Abstract
Background
This study aimed to discuss the diagnostic value of multi-parameter ultrasound evaluation in sepsis complicated with acute kidney injury (AKI).
Methods
Patients were divided into an AKI group (n = 50) and a non-injury group (n = 50) based on the presence of AKI. The clinical characteristics were collected, and renal function parameters between the two groups were compared, including 24-h urine volume, serum creatinine, urea, serum cystatin C (CysC), renal parenchymal thickness (RPT), renal artery resistance index (RI), and multi-parameter ultrasound scoring (MPUS). Additionally, logistic regression analysis was conducted to determine the influencing factors of sepsis complicated with AKI. The prediction value was evaluated using a receiver operating characteristic (ROC) curve.
Results
In the AKI group, creatinine, CysC, urea, MPUS score, RPT, and RI values were significantly higher, while the 24-h urine volume was lower than those in the non-injury group (p < 0.01). Moreover, multivariate logistic analysis indicated that high CysC and RI values were independent risk factors, whereas high 24-h urine volume and low MPUS were independent protective factors for sepsis-induced AKI. The ROC curve demonstrated that RI (AUC = 0.906) was more effective than 24-h urine volume (AUC = 0.797), CysC (AUC = 0.730), and MPUS (AUC = 0.794) in identifying sepsis-induced AKI.
Conclusion
High RI values increase the risk of sepsis-induced AKI, whereas low MPUS may reduce it. RI showed high diagnosis values for sepsis complicated with AKI.
Introduction
Sepsis is a systemic response to infection with some degree of organ dysfunction [Citation1]. Generally, sepsis often occurs in patients with infection, trauma, burns, surgery, urinary tract infection, or multiple chronic diseases [Citation2,Citation3]. If the septic symptoms worsen, the patients may further experience organ hypoperfusion, organ dysfunction, or hypotension [Citation4]. Acute kidney injury (AKI), a very common complication of sepsis, is a sudden decrease in renal function within a short period caused by various etiologies [Citation5]. Studies have shown that patients in the intensive care unit (ICU) are more likely to develop AKI due to clinical instability and previous risk factors, including advanced age, sepsis, hypovolemia, surgery, and nephrotoxic medication [Citation6,Citation7]. Up to 50% of AKI cases are associated with sepsis, and up to 60% of patients with sepsis develop AKI [Citation8,Citation9]. Reportedly, among all ICU cases, 2%–3% of patients with septic AKI require dialysis intervention, with a mortality rate of 30%–60% [Citation10]. However, the pathophysiology of septic AKI remains poorly understood. Sepsis-induced AKI has been suggested to be associated with factors, including microcirculatory dysfunction, inflammatory response, and changes in bioenergetics [Citation11,Citation12]. Presently, septic AKI has emerged as a significant medical issue for healthcare workers. However, early diagnosis of sepsis complicated with AKI allows for prompt clinical intervention to lower the risk of mortality. Besides aiding in the prevention and treatment of septic AKI, early diagnosis also plays an important role in improving the prognosis of patients [Citation13]. AKI diagnosis currently depends on renal function indicators such as serum creatinine level and 24-h urine volume. However, due to several significant factors, both serum creatinine level and 24-h urine volume may delay the diagnosis and treatment of some patients with AKI [Citation14,Citation15]. The renal artery resistance index (RI) is a noninvasive tool for evaluating renal function, and represents a pulsatility and vascular compliance index [Citation16]. Based on some reports, ultrasound exploration showed elevated RI, pulsatility index (PI), and renal parenchymal thickness (RPT) in patients with AKI, suggesting the high clinical significance of ultrasound-related parameters for the diagnosis of AKI [Citation17]. Besides, PI and RPT can be quantitatively assessed according to the degree of renal injury in patients, and the construction of the multi-parameter ultrasound scoring (MPUS) system can help avoid the defects of a single evaluation in RI or RPT [Citation18]. Reportedly, MPUS has been used to predict hydronephrosis [Citation19,Citation20]. Meanwhile, some studies have predicted kidney injury using MPUS [Citation21,Citation22]. However, the diagnostic efficacy of MPUS in patients with sepsis-induced AKI remains unclear. Therefore, this study mainly aimed to discuss the diagnostic value of MPUS in sepsis complicated with AKI.
Materials and methods
General information
The medical history data of 100 patients with sepsis admitted to the ICU in Sinopharm Dongfeng General Hospital on the first day from May 2020 to May 2022 were retrospectively analyzed. According to the statistical principle as well as inclusion and exclusion criteria, 50 septic patients with AKI were selected and categorized as the AKI group, whereas the remaining 50 septic patients without AKI were categorized as the non-injury group. Baseline data of the two groups, including age, gender, kidney disease (yes/no), diabetes mellitus (yes/no), hypertension (yes/no), retinopathy (yes/no), peripheral neuropathy (yes/no), body mass index (BMI), uric acid, glycosylated hemoglobin (MQ-6000 Runda Medical, China), blood calcium, serum phosphorus, total cholesterol, triacylglycerol, serum albumin, hemoglobin (BC6800, Maindray, China), urine protein quantitation (ABBOTT ARCHITECT C16200 automatic biochemistry analyzer), urine red blood cell count (Mejer180, SHENZHEN, China), estimated glomerular filtration rate (eGFR), blood urea nitrogen, and serum creatinine, were collected. The ethics committee of Sinopharm Dongfeng General Hospital approved this study (LW-2022-032).
Inclusion criteria included the following [Citation1]: age: 18–80 years [Citation2]; patients who met the diagnostic criteria for sepsis-3 in the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) (2016) [Citation23] and for AKI in KDIGO Clinical Practice Guideline for Acute Kidney Injury [Citation24] (Increased blood creatinine level ≥ 26.5 μmol/L within 48 h)—KDIGO is an acronym for Kidney Disease: Improving Global Outcomes; and [Citation3] patients with perfected renal multi-parameter ultrasound evaluation. Only subjects with complete data for all variables were considered. The exclusion criteria included the following [Citation1]: history of chronic kidney disease (stage 4–5) or continuous renal replacement therapy (RRT) [Citation2]; history of AKI caused by noninfectious factors (including acute trauma, transplant rejection, and autoimmune disease) [Citation1,Citation3]; pregnant and lactating women; and [Citation4] incomplete clinical data.
Outcome measures
Patients’ blood was collected from the antecubital vein upon admission to the ICU. Blood samples were centrifuged (2500 rpm, 10 min, 4 °C) to obtain serum. Serum creatinine (C011-2-1), urea (C013-2-1), and serum cystatin C (H336-1-1) levels were measured using corresponding detection kits (Nanjing Jian Cheng Institute of Biotechnology, Nanjing, China). We collected patients’ renal function indicators: 24-h urine volume, serum creatinine, urea, and serum cystatin C. Renal ultrasound parameters, including RPT, RI, and renal pelvis anteroposterior diameter (APD), were collected from patients through ultrasonography. The interlobar artery was used to calculate RI. The MPUS standard was adjusted based on previous research [Citation20,Citation21]. The MPUS score was calculated as the sum of the scores of the three renal ultrasound parameters, i.e., MPUS = RPT + RI + APD (). Acute physiological and chronic health evaluation II (APACHE II) and sequential organ failure assessment (SOFA) were used to evaluate body condition.
Table 1. Renal ultrasound feature scoring criteria.
Statistical analysis
Data were statistically analyzed using SPSS 26.0. Enumeration data are expressed as n, and the χ2 test was used for comparison between groups. The Shapiro-Wilk test was used to evaluate the normality of the measurement data. Measurement data with normal distribution are expressed as mean ± standard deviation, and an independent sample t-test was used for comparison between the two groups. Non-normally distributed continuous data are expressed as median (interquartile spacing, IQR) and analyzed using the Kruskal–Wallis H-test. Logistic multivariate regression was used to analyze the influencing factors of sepsis complicated with AKI. The receiver operating characteristic (ROC) curve was plotted to predict the diagnostic values of the influencing factors for sepsis complicated with AKI. Additionally, a statistically significant difference was at p < 0.05.
Results
Baseline characteristics of the patients
shows that there were no significant differences between the two groups in age, gender, kidney disease (yes/no), diabetes mellitus (yes/no), hypertension (yes/no), retinopathy (yes/no), peripheral neuropathy (yes/no), BMI, uric acid, glycosylated hemoglobin, blood calcium, serum phosphorus, total cholesterol, triacylglycerol, serum albumin, hemoglobin, urine protein quantitation, urine red blood cell count, eGFR, and blood urea nitrogen (p > 0.05).
Table 2. Comparisons of baseline characteristics.
Comparison of renal function
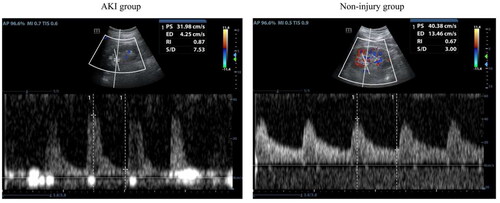
Patients in the AKI group showed much higher levels of serum creatinine, urea, serum cystatin C, RPT, RI, APD, and MPUS but lower 24-h urine volume than those in the non-injury group (p < 0.05) (). The ultrasound image of the kidney in shows the changes in RI values between the two groups. It was observed that the non-injury group had lower RI values than the AKI group ().
Table 3. Comparisons of renal function in the patients.
Logistic multivariate regression analysis for factors influencing sepsis complicated by AKI
Logistic multivariate regression analysis was conducted to evaluate the factors influencing AKI in patients with sepsis, such as 24-h urine volume, serum cystatin C, RPT, RI, MPUS, APACHE II score, and SOFA score (). High serum cystatin C (OR = 2.376, 95% CL, 1.477–3.820) and high RI values (OR = 15.288, 95% CL, 4.416–33.437) were independent risk factors affecting sepsis complicated with AKI, whereas high 24-h urine volume (OR = 3.126, 95% CL, 1.256–8.263) and low MPUS (OR = 8.245, 95% CL, 2.365–25.623) were independent protection factors (p < 0.05). However, RPT, APACHE II score, and SOFA score exhibited no significant effects on sepsis complicated with AKI (p > 0.05).
Table 4. Logistic multivariate regression analysis for influencing factors of sepsis complicated with acute kidney injury.
Receiver operating characteristic (ROC) curve analysis for 24-h urine volume, serum cystatin C, renal artery resistance index (RI), and multi-parameter ultrasound scoring (MPUS)
The ROC curve was plotted to analyze the application of the significant influencing factors (24-h urine volume, serum cystatin C, RI, and MPUS) in predicting sepsis-induced AKI (). RI (area under the curve, AUC = 0.906) exhibited a better effect than 24-h urine volume (AUC = 0.797), serum cystatin C (AUC = 0.730), and MPUS (AUC = 0.794) on diagnosing sepsis complicated with AKI (p < 0.05) (, ).
Figure 2. Receiver operating characteristic (ROC) curve for 24-h urine volume, serum cystatin C, renal artery resistance index (RI), and multi-parameter ultrasound scoring (MPUS).
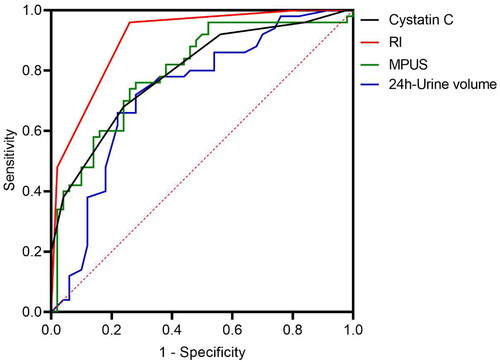
Table 5. Receiver operating characteristic (ROC) curve analysis for related indicators of patients with septic AKI.
Discussion
In this study, patients in the AKI group showed significantly higher serum creatinine, urea, and serum cystatin C levels but lower 24-h urine volume than those in the non-injury group. Logistic multivariate regression analysis revealed that high serum cystatin C levels were an independent risk factor for sepsis complicated with AKI, whereas 24-h urine volume served as an independent protective factor. The results agree with those of previous studies [Citation25]. Patients with AKI usually present with decreased eGFR, retention of creatinine and urea nitrogen, disorders of water, electrolyte, and acid–base balance, and dysfunction of multiple systemic organs. Dialysis or kidney transplantation may be required as treatment options if severe renal function impairment progresses to renal failure [Citation26]. More terribly, patients tend to exhibit no clinical symptoms or signs in the early stages of renal impairment due to kidney’s strong compensatory function [Citation27]. Currently, renal function indicators, including 24-h urine volume, urea nitrogen, serum creatinine, serum cystatin C, and urine microalbumin (mAlb), are commonly applied markers for renal function evaluation in clinical practice [Citation25,Citation28]. Patients with AKI experience a significantly increased serum creatinine level within 48 h, a decreased 24-h urine volume for more than 6 h, and a continuously increasing serum cystatin C level [Citation29]. Cystatin C is reportedly an effective alternative marker that can predict renal function decline earlier than serum creatinine in the general ICU population [Citation30]. Therefore, among renal function indicators, serum cystatin C and 24-h urine volume can be adopted as predictive factors for diagnosing patients with sepsis-induced AKI. Overall, early detection of renal function impairment indicators, timely assessment of renal function, and early intervention treatment are significant for the prognosis of patients with sepsis-induced AKI [Citation31].
Renal ultrasound has also been shown to reflect renal function in patients with renal impairment [Citation32]. Renal ultrasound measurements mainly include renal length, renal width, renal cortical thickness, renal medullary pyramid thickness, RPT, renal volume, and RI [Citation33]. Some studies have revealed the important predictive value of renal ultrasound-related parameters in fetal hydronephrosis [Citation10]. Among these parameters, RPT and RI values exhibited higher relevance to AKI [Citation34]. Currently, RPT and RI have been used clinically to assess renal function in patients with renal impairment [Citation35]. A meta-analysis showed that RI can predict the occurrence of AKI after a major surgery. Patients with AKI have higher RI values (pooled sensitivity: 81.8%, specificity: 77.6%, the area under the curve: 0.866) [Citation16]. A previous study also reported that RI could serve as an early predictor of AKI in critically ill patients [Citation36]. In this study, patients in the AKI group exhibited much higher RI, RPT, and MPUS values than those in the non-injury group. Additionally, logistic multivariate regression analysis showed that a high RI value was an independent risk factor for patients with sepsis-induced AKI. The aforementioned findings, which agree with those of other studies, suggest that RI and RPT values can reflect the pathological process of renal function decline in patients with AKI [Citation37,Citation38]. In contrast, a study did not support the predictive value of RI in patients with persistent AKI [Citation39], as it is related to the inclusion of different research subjects. Furthermore, patients in the AKI group had significantly higher MPUS than those in the non-injury group, and logistic multivariate regression analysis also showed that low MPUS was an independent protective factor affecting the occurrence of AKI in patients with sepsis. The above findings indicate that MPUS can also serve as an important parameter for ultrasound diagnostic assessment in patients with sepsis-induced AKI. The MPUS system was constructed through the assignment of APD, RPT, and RI; this construction provides more comprehensive information for diagnostic evaluation and avoids the limitations of single-parameter diagnostic evaluation [Citation40]. More significantly, data quantification also makes diagnostic evaluation more convenient, and the accuracy of diagnostic evaluation can be ensured by setting scoring criteria according to clinical experience [Citation38]. Regarding the ROC curve analysis in this study, it was found that RI was superior to 24-h urine volume, serum cystatin C, and MPUS in detecting sepsis-induced AKI. Alternatively, the ROC curve analysis further verified the good diagnostic value of RI for sepsis complicated with AKI. Previous studies have reported significantly increased RI values in patients with stage 3 AKI compared with those without AKI [Citation41], which was similar to our findings. Additionally, this study constructed a column chart to predict stage 3 AKI, including serum creatinine, urine volume, lactate, and power Doppler ultrasound and renal venous Doppler waveform scores [Citation41]. RI was excluded from the column chart, and its role in AKI staging remains to be studied in the following study.
This study has some limitations. This study was retrospective with limited samples. We also did not focus on the prognosis of patients with AKI, and it was unclear whether these indicators are related to the renal recovery of sepsis in patients with AKI. This still requires further exploration in the following research. Additionally, our results suggest that MPUS is a protective factor for patients with sepsis-induced AKI, but its diagnostic efficacy for AKI is lower than that of RI. This result was inconsistent with our initial speculation that MPUS could be used for AKI diagnosis in patients with sepsis. This may be limited by the current research design. Therefore, further prospective clinical cohort studies with larger sample sizes are required to validate the current findings.
Conclusion
High serum cystatin C and high RI values were independent risk factors affecting sepsis complicated with AKI, whereas high 24-h urine volume and low MPUS values were independent protection factors. RI has positive diagnostic and treatment values for patients with sepsis complicated with AKI.
Authors’ contributions
Liu Wang designed the study. Xiang Wang collated the data, carried out data analyses and produced the initial draft of the manuscript. Liu Wang and Xiang Wang contributed to drafting the manuscript. All authors have read and approved the final submitted manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Datasets used in this article are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Vincent JL, Opal SM, Marshall JC, et al. Sepsis definitions: time for change. Lancet. 2013;381(9868):1–7. doi: 10.1016/S0140-6736(12)61815-7.
- Walkey AJ, Lagu T, Lindenauer PK. Trends in sepsis and infection sources in the United States: a population-based study. Ann Am Thorac Soc. 2015;12(2):216–220. doi: 10.1513/AnnalsATS.201411-498BC.
- Dellinger RP, Levy MM, Carlet JM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008;34(1):17–60. doi: 10.1007/s00134-007-0934-2.
- Cawcutt KA, Peters SG. Severe sepsis and septic shock: clinical overview and update on management. Mayo Clin Proc. 2014;89(11):1572–1578. doi: 10.1016/j.mayocp.2014.07.009.
- Bagshaw SM, Uchino S, Bellomo R, et al. Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin J Am Soc Nephrol. 2007;2(3):431–439. doi: 10.2215/CJN.03681106.
- Santana KYA, Santos APA, Magalhães FB, et al. Prevalence and factors associated with acute kidney injury in patients in intensive care units. Rev Bras Enferm. 2021;74(2):e20200790. doi: 10.1590/0034-7167-2020-0790.
- Al-Kuraishy HM, Al-Gareeb AI, Al-Nami MS. Irbesartan attenuates gentamicin-induced nephrotoxicity in rats through modulation of oxidative stress and endogenous antioxidant capacity. Int J Prev Med. 2020;11:16. doi: 10.4103/ijpvm.IJPVM_567_18.
- Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–818. doi: 10.1001/jama.294.7.813.
- Bagshaw SM, Lapinsky S, Dial S, et al. Acute kidney injury in septic shock: clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Med. 2009;35(5):871–881. doi: 10.1007/s00134-008-1367-2.
- Palevsky PM, Zhang JH, O'Connor TZ, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359(1):7–20.
- Manrique-Caballero CL, Del Rio-Pertuz G, Gomez H. Sepsis-associated acute kidney injury. Crit Care Clin. 2021;37(2):279–301. doi: 10.1016/j.ccc.2020.11.010.
- Skube SJ, Katz SA, Chipman JG, et al. Acute kidney injury and sepsis. Surg Infect (Larchmt). 2018;19(2):216–224. doi: 10.1089/sur.2017.261.
- Godin M, Murray P, Mehta RL. Clinical approach to the patient with AKI and sepsis. Semin Nephrol. 2015;35(1):12–22. doi: 10.1016/j.semnephrol.2015.01.003.
- Fan YW, Jiang SW, Chen JM, et al. A pulmonary source of infection in patients with sepsis-associated acute kidney injury leads to a worse outcome and poor recovery of kidney function. World J Emerg Med. 2020;11(1):18–26. doi: 10.5847/wjem.j.1920-8642.2020.01.003.
- Hasson D, Menon S, Gist KM. Improving acute kidney injury diagnostic precision using biomarkers. Pract Lab Med. 2022;30:e00272. doi: 10.1016/j.plabm.2022.e00272.
- Bellos I, Pergialiotis V, Kontzoglou K. Renal resistive index as predictor of acute kidney injury after major surgery: a systematic review and meta-analysis. J Crit Care. 2019;50:36–43. doi: 10.1016/j.jcrc.2018.11.001.
- Liu C, Wang X. Clinical utility of ultrasonographic evaluation in acute kidney injury. Transl Androl Urol. 2020;9(3):1345–1355. X doi: 10.21037/tau-20-831.
- Chalmers DJ, Meyers ML, Brodie KE, et al. Inter-rater reliability of the APD, SFU and UTD grading systems in fetal sonography and MRI. J Pediatr Urol. 2016;12:305 e301–305 e305.
- Kıllı İ, Avlan D, Taşkınlar H, et al. Effective predictors for surgical decision in antenatal hydronephrosis: a prospective multiparameter analysis. Turk J Urol. 2017;43(3):361–365. doi: 10.5152/tud.2017.81568.
- Xiang YC, Yang J, Yu SW, et al. Application of multi-parameter ultrasound scoring in prenatal diagnosis and prognosis assessment of fetal hydronephrosis. Pract J Clin Med. 2021;18(2):53–56.
- Liu BY, Zhao LX, Zheng SG, et al. Ultrasound multi-parameter scoring in diagnosis of chronic kidney disease. Chin J Med Imag Technol. 2021;37:5.
- Liu PQ, Ding CW, Zhang YC, et al. Diagnostic value of multi-parameter ultrasound in patient with septic acute kidney injury. Chin J Med Imag. 2023;31:653–657.
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287.
- Khwaja A. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138.
- Song S, Meyer M, Türk TR, et al. Serum cystatin C in mouse models: a reliable and precise marker for renal function and superior to serum creatinine. Nephrol Dial Transplant. 2009;24(4):1157–1161. doi: 10.1093/ndt/gfn626.
- Kellum JA, Romagnani P, Ashuntantang G, et al. Acute kidney injury. Nat Rev Dis Primers. 2021;7(1):52. doi: 10.1038/s41572-021-00284-z.
- Lo LJ, Go AS, Chertow GM, et al. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int. 2009;76(8):893–899. doi: 10.1038/ki.2009.289.
- Stevens LA, Levey AS. Measurement of kidney function. Med Clin North Am. 2005;89(3):457–473. doi: 10.1016/j.mcna.2004.11.009.
- Kellum JA, Sileanu FE, Murugan R, et al. Classifying AKI by urine output versus serum creatinine level. J Am Soc Nephrol. 2015;26(9):2231–2238. doi: 10.1681/ASN.2014070724.
- Nejat M, Pickering JW, Walker RJ, et al. Rapid detection of acute kidney injury by plasma cystatin C in the intensive care unit. Nephrol Dial Transplant. 2010;25(10):3283–3289. doi: 10.1093/ndt/gfq176.
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–184. doi: 10.1159/000339789.
- Lin HY, Lee YL, Lin KD, et al. Association of renal elasticity and renal function progression in patients with chronic kidney disease evaluated by real-time ultrasound elastography. Sci Rep. 2017;7(1):43303. doi: 10.1038/srep43303.
- Beland MD, Walle NL, Machan JT, et al. Renal cortical thickness measured at ultrasound: is it better than renal length as an indicator of renal function in chronic kidney disease? AJR Am J Roentgenol. 2010;195(2):W146–149. doi: 10.2214/AJR.09.4104.
- Faubel S, Patel NU, Lockhart ME, et al. Renal relevant radiology: use of ultrasonography in patients with AKI. Clin J Am Soc Nephrol. 2014;9(2):382–394. doi: 10.2215/CJN.04840513.
- Gazhonova VE, Zykova AS, Chistyakov AA, et al. Prognostic value of renal resistance index in estimating the progression of chronic kidney disease. Ter Arkh. 2015;87(6):29–33. doi: 10.17116/terarkh201587629-33.
- Haitsma Mulier JLG, Rozemeijer S, Röttgering JG, et al. Renal resistive index as an early predictor and discriminator of acute kidney injury in critically ill patients; a prospective observational cohort study. PloS One. 2018;13(6):e0197967. doi: 10.1371/journal.pone.0197967.
- Gasser B, Uscategui RAR, Maronezi MC, et al. Clinical and ultrasound variables for early diagnosis of septic acute kidney injury in bitches with pyometra. Sci Rep. 2020;10(1):8994. doi: 10.1038/s41598-020-65902-4.
- Kalantarinia K. Novel imaging techniques in acute kidney injury. Curr Drug Targets. 2009;10(12):1184–1189. doi: 10.2174/138945009789753246.
- Fu Y, He C, Jia L, et al. Performance of the renal resistive index and usual clinical indicators in predicting persistent AKI. Ren Fail. 2022;44(1):2028–2038. doi: 10.1080/0886022X.2022.2147437.
- Spiesecke P, Münch F, Fischer T, et al. Multiparametric ultrasound findings in acute kidney failure due to rare renal cortical necrosis. Sci Rep. 2021;11(1):2060. doi: 10.1038/s41598-021-81690-x.
- Zhi HJ, Cui J, Yuan MW, et al. Predictive performance of renal resistive index, semiquantitative power doppler ultrasound score and renal venous doppler waveform pattern for acute kidney injury in critically ill patients and prediction model establishment: a prospective observational study. Ren Fail. 2023;45:2258987.