Abstract
Background
Acute kidney injury (AKI) is increasingly prevalent in children with nephrotic syndrome (NS). It is associated with adverse outcomes in NS, especially steroid-resistant nephrotic syndrome (SRNS). The incidence, risk factors and outcomes of AKI in secondary SRNS remain undefined. The main objectives of this study were to determine the risk factors and prognosis of AKI in hospitalized children with secondary SRNS.
Material and methods
This retrospective study was conducted from January 2014 to December 2019, involving 172 hospitalizations with secondary SRNS admitted to the First Affiliated Hospital of Sun Yat-sen University. AKI was defined and classified in accordance with the 2012 Kidney Disease Improving Global Outcomes (KDIGO) guidelines.
Results
AKI was found in 67 (39.0%) of 172 hospitalizations with secondary SRNS. Average age of onset in our group is 4.4 (3.1, 6.7) years with AKI and 3.7 (1.8, 5.6) years without AKI. Urea nitrogen level is 5.9 (4.1, 10.0) mmol/L with AKI and 5.1 (3.7, 7.0) mmol/L. Uric acid level is 446.0 (340.0, 567.0) umol/L with AKI and 401.0 (303.0, 496.0) umol/L. 24-h urinary protein level is 4.14 (2.9, 6.5) g with AKI and 2.5 (1.3, 5.3) without AKI. Multivariate logistic regression revealed that infection (OR = 5.287; 95% confidence interval, 2.349 to 11.899; p < 0.001), age at onset (OR = 1.180; 95% confidence interval, 1.032 to 1.349; p = 0.015) and uric acid level (OR = 1.003; 95% confidence interval, 1.000 to 1.006; p = 0.031) were significantly associated with the development of AKI in children with secondary SRNS. Among 72 children with secondary SRNS, six went to end-stage kidney disease (ESKD). Children in the AKI group were more likely to progress to ESKD compared with children in the non-AKI group (p = 0.017) with a median follow‐up of 48.5months.
Conclusion
AKI occurred in 39.0% of total hospitalizations associated with secondary SRNS. Risk factors including infection, age of onset, and uric acid level are associated with AKI in children with secondary SRNS. Furthermore, AKI was identified as a risk factor for the progression of secondary SRNS to ESKD.
Introduction
Nephrotic syndrome (NS) is a common kidney disease in childhood, affecting 1.15 to 16.9 per 100,000 children [Citation1,Citation2]. It is characterized by massive proteinuria, hypoalbuminemia or edema [Citation3]. Depending on the initial response to steroids, NS can be classified into steroid-sensitive nephrotic syndrome (SSNS) and steroid-resistant nephrotic syndrome (SRNS). However, steroid responsiveness can change over time, and some patients initially responsive to steroids may develop steroid resistance in subsequent relapses, called secondary SRNS or secondary steroid resistance [Citation3,Citation4]. According to previous studies, secondary SRNS accounts for 13.8% to 35.9% of SRNS [Citation5–7]. Patients with secondary SRNS are commonly treated with immunosuppressant medications, such as cyclosporine or tacrolimus [Citation3]. Patients with NS are vulnerable to severe complications, such as infection, venous thromboembolism (VTE), and acute kidney injury (AKI). An American study revealed that the frequency of AKI among hospitalized children with NS increased by 158% between 2000 and 2009, while the rates of infection and VTE remained stable overall [Citation8]. AKI in children with NS is reversible in most cases, but it can prolongs hospital lengths of stay or progress to chronic kidney disease (CKD) [Citation9,Citation10]. Compared to SSNS, AKI is more commonly observed in steroid-dependent NS (SDNS) and SRNS [Citation11]. However, there is a paucity of research on risk factors and outcomes of AKI in children with SRNS, not to mention secondary SRNS. As a result, our study aimed to explore the prevalence, clinical features, risk factors, and prognosis of AKI in hospitalized children with secondary SRNS.
Materials and methods
Study population
This single-center retrospective study was conducted in the Department of Pediatric Nephrology and Rheumatology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China. All hospitalizations for children with secondary SRNS ≤14 years of age at our center between January 1, 2014 and December 31, 2019 were included. Hospitalizations were excluded if the child was: 1) congenital or secondary NS; 2) chronic kidney disease stage III or higher at admission [Citation9]; 3) admitted only for kidney biopsy, evaluation, prescheduled NS therapy (intravenous methylprednisolone or cyclophosphamide or rituximab) or causes unrelated to NS; 4) underwent less than two serum creatinine (SCr) tests during hospitalization.
Definitions
NS is defined as nephrotic-range proteinuria (first morning or 24-h protein creatinine ratio (PCR) ≥2 g/g or ≥3+ dipstick) and either hypoalbuminemia (serum albumin <30 g/L) or edema. SDNS is defined as two consecutive relapses during therapy with prednisone or prednisolone (either at full dose or during tapering) or within 15 days of prednisone or prednisolone discontinuation. Secondary SRNS is defined as a SSNS patient at disease onset who at a subsequent relapse fails to achieve remission after 4 weeks of therapy with daily prednisone or prednisolone at standard dose. Complete remission (CR) was defined as a first morning or 24-h PCR ≤ 200 mg/g (or > 20 mg/mmol or negative or trace dipstick) on three or more consecutive occasions. SSNS was defined as CR after 4 weeks of prednisone or prednisolone at the standard dose (60 mg/m2/d or 2 mg/kg/d with a maximum of 60 mg/d), while those who failed to achieve CR were defined as SRNS. Relapse was defined as recurrence of nephrotic-range proteinuria. Frequent relapsing NS was defined as ≥2 relapses per 6 months within 6 months of NS onset or ≥4 relapses per year in any subsequent 12-month period, while infrequent relapsing NS was defined as <2 relapses per 6 months or <4 relapses per year [Citation3].
AKI was defined and classified in accordance with the 2012 Kidney Disease Improving Global Outcomes (KDIGO) guidelines [Citation12]. We only used serum creatine (SCr) for defining AKI. Baseline SCr level was defined as the latest creatinine value before admission obtained within the prior 6 months. If no prior creatinine value was available, the lowest creatinine value obtained during the hospitalization was defined as the baseline value [Citation9,Citation13]. If the highest serum creatinine level during an episode was below 0.5 mg/dL, the episode is not considered AKI [Citation13,Citation14]. The recovery of AKI is defined as the recovery of the child’s creatinine level to 1.5 times the baseline value [Citation15,Citation16]. The estimated glomerular filtration rate was calculated using the bedside Schwartz formula [Citation17]. Chronic kidney disease was defined as abnormalities of kidney structure or function, present for >3 months, with health implications, and classified based on GFR category (G1-G5) [Citation3]. Laboratory data was collected from the time points before the AKI occurrence within 1 month or at the time of admission.
The main cause of AKI is discussed and decided by our medical team based on medical history. Nephrotoxic medication exposure is defined as administration within one week prior to the onset of AKI. Even a single dose is enough to be considered as nephrotoxic exposure. The definition of Nephrotoxic medications refers to Rheault’s research [Citation9], including calcineurin inhibitors (CNI), renin-angiotensin system (RAS) blockers, nephrotoxic antibiotics, antiviral (acyclovir) and NSAIDs, etc. Nephrotoxic antibiotics include vancomycin, piperacillin/tazobactam, ceftazidime/cefotaxime/cefuroxime and Gentamicin/tobramycin/amikacin, etc.
Clinical data collection and follow-up
We reviewed clinical records to gather data, which included basic information, laboratory characteristics, biopsy findings, complications (AKI or infection), and medication regimen. The study endpoint was May 31, 2020, end-stage kidney disease (ESKD) or death, which was determined via clinical records and telephone tracing.
Statistical analyses
The data were analyzed using SPSS version 25.0 (IBM Corp., Armonk, NY, USA). Continuous data were expressed as median (interquartile range (IQR), Q1-Q3) and analyzed using the Mann–Whitney U-test. Categorical data were expressed as frequency (percentage), and groups were compared using the chi-square test or Fisher exact test when appropriate. Missing data were limited to laboratory values. Where the data were missing, the values are filled using multiple imputation. Multivariate logistic regression was applied to identify the risk factors for AKI in childhood secondary SRNS. Kaplan–Meier analysis and Log-rank test were used to analyze time to ESKD. The multivariable model incorporated variables significant to P value < 0.05 in the univariate analyses and those considered to be related according to clinical experiences. Two-tailed P value < 0.05 was considered statistically significant.
Results
Cohort demographic and clinical characteristics
This study involved 172 hospitalizations that occurred among 72 children with secondary SRNS, admitted to the Department of Pediatric Nephrology and Rheumatology, The First Affiliated Hospital, Sun Yat-sen University, between January 1, 2014 and December 31, 2019. The median age at admission was 7.42 years (IQR, 5.44-10.33 years) with 121 (70.3%) boys and 51 (29.7%) girls. Among the cases, 33 (45.8%) were hospitalized once, 17 (23.6%) were hospitalized twice, 9 (12.5%) were hospitalized three times, and 13 (18.0%) were hospitalized four or more times.
AKI incidence and risk factors
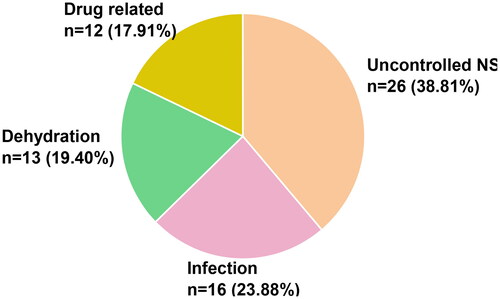
According to KDIGO guidelines, AKI occurred in 67 (39.0%) of hospitalizations among 42(58.3%) children with secondary SRNS, with 25 (37.3%) cases were in stage 1, 16 (23.8%) cases were in stage 2, and 26 (38.8%) cases were in stage 3. The pathologic diagnosis revealed focal segmental glomerulosclerosis (FSGS) in 7 (31.2%) cases in stage 1, 5 (31.2%) cases in stage 2, and 12 (46.2%) cases in stage 3. The causes of AKI were NS deterioration in 26 (38.8%) cases, infections in 16 (23.9%) cases, dehydration in 13 (19.4%) cases, and drug-related causes in 12 (17.9%) cases ().
Patients in the AKI group were older at onset compared to those in the non-AKI group (p = 0.036). However, there were no significant differences in gender, admission age, time to secondary SRNS or frequency of relapses before secondary SRNS between AKI and non-AKI groups (). Children with secondary SRNS accompanied by infection were more likely to develop AKI (p < 0.001). A total of 54 (80.6%) hospitalizations in the AKI group were infected, including upper respiratory tract infection (n = 16), acute bronchitis (n = 12), pneumonia (n = 12), sepsis (n = 5), cellulitis (n = 4), sinusitis (n = 3), peritonitis (n = 1), and gastrointestinal infection (n = 1).
Table 1. Patient characteristics of hospitalized children with secondary SRNS.
The AKI group exhibits different biopsy diagnosis compared with the non-AKI group in children with secondary SRNS (p = 0.009). In the AKI group, 19(28.4%) children have minimal change disease (MCD) and 24 (35.8%) children have FSGS, while in the non-AKI group, 57 (54.3%) children have MCD and 21 (20.0%) children have FSGS. The use of nephrotoxic drugs (p = 0.055) and calcineurin inhibitor (CNI) drugs (p = 0.199) did not significantly differ between AKI and non-AKI groups.
For laboratory tests, the AKI group had significantly higher baseline creatinine level (p = 0.041), urea nitrogen level (p = 0.018), uric acid level (p = 0.016), and 24-h urine protein level (p < 0.001) compared with the non-AKI group. However, serum albumin was no statistically significant difference between the two groups ().
Univariate logistic regression revealed that infection (OR = 3.635; 95% confidence interval, 1.775 to 7.442; p < 0.001), age at onset (OR = 1.136; 95% confidence interval, 1.015 to 1.273; p = 0.027), uric acid level (OR = 1.003; 95% confidence interval, 1.001 to 1.005; p = 0.006), 24-h urinary protein (OR = 1.144; 95% confidence interval, 1.028 to 1.273; p = 0.014), urea nitrogen (OR = 1.088; 95% confidence interval, 1.013 to 1.168; p = 0.021) were significant risk factors of AKI in hospitalized children with secondary SRNS. Multivariate logistic regression revealed that infection (OR = 5.287; 95% confidence interval, 2.349 to 11.899; p < 0.001), age at onset (OR = 1.180; 95% confidence interval, 1.032 to 1.349; p = 0.015) and uric acid level (OR = 1.003; 95% confidence interval, 1.000 to 1.006; p = 0.031) were significantly associated with the development of AKI in children with secondary SRNS ().
Table 2. Factors associated with AKI in hospitalized children with secondary SRNS.
Outcomes of AKI
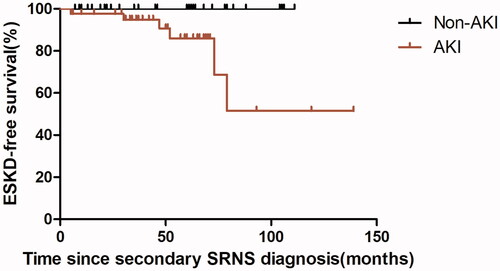
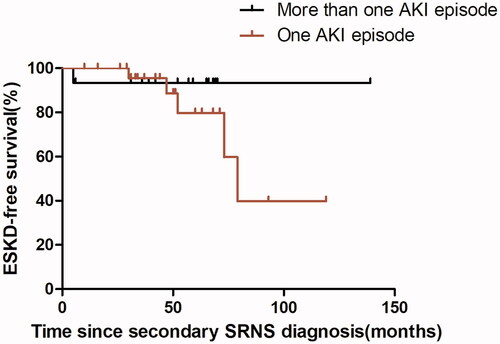
Among 67 hospitalizations in the AKI group, 62 had a median recovery time of 13 days (IQR,5.25-25 days), 1 died, 2 developed ESKD during hospitalization and 2 have incomplete recovery data. The two hospitalizations who developed ESKD both with stage 3 AKI had a histological diagnosis of FSGS. In 26 hospitalizations with stage 3 AKI, 5 (19.2%) developed ESKD at the end of follow-up. Additionally, 2 (12.5%) of 16 hospitalizations with stage 2 AKI, and 2 (8%) of 25 hospitalizations with stage 1 AKI progressed to ESKD at the end of follow-up. Additionally, the duration of hospitalization was significantly longer in the AKI group (p < 0.001). Among 72 children with secondary SRNS, six went to end-stage kidney disease (ESKD), Kaplan-Meier analysis reveals that children with secondary SRNS in our study who have had a previous episode of AKI was significantly more likely to proceed to ESKD (p = 0.017) with a median follow‐up of 48.5 months (). 15 children with secondary SRNS in our group experienced more than one AKI episode, one developed ESKD; 27 patients experienced one AKI episode, 5 developed ESKD. Kaplan-Meier analysis reveals no difference in the occurrence of ESRD between the two groups (p = 0.360) ().
Discussion
This study included a total of 72 children with secondary SRNS and 172 hospitalizations. According to KDIGO guidelines, AKI accounted for 67 (39.0%) hospitalizations. We exclude children with CKD III or higher in this study. Children with CKD III or higher have more complications such as anemia, hypertension, and heart failure. Furthermore, treatments for children with CKD III or higher are also significantly different from those for children with CKD II or lower. Several recent studies have focused on the incidence of AKI in children with NS [Citation9,Citation11,Citation13,Citation16,Citation17]. A multicenter study in Korea showed that according to the definition of KDIGO guidelines, the incidence of AKI in hospitalized children with NS was 16.2%, and the incidence of AKI in SSNS was 13.4% [Citation13]. A study on hospitalized children with NS in China showed that the incidence of AKI was 34.2%, and that in cases of SSNS was 27.0% [Citation18]. In our study, the incidence of AKI in hospitalized children with secondary SRNS was 39.0% which is higher than the aforementioned studies. According to a Japanese study of AKI in children with SRNS, the incidence of severe AKI, defined as KDIGO stage 2 or 3 AKI, was 25.8% [Citation19]. Our data are 23.9%, which is lower than the data above.
Several factors relate to the occurrence of AKI in children, including infections, hypoproteinemia, nephrotoxic drugs, 24-h urinary protein, et al. [Citation9,Citation11,Citation20]. In our cohort, multivariate logistic regression analysis revealed that infections were associated with AKI. Viruses and bacteria may cause direct injuries on the tubular epithelial cells, and systemic inflammation caused by infections is also associated with the development of AKI [Citation21,Citation22]. Therefore, it is crucial to avoid infection in children with secondary SRNS. Proteinuria is also recognized as an important risk factor for the development of AKI in the past researches [Citation23,Citation24]. In our study, univariate logistic regression revealed proteinuria is a significant risk factor of AKI in hospitalized children with secondary SRNS. But multivariate logistic regression reveals that it is not a risk factor of AKI in hospitalized children with secondary SRNS. Large sample randomized controlled studies are needed to determine the role of proteinuria impact of urinary protein on AKI occurrence in children with secondary SRNS. Renal pathology has been considered to be closely related to the occurrence of AKI in many previous studies [Citation13,Citation25–27]. In our study, the AKI group displayed distinct renal pathologies compared to the non-AKI group in secondary SRNS children (p = 0.009). In the AKI group, 19(28.4%) children have minimal change disease (MCD) and 24 (35.8%) children have FSGS, while in the non-AKI group, 57 (54.3%) children have MCD and 21 (20.0%) children have FSGS. Further studies are required to explore the relationship between renal pathology and AKI, along with the underlying mechanisms. Previous studies have shown mixed results regarding the association between CNI use and the occurrence of AKI in pediatric nephrotic syndrome [Citation13,Citation20]. There was no statistically significant difference in nephrotoxic drugs or CNI between the AKI group and the non-AKI group in this study in secondary SRNS children. Furthermore, there was no difference in lower albumin level between AKI and non-AKI groups. Yang et al. [Citation13] discovered that lower albumin level was a risk factor for AKI in children hospitalized with nephrotic syndrome. However, Rheault et al. [Citation9] and Kim et al. [Citation20] showed that serum albumin level at admission was not associated with AKI in children with NS. Therefore, whether hypoproteinemia or CNI use is a risk factor for AKI in children with secondary SRNS still needs to be studied in larger samples. In addition, both univariate and multivariate regression analysis demonstrated that high serum uric acid level was a risk factor for AKI in our group, in agreement with other reported NS studies [Citation20].
Hyperuricemia is known to induce renal injury through crystal formation or reactive oxygen radical production, and inflammatory mediator-related mechanisms [Citation28,Citation29]. Particular attention should be paid to children with secondary SRNS who also have high uric acid level. Previous studies in children and adult patients have suggested that age could be a risk factor for AKI in NS patients [Citation26, Citation30]. Further, our study indicates that older age of onset is a risk factor for AKI in children with secondary SRNS. Previous studies have found a correlation between older age at admission and AKI in children with NS [Citation13, Citation20]. In this study population, we discovered that the median age at admission was higher among children with AKI than those without AKI, although there was no statistical difference. In addition, we analyzed the time to secondary SRNS and the frequency of relapses before secondary SRNS, neither of which were related to the occurrence of AKI.
Many studies have shown that AKI is a risk factor for CKD progression and future mortality [Citation10,Citation31–34]. However, the relationship between the occurrence of AKI and CKD progression in secondary SRNS children remains unclear. According to the Kaplan-Meier analysis, our study observed a significantly lower cumulative renal survival rate in the children with secondary SRNS have had a previous episode of AKI (p = 0.017). This suggests that the occurrence of AKI increases the risk of ESKD progression in children with secondary SRNS.
There are several limitations in this study. First, it was a single-center study. Second, owing to the retrospective nature of data collection, some information bias and missing data might be unavoidable. A prospective multicenter study with a large sample is needed to validate our findings.
In conclusion, the study revealed that the incidence of AKI in children with secondary SRNS at our center was 39.0%. Secondary SRNS children with AKI were more prone to developing ESKD, and older age of onset, infections, and higher uric acid level were independent risk factors for the development of AKI in secondary SRNS children.
Acknowledgments
The authors thank all participants for their support.
Ethics approval and consent participants
The study was approved by the ethics committee of the First Affiliated Hospital of Sun Yat-sen University (No. [2022]487) and the exemption from informed consent was granted.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Noone DG, Iijima K, Parekh R. Idiopathic nephrotic syndrome in children. Lancet. 2018;392(10141):1–8. doi:10.1016/S0140-6736(18)30536-1.
- Veltkamp F, Rensma LR, Bouts AHM, LEARNS consortium. Consortium L. Incidence and relapse of idiopathic nephrotic syndrome: meta-analysis. Pediatrics. 2021;148(1):e2020029249. doi:10.1542/peds.2020-029249.
- Rovin BH, Adler SG, Barratt J, et al. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4):S1–S276. doi:10.1016/j.kint.2021.05.021.
- Trautmann A, Boyer O, Hodson E, et al. IPNA clinical practice recommendations for the diagnosis and management of children with steroid-sensitive nephrotic syndrome. Pediatr Nephrol. 2023;38(3):877–919. doi:10.1007/s00467-022-05739-3.
- Bierzynska A, McCarthy HJ, Soderquest K, et al. Genomic and clinical profiling of a national nephrotic syndrome cohort advocates a precision medicine approach to disease management. Kidney Int. 2017;91(4):937–947. doi:10.1016/j.kint.2016.10.013.
- Kim JS, Bellew CA, Silverstein DM, et al. High incidence of initial and late steroid resistance in childhood nephrotic syndrome. Kidney Int. 2005;68(3):1275–1281. doi:10.1111/j.1523-1755.2005.00524.x.
- Sato M, Ishikura K, Ando T, et al. Prognosis and acute complications at the first onset of idiopathic nephrotic syndrome in children: a nationwide survey in Japan (JP-SHINE study). Nephrol Dial Transplant. 2021;36(3):475–481. doi:10.1093/ndt/gfz185.
- Rheault MN, Wei CC, Hains DS, et al. Increasing frequency of acute kidney injury amongst children hospitalized with nephrotic syndrome. Pediatr Nephrol. 2014;29(1):139–147. doi:10.1007/s00467-013-2607-4.
- Rheault MN, Zhang L, Selewski DT, et al. AKI in children hospitalized with nephrotic syndrome. Clin J Am Soc Nephrol. 2015;10(12):2110–2118. doi:10.2215/CJN.06620615.
- Yaseen A, Tresa V, Lanewala AA, et al. Acute kidney injury in idiopathic nephrotic syndrome of childhood is a major risk factor for the development of chronic kidney disease. Ren Fail. 2017;39(1):323–327. doi:10.1080/0886022X.2016.1277743.
- Sharma M, Mahanta A, Barman AK, et al. Acute kidney injury in children with nephrotic syndrome: a single-center study. Clin Kidney J. 2018;11(5):655–658. doi:10.1093/ckj/sfy024.
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. Section 2: AKI definition. Kidney Int Suppl. 2012;2(1):19–36. doi:10.1038/kisup.2011.32.
- Yang EM, Yoo KH, Ahn YH, et al. Lower albumin level and longer disease duration are risk factors of acute kidney injury in hospitalized children with nephrotic syndrome. Pediatr Nephrol. 2021;36(3):701–709. doi:10.1007/s00467-020-04740-y.
- Downes KJ, Cowden C, Laskin BL, et al. Association of acute kidney injury with concomitant vancomycin and piperacillin/tazobactam treatment among hospitalized children. JAMA Pediatr. 2017;171(12):e173219. doi:10.1001/jamapediatrics.2017.3219.
- Long TE, Sigurdsson MI, Sigurdsson GH, et al. Improved long‐term survival and renal recovery after acute kidney injury in hospitalized patients: a 20 year experience. Nephrology (Carlton). 2016;21(12):1027–1033. doi:10.1111/nep.12698.
- Hollander SA, Montez-Rath ME, Axelrod DM, et al. Recovery from acute kidney injury and CKD following heart transplantation in children, adolescents, and young adults: a retrospective cohort study. Am J Kidney Dis. 2016;68(2):212–218. doi:10.1053/j.ajkd.2016.01.024.
- Schwartz GJ, Muñoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–637. doi:10.1681/ASN.2008030287.
- Gao J, Chen CY, Tu J. Analysis of related factors of primary nephrotic syndrome complicated with acute kidney injury in children. Chinese Journal of Medicine. 2020;55(2):217–220. (In Chinese).
- Ishiwa S, Sato M, Kamei K, et al. Risks and renal outcomes of severe acute kidney injury in children with steroid-resistant nephrotic syndrome. Clin Exp Nephrol. 2022;26(7):700–708. doi:10.1007/s10157-022-02198-w.
- Kim MY, Cho MH, Kim JH, et al. Acute kidney injury in childhood-onset nephrotic syndrome: incidence and risk factors in hospitalized patients. Kidney Res Clin Pract. 2018;37(4):347–355. doi:10.23876/j.krcp.18.0098.
- Poston JT, Koyner JL. Sepsis associated acute kidney injury. BMJ. 2019;364:k4891. doi:10.1136/bmj.k4891.
- Park J, Kim SU, Choi HJ, et al. Predictive role of the D-Dimer level in acute kidney injury in living donor liver transplantation: a retrospective observational cohort study. J Clin Med. 2022;11(2):450. doi:10.3390/jcm11020450.
- Huang TM, Wu VC, Young GH, National Taiwan University Hospital Study Group of Acute Renal Failure., et al. Preoperative proteinuria predicts adverse renal outcomes after coronary artery bypass grafting. J Am Soc Nephrol. 2011;22(1):156–163. doi:10.1681/ASN.2010050553.
- Wahl TS, Graham LA, Morris MS, et al. Association between preoperative proteinuria and postoperative acute kidney injury and readmission. JAMA Surg. 2018;153(9):e182009. doi:10.1001/jamasurg.2018.2009.
- Meyrier A, Niaudet P. Acute kidney injury complicating nephrotic syndrome of minimal change disease. Kidney Int. 2018;94(5):861–869. doi:10.1016/j.kint.2018.04.024.
- Lin SP, Zhu FG, Meng JL, et al. Clinical features of acute kidney injury in patients with nephrotic syndrome and minimal change disease: a retrospective, cross-sectional study. Chin Med J (Engl). 2021;134(2):206–211. doi:10.1097/CM9.0000000000001218.
- Li ZZ, Weng MJ, Lin LZ, et al. Acute kidney injury in patients with idiopathic membranous nephropathy: influencing factors and prognosis. Ren Fail. 2023;45(1):2194451.
- Xu X, Hu J, Song N, et al. Hyperuricemia increases the risk of acute kidney injury: a systematic review and meta-analysis. BMC Nephrol. 2017;18(1):27. doi:10.1186/s12882-016-0433-1.
- Hahn K, Kanbay M, Lanaspa MA, et al. Serum uric acid and acute kidney injury: a mini review. J Adv Res. 2017;8(5):529–536. doi:10.1016/j.jare.2016.09.006.
- Guan N, Yao Y, Xiao H, et al. Factors predicting the recovery from acute kidney injury in children with primary nephrotic syndrome. Clin Exp Nephrol. 2021;25(9):1011–1017. doi:10.1007/s10157-021-02074-z.
- Sigurjonsdottir VK, Chaturvedi S, Mammen C, et al. Pediatric acute kidney injury and the subsequent risk for chronic kidney disease: is there cause for alarm? Pediatr. Pediatr Nephrol. 2018;33(11):2047–2055. doi:10.1007/s00467-017-3870-6.
- Selby NM, Taal MW. Long-term outcomes after AKI—a major unmet clinical need. Kidney Int. 2019;95(1):21–23. doi:10.1016/j.kint.2018.09.005.
- Goldstein SL, Devarajan P. Acute kidney injury in childhood: should we be worried about progression to CKD? Pediatr Nephrol. 2011;26(4):509–522. doi:10.1007/s00467-010-1653-4.
- Siew ED, Parr SK, Abdel-Kader K, et al. Predictors of recurrent AKI. J Am Soc Nephrol. 2016;27(4):1190–1200. doi:10.1681/ASN.2014121218.