rate for HBsAg, with a lower prevalence in children under 5 years old (0.77%) compared to adults (5.69%–11.22%). In contrast, the seroprevalence of HBsAg in other Asian countries, such as South Korea (4.0%) [Citation3]and Singapore (3.6%, 95% CI: 2.9–4.2%) [Citation4], is lower. In Europe, countries like the UK and the Netherlands report even lower seroprevalence rates for HBsAg, typically less than 1% [Citation5].
Hepatitis B infection exhibits diverse clinical manifestations and can lead to liver failure. In China, extra-hepatic manifestations of hepatitis B, including HBV-associated glomerulonephritis (HBV-GN), have gained recognition. HBV-GN is now one of the most prevalent secondary glomerular diseases in China, accounting for 0.25% of renal biopsies [Citation6,Citation7]. Diagnosis of HBV-GN relies on positive HBV serum markers and the presence of HBV antigens in kidney biopsy specimens [Citation8]. Zhang et al. utilizing renal biopsy data from 329 cases, demonstrated an association between serological HBV markers and renal pathological changes, with mesangioproliferative glomerulonephritis being the most common pathological subtype of HBV-GN [Citation9]. However, renal biopsy is typically performed in patients with evidence of both renal damage and HBV infection, neglecting serum HBsAg-negative HBV-GN patients [Citation10]. Kong et al. examined 500 glomerulonephritis patients without serological evidence of HBV infection and identified that 0.6% of these patients had occult HBV infection as shown by immunohistochemistry of frozen renal tissue specimens [Citation11]. Li et al. conducted a study on 18 seronegative HBV-GN patients, highlighting that proteinuria was the predominant clinical manifestation, and renal histopathology predominantly displayed membranous nephropathy. This finding is consistent with an anecdotal report of 5 pediatric cases of HBsAg-negative HBV-GN, all of whom were diagnosed with membranous nephropathy [Citation12].
The clinical utility of these findings is hindered by the limited sample size and anecdotal nature of previous studies on seronegative HBV-GN patients. Our prior investigation involving 196 cases of HBV-associated membranous nephropathy revealed some associations between clinicopathologic variables and HBV markers [Citation13], though a definitive relationship was not established. In the present study, we conducted a retrospective review of the clinical, pathological, and serological data from 420 patients with pathologically confirmed HBV-MN. We aimed to assess the prevalence of seropositive and seronegative HBV-MN in this cohort and conducted an in-depth analysis of the immunopathologic characteristics of HBV-MN patients.
Patients and methods
Patients
We retrospectively analyzed clinicopathologic and serologic data from patients who underwent renal biopsy at China-Japan Friendship Hospital between January 2014 and July 2021. The diagnosis of HBV-GN was based on established criteria [Citation14], including the presence of pathologically confirmed glomerulonephritis, with the exclusion of conditions such as lupus nephritis and other secondary nephritises. Diagnosis also required the presence of positive HBV antigens, specifically HBsAg or hepatitis B core antigen (HBcAg), as determined by immunohistochemistry in kidney tissue. Patients with available serological data on HBV antigens were included. Renal pathologic categories were classified according to the WHO Pathological Categories of Renal Glomerular Disease [Citation15] (amended version), with two experienced pathologists (GZ and WZ) providing independent assessments.
Clinical data
Patient data obtained at the time of renal biopsy included age, sex, history of hepatitis B (documented or suspected), liver and kidney ultrasound results, as well as laboratory findings such as urinalysis, 24-h protein excretion, alanine aminotransferase (ALT), serum creatinine, and immunoglobulin levels. Serum HBV markers (HBsAg, HBeAg, HBsAb, HBeAb, and HBcAb) and HBV-DNA were determined using chemiluminescence immunoassay (CLIA) and real-time fluorescence quantitative polymerase chain reaction (RTFQ PCR). The estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI formula [Citation16]. Renal function was classified into chronic kidney disease (CKD) stages 1–5 based on eGFR values [Citation17]. CKD1: eGFR > 90 mL/min/1.73 m2, CKD2: GFR 60–89 mL/min/1.73 m2, CKD3: GFR 30–59 mL/min/1.73 m2 (CKD3a: GFR 45–59 mL/min/1.73m2, CKD3b: GFR 30–44 mL/min/1.73 m2, CKD 4: GFR 15–29 mL/min/1.73 m2, and CKD5: GFR < 15 mL/min/1.73 m2 or dialysis.
Analysis of kidney biopsies
Percutaneous renal puncture was performed and kidney tissue specimens were obtained in 3 parts in all cases for light microscopy, immunofluorescence and electron microscopy. (1) Light microscopy: light microscopic specimens all contained more than 10 glomeruli, were paraffin-embedded, sectioned 2 μm thick, and stained with HE, PAS, PASM, and MASSON, respectively. (2) Immunofluorescence: Frozen sections were used to detect IgG, IgA, IgM, C3, C1q, FRA and IgG subclasses (IgG1, IgG2, IgG3 and IgG4) by direct immunofluorescence. (3) Electron microscopy: All kidney biopsy specimens were sent to the electron microscopy laboratory of China-Japan Friendship Hospital for examination. Renal pathological typing and diagnosis were referred to our pathological standards for renal biopsies.
Serum testing for anti-PLA2R antibody
The patient’s serum was collected 1–3 d before renal biopsy and tested directly after collection with a human anti-phospholipase A2 receptor antibody enzyme immunoassay kit (Shanghai Lianshuo Biological), and each step was performed according to the instructions. Serum anti-PLA2R antibody ≥20 IU/ml was considered positive.
Statistical analysis
Statistical analysis was performed using the SPSS statistical software package, version 19.0 (SPSS Inc., Chicago, IL). Normally distributed variates were expressed as the mean ± SD, and continuous variates of unnormal distribution were expressed as the median with inter quartile range (IQR). Categorical variables were expressed as absolute values and percentages. For continuous variates, comparisons between two groups were performed using the Student’s t test for normally distributed data and the Wilcoxon rank-sum test for non parametric data; For categorical variables, Chi-square, Fisher exact test or Pearson Chi-square test were performed appropriately.
Results
Prevalence of seropositive and seronegative HBV-GN
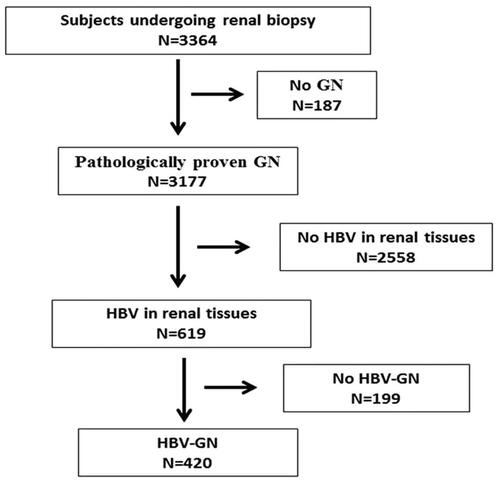
The study flowchart, depicted in , illustrates the patient selection process. Among 3364 patients who underwent renal biopsy at our institution during the study period, 3177 (94.4%) were diagnosed with pathologically-proven glomerulonephritis. Those without HBV in renal tissues and individuals lacking HBV-GN were excluded from the subsequent analysis. Ultimately, 420 (12.5%) patients with pathologically-proven HBV-MN were considered in this retrospective investigation.
Figure 1. The study flowchart. GN: glomerulonephritis; HBV, hepatitis B virus; MN: membranous nephropathy.
The distribution of serum HBV markers in patients with pathologically proven glomerulonephritis is detailed in . Out of the cohort, 230 (54.8%) patients were seropositive for HBV, including 29 (6.9%) who were HBsAg-positive. The remaining 190 (45.2%) were seronegative for HBV. HBV antigens were detected in renal tissue specimens from all 420 (100%) patients. Among them, 89 (21.2%) were positive for HBsAg, 28 (6.7%) for HBcAg, and 303 (72.1%) for both HBsAg and HBcAg. Immunohistochemical staining confirmed the presence of HBsAg and HBcAg antigens in the glomeruli, as depicted in .
Figure 2. Detection of viral antigens and particles in renal tissues. Immunohistochemical staining of frozen renal tissue sections. (A) HBsAg and (B) HBcAg antigens are located in the glomeruli as indicated by brown staining in the glomeruli.
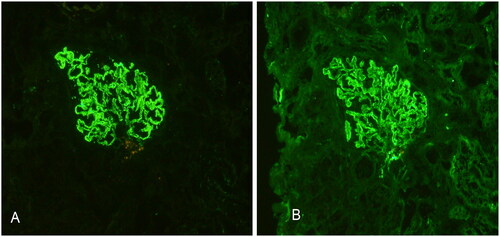
Table 1. Distribution of serum HBV markers among patients with pathologically proven glomerulonephritis (N = 420).
Demographic and baseline characteristics of the study population
summarizes the demographic and baseline characteristics of the study population. The mean age of the patients was 45.1 ± 15.6 years (range 14–82), with a slight male predominance (234, 55.7%). The median duration of HBV-MN was 7.76 months, ranging from 0.1 to 144. Hematuria was prevalent in the majority of patients (80.5%). The average 24-h urinary protein excretion was 4.73 ± 2.59 g/day, varying from 0.37 to 14.38, and around two-thirds (68.1%) had nephrotic syndrome. Additionally, most patients (84.5%) had CKD1. The blood creatinine values and incidence of liver function abnormalities was higher in the serology-positive group than in the serology-negative group.
Table 2. Demographic And baseline characteristics of the study subjects.
Immunopathologic characteristics of the study population
displays the immunopathologic characteristics of the study population. All 420 subjects were diagnosed with membranous nephropathy, with the vast majority (93.3%) having uncomplicated membranous nephropathy. In the remaining 28 patients, membranous nephropathy was complicated by ischemic nephropathy in 13 patients, tubulointerstitial lesions in 9 patients, IgA nephropathy in 3 patients, obesity-associated nephropathy in 2 patients, and diabetic nephropathy in 1 patient. Among the 348 patients for whom IgG subtypes were determined at the time of biopsy, 96.0% were positive for IgG1, and 97.7% for IgG4. Approximately half (49.7%) were positive for IgG1, IgG2, and IgG4. Thirty-seven patients (10.6%) were positive for IgG1, IgG3, and IgG4, and one-fourth (25.6%) were positive for IgG1, IgG2, IgG3, and IgG4. Patients with seropositive and seronegative HBV-GN exhibited comparable immunopathologic characteristics.
Table 3. Pathological characteristics of the study population.
Positivity of anti-PLA2R antibodies in the study population
We conducted serum anti-PLA2R antibody testing on 280 patients from August 2018 to July 2021. Of the 165 patients in the seropositive HBV-MN group, 53 (32.1%) were positive for serum anti-PLA2R antibodies. In the seronegative HBV-MN group of 115 patients, 72 (62.6%) were positive for serum anti-PLA2R antibodies. The overall positive rate of anti-PLA2R antibodies in HBV-MN patients was 44.6%, and the seronegative group exhibited a significantly higher positivity rate compared to the seropositive group (p < .01).
Table 4. Comparative analysis of the clinicopathological data between the anti-PLA2R antibody-positive and negative groups.
Based on serum anti-PLA2R antibody levels, patients were categorized into two groups: negative (<20 IU/ml, n = 155) and positive (≥20 IU/ml, n = 125). The positive group displayed significantly higher levels of urine protein (p < .05) and serum cholesterol (p < .01) compared to the negative group. Furthermore, the positive group had significantly lower levels of serum albumin and IgG than the negative group (p < .01). The rate of IgG4 in renal tissue among patients with positive serum anti-PLA2R antibodies was significantly higher than that in the negative group (p < .05), with no significant difference in other IgG subclasses between the two groups ().
Discussion
HBV-GN is highly prevalent in China, constituting a common secondary glomerular disease among its population. Diagnosis traditionally relies on HBV seropositivity and the presence of HBV antigens in renal biopsy specimens, potentially leading to underdiagnosis. The recognition of seronegative HBV-GN’s importance is growing, yet current evidence remains limited, mainly derived from retrospective studies and anecdotal case reports [Citation9–12].
In our study, we retrospectively analyzed a substantial cohort of 3364 patients who underwent renal biopsies over five years. Among these patients, a vast majority (94.4%) had pathologically-proven glomerulonephritis. Interestingly, 76.9% showed no evidence of HBV in renal tissues, while 420 (12.5%) were confirmed cases of HBV-MN. Among them, 230(54.8%) were seropositive for HBV, and 190 (45.2%) were seronegative for HBV. Our findings indicate that seronegative HBV-MN might be more prevalent than previously recognized, particularly when compared to Kong et al.’s study, where only 0.6% of 500 HBV-GN patients were found to have occult HBV infection [Citation11].
The pathogenesis of HBV-GN remains incompletely understood. It is generally attributed to chronic HBV infection [Citation18], leading to immune complex deposition in glomeruli [Citation19]. In our study, both seropositive and seronegative HBV-MN patients exhibited renal tissue presence of HBV antigens and various IgG subtypes, indicating direct virally-induced pathological changes and ongoing immunological damage. Notably, IgG1 and IgG4 were nearly universally present in renal tissue specimens at the time of biopsy, suggesting widespread IgG subtype deposition in glomeruli.
Previous research by Zhou et al. also observed the presence of HBV-DNA in renal tissues, often coinciding with HBV antigen presence, suggesting potential local and systemic origins of these antigens, which contribute to cytopathologic damage and abnormal immune responses [Citation20]. Our findings, demonstrating the presence of HBV antigens and IgG subtypes in seronegative HBV-MN patients, further support the notion of locally initiated immunological damage within the kidneys of HBV-MN patients.
Membranous nephropathy is the predominant manifestation of HBV-GN [Citation21] and earlier studies have demonstrated the strongest association of HBV infection with membranous nephropathy [Citation22–27], as observed in all 420 patients in our study. In adults, proteinuria and nephrotic syndrome are the primary clinical features of HBV-MN [Citation28]. Our findings align with this pattern, as 95% of our HBV-MN patients presented with CKD1 and CKD2, indicating preserved renal function upon biopsy. Nevertheless, severe proteinuria, along with hematuria, was prevalent, and nephrotic syndrome developed in two-thirds of these patients.
We have shown that the prevalence of seronegative HBV-MN is similar to that of seropositive HBV-MN. The current diagnostic criteria for HBV-GN in China do not mandate HBV seropositivity. However, the strict criteria may leave a significant proportion of HBV-GN patients undiagnosed and untreated, particularly as HBV-GN accounts for a larger share of nephritis cases in China compared to regions with lower HBV infection rates like North America and Western Europe. Therefore, it is crucial to evaluate both serological HBV markers and HBV markers in renal tissues for patients with unexplained renal diseases, including membranous nephropathy.
Serum HBV-DNA >1*103 IU/mL was observed in less than 4.6% of our patients. Unfortunately, we could not assess the correlation between serum HBV DNA levels and patient clinicopathologic variables. A study by Jiang and Liu [Citation29] found a connection between renal injury and the replication level of HBV DNA in HBV-GN patients. They further demonstrated that a higher serum HBV DNA level was associated with increased deposition of HBV antigens in renal tissues and more progressive renal injury in HBV-GN patients [Citation30]. This study is limited by its retrospective nature and lack of follow-up data. Additionally, our patient cohort is from a tertiary care center in China, which may not fully represent community care center settings.
In 2009, Beck [Citation31] detected M-type PLA2R antibodies in the blood of idiopathic membranous nephropathy (IMN) patients, present in about 70–80% of IMN patients and strongly linked to disease activity and prognosis. These antibodies were not found in patients with secondary membranous nephropathy. Additionally, they observed that anti-PLA2R antibody titers correlated with disease activity, disappearing during remission and increasing upon recurrence [Citation31,Citation32].
Recent studies found positive anti-PLA2R antibodies in the serum of patients with atypical membranous nephropathy and HBV-MN [Citation33–35]. Both IMN and HBV-MN patients had serum anti-PLA2R antibody expression, with no difference in the positive rate between these groups [Citation36]. In our study, the total anti-PLA2R antibody positivity in HBV-MN patients was 44.6%, and the serology-negative group had a significantly higher positive rate compared to the serology-positive group (p < .01).
In conclusion, seronegative HBV-MN is more prevalent than previously believed and may equal seropositive HBV-MN in tertiary care settings in China. The blood creatinine values and incidence of liver function abnormalities was higher in the serology-positive group than in the serology-negative group. Notably, the serology-negative group displayed a significantly higher positive rate of anti-PLA2R antibodies compared to the seropositive group. While our study is the largest clinical cohort for HBV-MN, further confirmation through rigorous prospective studies is warranted.
Ethical approval
This study was approved by the Ethics Committee of China-Japan Friendship Hospital (approval number: 2019-17-K12)
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- WHO. Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021. Accountability for the global health sector strategies 2016–2021: Actions for impact. Available from: https://www.who.int/publications/i/item/9789240027077
- Huang P, Zhu LG, Zhu YF, et al. Seroepidemiology of hepatitis B virus infection and impact of vaccination. World J Gastroenterol. 2015;21(25):1–7. doi: 10.3748/wjg.v21.i25.7842.
- Lee BS, Cho YK, Jeong SH, et al. Nationwide seroepidemiology of hepatitis B virus infection in South Korea in 2009 emphasizes the coexistence of HBsAg and anti-HBs. J Med Virol. 2013;85(8):1327–1333. doi: 10.1002/jmv.23594.
- Ang LW, Cutter J, James L, et al. Seroepidemiology of hepatitis B virus infection among adults in Singapore: a 12-year review. Vaccine. 2013;32(1):103–110. doi: 10.1016/j.vaccine.2013.10.057.
- Nardone A, Anastassopoulou CG, Theeten H, et al. A comparison of hepatitis B seroepidemiology in ten European countries. Epidemiol Infect. 2009;137(7):961–969. doi: 10.1017/S0950268808001672.
- Zhang X, Liu S, Tang L, et al. Analysis of pathological data of renal biopsy at one single center in China from 1987 to 2012. Chin Med J. 2014;127(9):1715–1720. doi: 10.3760/cma.j.issn.0366-6999.20132765.
- Li LS, Liu ZH. Epidemiologic data of renal diseases from a single unit in China: analysis based on 13,519 renal biopsies. Kidney Int. 2004;66(3):920–923. doi: 10.1111/j.1523-1755.2004.00837.x.
- Khedmat H, Taheri S. Hepatitis B virus-associated nephropathy: an international data analysis. Iran J Kidney Dis. 2010;4(2):101–105.
- Zhang L, Meng H, Han X, et al. The relationship between HBV serum markers and the clinicopathological characteristics of hepatitis B virus-associated glomerulonephritis (HBV-GN) in the northeastern Chinese population. Virol J. 2012;9(1):200. doi: 10.1186/1743-422X-9-200.
- Li D, Gao G, Jiang H, et al. Hepatitis B virus-associated glomerulonephritis in HBsAg serological-negative patients. Eur J Gastroenterol Hepatol. 2015;27(1):65–69. doi: 10.1097/MEG.0000000000000236.
- Kong D, Wu D, Wang T, et al. Detection of viral antigens in renal tissue of glomerulonephritis patients without serological evidence of hepatitis B virus and hepatitis C virus infection. Int J Infect Dis. 2013;17(7):e535–e538. doi: 10.1016/j.ijid.2013.01.017.
- Liu T, Yang S, Yue Z, et al. Clinical and pathological characteristics of 5 children with HBV surface antigen (HBsAg)-negative hepatitis B virus-associated glomerulonephritis. J Clin Virol. 2015;66:1–5. doi: 10.1016/j.jcv.2015.02.012.
- Tan Z, Fang J, Lu JH, et al. HBV serum and renal biopsy markers are associated with the clinicopathological characteristics of HBV-associated nephropathy. Int J Clin Exp Pathol. 2014;7:8150–8154.
- Medicine TEBoJoI. Summary of the forum about hepatitis B virus associated glomerulonephritis. Chin J Intern Med. 1990;29:519.
- Churg J, Bj, Glassock RJ. Renal disease: classification and atlas of glomerular disease. 2nd ed. New York (NY): Ikagu-Shoin. 1995.
- Stevens LA, Claybon MA, Schmid CH, et al. Evaluation of the chronic kidney disease epidemiology collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int. 2011;79(5):555–562. doi: 10.1038/ki.2010.462.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-Mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2009;113:S1–S130. doi: 10.1038/ki.2009.188.
- Zhiqiang G, Zhaohui D, Qinhuan W, et al. Cost of chronic hepatitis B infection in China. J Clin Gastroenterol. 2004;38(10 Suppl 3):S175–S178. doi: 10.1097/00004836-200411003-00010.
- Lin CY. Hepatitis B virus-associated membraneous nephropathy: clinical features, immunological profiles and outcome. Nephron. 1990;55(1):37–44. doi: 10.1159/000185916.
- Zhou SD, Zy Guo MY, Fang LJ, et al. The study of the significance of the appearance of HbcAg in glomerulonephritis. Chin J Nephrol. 1995;11:104.
- Lai KN, Li PK, Lui SF, et al. Membranous nephropathy related to hepatitis B virus in adults. N Engl J Med. 1991;324(21):1457–1463. doi: 10.1056/NEJM199105233242103.
- Coovadia HM, Adhikari M, Morel-Maroger L. Clinico-pathological features of the nephrotic syndrome in South African children. Q J Med. 1979;48(189):77–91.
- Wong VC, Ip HM, Reesink HW, et al. Prevention of the HBsAg carrier state in newborn infants of mothers who are chronic carriers of HBsAg and HBeAg by administration of hepatitis-B vaccine and hepatitis-B immunoglobulin. Double-blind randomised placebo-controlled study. Lancet. 1984;1(8383):921–926. doi: 10.1016/s0140-6736(84)92388-2.
- Takekoshi Y, Tanaka M, Shida N, et al. Strong association between membranous nephropathy and hepatitis-B surface antigenaemia in Japanese children. Lancet. 1978;2(8099):1065–1068. doi: 10.1016/s0140-6736(78)91801-9.
- Tadokoro M. The clinico-pathological studies of hepatitis B virus nephropathy in adults. Nihon Jinzo Gakkai Shi. 1991;33(3):257–266.
- Hsu HC, Lin GH, Chang MH, et al. Association of hepatitis B surface (HBs) antigenemia and membranous nephropathy in children in Taiwan. Clin Nephrol. 1983;20(3):121–129.
- Slusarczyk J, Michalak T, Nazarewicz-de Mezer T, et al. Membranous glomerulopathy associated with hepatitis B core antigen immune complexes in children. Am J Pathol. 1980;98(1):29–43.
- Lai KN, Lai FM, Chan KW, et al. The clinico-pathologic features of hepatitis B virus-associated glomerulonephritis. Q J Med. 1987;63(240):323–333.
- Jiang W, Liu LQ. Effect of content of hepatitis B virus DNA in the serum on the pathologic change in hepatitis B virus associated-glomerulonephritis. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2008;33(9):857–860.
- Jiang W, Liu T, Dong H, et al. Relationship between serum DNA replication, clinicopathological characteristics and prognosis of hepatitis B virus-associated glomerulonephritis with severe proteinuria by lamivudine plus adefovir dipivoxil combination therapy. Biomed Environ Sci. 2015;28:206–213.
- Beck LH, Bonegio RGB, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361(1):11–21. doi: 10.1056/NEJMoa0810457.
- Qin W, Beck LH, Zeng C, et al. Anti-phospholipase A2 receptor antibody in membranous nephropathy. J Am Soc Nephrol. 2011;22(6):1137–1143., doi: 10.1681/ASN.2010090967.
- Xie Q, Li Y, Xue J, et al. Renal phospholipase A2 receptor in hepatitis B virus-associated membranous nephropathy. Am J Nephrol. 2015;41(4-5):345–353. doi: 10.1159/000431331.
- Gunnarsson I, Schlumberger W, Rönnelid J. Antibodies to M-type phospholipase A2 receptor (PLA2R) and membranous lupusnephritis. Am J Kidney Dis. 2012;59(4):585–586. doi: 10.1053/j.ajkd.2011.10.044.
- Lönnbro-Widgren J, Ebefors K, Mölne J, et al. Glomerular IgG subclasses in idiopathic and malignancy-associated membranous nephropathy. Clin Kidney J. 2015;8(4):433–439. doi: 10.1093/ckj/sfv049.
- Jiang Z, Cai M, Dong B, et al. Clinicopathological features of atypical membranous nephropathy with unknown etiology in adult Chinese patients. Medicine. 2018;97(32):32(e11608. doi: 10.1097/MD.0000000000011608.

