Abstract
Background
This study aims to undertake a comprehensive assessment of the effectiveness and safety profile of Mahuang Fuzi and Shenzhuo Decoction (MFSD) in the management of primary membranous nephropathy (PMN), within the context of a prospective clinical investigation.
Methods
A multicenter, open-label clinical trial was executed on patients diagnosed with PMN. These individuals were subjected to MFSD therapy for a duration of at least 24 months, with primary outcome of clinical remission rates. The Cox regression analysis was employed to discern the pertinent risk factors exerting influence on the efficacy of MFSD treatment, with scrupulous monitoring of any adverse events.
Results
The study comprised 198 participants in total. Following 24 months of treatment, the remission rate was 58.6% (116/198). Among the subgroup of 130 participants subjected to a 36-month follow-up, the remission rate reached 70% (91/130). Subgroup analysis revealed that neither a history of immunosuppressive therapy (HIST) nor an age threshold of ≥60 years exhibited a statistically significant impact on the remission rate at the 24-month mark (p > .05). Multivariate Cox regression analyses elucidated HIST, nephrotic syndrome, or mass proteinuria, and a high-risk classification as noteworthy risk factors in the context of MFSD treatment. Remarkably, no fatalities resulting from side effects were documented throughout the study’s duration.
Conclusions
This trial establishes the efficacy of MFSD as a treatment modality for membranous nephropathy. MFSD demonstrates a favorable side effect profile, and remission rates are consistent across patients, irrespective of HIST and age categories.
Introduction
Membranous nephropathy (MN) is a major cause of nephrotic syndrome in adults, with a higher prevalence in China [Citation1]. Among primary glomerular diseases, MN ranks second in prevalence, and accounts for 35% of biopsies in elderly patients with nephrotic syndrome [Citation2]. MN is an autoantibody-mediated glomerular disease, characterized by subepithelial immune deposition of glomerular capillaries, leading to diffuse thickening of the basement membrane [Citation3]. MN can be classified into primary membranous nephropathy (PMN) of undetermined etiology, and secondary membranous nephropathy (SMN) caused by other systemic diseases such as HBV infection and SLE. Since the identification of the M-type antiphospholipid A2 receptor (PLA2R) by Beck et al., several autoantigens have been identified, deepening the understanding of PMN [Citation4]. Currently, PLA2R and thrombospondin type-1 domain containing 7A (THSD7A) are the dominant autoantigens, accounting for 70–80% and 1–3% of cases, respectively [Citation5,Citation6]. Approximately, one-third of PMN patients enter spontaneous remission, while another third progress to end-stage kidney disease (ESKD) [Citation7].
In accordance with the 2021 clinical practice guidelines of kidney disease: improving global outcomes (KDIGO), PMN patients are currently treated with optimal supportive therapy and/or immunosuppressive therapy based on their risk level. The risk of progressive loss of renal function in PMN patients needs to be assessed based on clinical and laboratory tests, and classified into four stages: low, moderate, high, and very high risk. Patients with low risk and partially moderate risk may be first given watchful waiting and supportive therapy, and if the disease progresses, immunosuppressive therapy may be considered. Immunosuppressive therapy is recommended for high-risk and very high-risk patients [Citation8]. The immunosuppressive regimens currently recommended include rituximab (RTX), calcineurin inhibitors (CNIs), such as cyclosporine or tacrolimus, and the modified Ponticelli regimen (methylprednisolone + cyclophosphamide (CTX)) [Citation8]. The efficacy of immunosuppressive therapy for PMN has been investigated in several randomized controlled trials (RCTs) from different countries [Citation9–13]. Recently, three RCTs, including the MENTOR, STARMEN, and RI-CYCLO trials, observed and compared the remission rates and side effects of different immunosuppressive regimens for PMN. The remission rates were 60–85% for the RTX regimen, 20–58% for the CNI regimen, and 81–84% for the modified Italian regimen [Citation14–16]. In all three trials, some patients did not remit or relapsed (12–27%), and the incidence of adverse events was high in all groups (21–97%). These adverse events include increased blood creatinine, hyperkalemia, diarrhea, leukopenia, pruritus, and infusion reactions. Tumors were also found in three patients in the RI-CYCLO trial [Citation16].
Previous research has provided evidence of the effectiveness of traditional Chinese medicine (TCM) in preventing and treating chronic kidney disease (CKD), including MN [Citation17]. In a multicenter randomized controlled clinical trial, the efficacy and safety of Shenqi particle in the treatment of idiopathic MN were evaluated. The study demonstrated that the efficacy of Shenqi particle was comparable to that of glucocorticoids (GCs) + CTX, with significantly lower side effects than those of immunosuppressive therapy [Citation18]. Another study examined the use of Jian Pi Qu Shi Formula in 15 MN patients who failed to respond to immunosuppressive therapy, and found that 80% of the patients achieved clinical remission with no occurrence of serious adverse events during the observation period [Citation19]. However, these trials had a limited sample size, and the observation period was not long enough to draw definitive conclusions.
Mahuang Fuzi and Shenzhuo Decoction (MFSD) is a formulation derived from the classical TCM text, ‘Synopsis of the Golden Chamber,’ by Dr. Zhong-jing Zhang. The herbal formula comprises six ingredients, namely, Ma Huang (Ephedra sinica Stapf.), Hei Fu Zi (Aconitum carmichaeli Debx.), Zhi Gan Cao (Glycyrrhiza uralensis Fisch.), Gan Jiang (Zingiber officinale Rosc.), Fu Ling (Poria cocos (Schw.) Wolf), and Chao Bai Zhu (Atractylodes macrocephala Koidz.) [Citation20]. Based on the clinical and laboratory characteristics of patients with MN, we have proposed MFSD therapy in accordance with TCM theory, and have successfully treated over 1000 MN patients in the past decade, with remarkable efficacy. In a previous multicenter retrospective study conducted by our team, the overall remission rate of MN patients treated with MFSD was found to be 61.4% after 36 months, including a complete remission (CR) rate of 16.7% [Citation20].
In this study, we conducted a prospective, multicenter, open-label clinical trial to evaluate the efficacy of MFSD for MN. Patients who participated in this trial were withdrawn from immunosuppressive therapy, but remained on supportive therapy such as angiotensin-converting enzyme inhibitors (ACEi), or angiotensin receptor blockers (ARBs). This study was conducted over a period of 24 months, with some patients followed up to 36 months. Our results show that the remission rate of MN with MFSD is comparable to that in patients with or without a history of immunosuppressive therapy (HIST), and is similar in both elder and younger patients, and has a lower incidence of adverse events. These results suggest that MFSD treatment regimens could be used as an alternative or adjunct to current treatment regimens for MN.
Methods
Study design and participants
This study employed a prospective, multicenter clinical trial design to evaluate the efficacy and safety of MFSD therapy for MN. The study was approved by the local ethics committee of the participating centers (2018BL-090-02) and was conducted in accordance with the guidelines of the International Coordinating Meeting on Good Clinical Practice (GCP) and applicable local laws and regulations. Prior to participation, all patients or their legal guardians were informed about the nature, purpose, and potential risks of the trial, and signed informed consent forms.
Participants were recruited from four centers, namely Beijing Hospital of Traditional Chinese Medicine (170 subjects), Shunyi Branch of Beijing Hospital of Traditional Chinese Medicine (18 subjects), Tangshan Fengrun District Hospital of Traditional Chinese Medicine (three subjects), and Zhangjiakou Hospital of Traditional Chinese Medicine (seven subjects). The trial was registered with www.Chictr.org.cn (ChiCTR1900021485).
Inclusion criteria
The inclusion criteria for this study were as follows: ① confirmed diagnosis of MN through renal biopsy, without secondary causes such as hepatitis B infection or systemic lupus erythematosus; or patients with clinical presentation of nephrotic syndrome (24-h urine protein > 3.5 g, albumin (ALB) <30 g/L and/or edema, hyperlipidemia) without renal biopsy and serum anti-phospholipase A2 receptor antibody (aPLA2R) >20 RU/mL; ② age between 18 and 80 years; ③ CKD stage 1–3b (estimated glomerular filtration rate, eGFR ≥ 30 mL/min/1.73 m2); and ④ provision of written informed consent.
Exclusion criteria
Exclusion criteria for this study were as follows: ① presence of other primary or secondary glomerular diseases, such as IgA nephropathy, diabetic nephropathy, etc.; ② simultaneous presence of cerebrovascular diseases (such as cerebral hemorrhage, cerebral infarction), cardiovascular diseases (such as coronary heart disease, arrhythmia), severe organ dysfunction (such as pulmonary heart disease, cardiac insufficiency, liver insufficiency, etc.), severe gastrointestinal diseases, psychiatric diseases, or malignant tumors; ③ persistent bacterial or viral infections, such as hepatitis B virus infection and persistent abnormal liver function, HIV infection; ④ hypersensitivity to any of the components of the drug being used; ⑤ pregnancy or lactation; ⑥ participation in other clinical trials. Any participant with any of the above conditions was excluded from this study.
Intervention protocol
For this study, we have defined the regular use of immunosuppressants for more than 6 months as HIST. Thus, all participants were treated with optimal supportive therapy and stopped taking immunosuppressants for more than three months. Those taking GCs gradually reduced the dose until discontinuing use. One week after completing the enrollment assessment, all enrolled participants commenced the MFSD regimen.
Optimal supportive therapy consisted of: (1) adequate dietary protein (0.8–1.0 g/kg per day), adequate caloric intake, and a low-salt diet (<3 g/day) if edema was present; (2) administration of ACEi/ARB to control blood pressure at approximately 125–130/75–80 mmHg; (3) use of statins (HMG CoA reductase inhibitors) to control hyperlipidemia in patients; (4) anticoagulation with low-molecular-weight heparin or warfarin if serum ALB is less than 20–25 g/L or if there are other risk factors for thrombosis; and (5) anti-infective therapy if infection is present, following the 2012 KDIGO guidelines [Citation21].
Interventional treatment involved the use of MFSD for optimal supportive therapy. The basic recipe of MFSD consisted of Ma Huang (Ephedra sinica Stapf.) (boiled first) 15 g, Hei Fu Zi (Aconitum carmichaeli Debx.) 15 g (boiled first), Zhi Gan Cao (Glycyrrhiza uralensis Fisch.) 6 g, Gan Jiang (Zingiber officinale Rosc.) 20 g, Fu Ling (Poria cocos (Schw.) Wolf) 30 g, and Chao Bai Zhu (Atractylodes macrocephala Koidz.) 30 g. This was administered once daily, with 500 mL of water decocted for 1 h, taken twice in the morning and evening, 250 mL each time, continuously for 6–36 months. The herbs come from the outpatient pharmacy of Beijing Hospital of Traditional Chinese Medicine affiliated with Capital Medical University. Patients can choose to decoct the herbs at the hospital or at home according to their conditions.
During patient visits, the herbal medicine was distributed and patient-related information was collected. Patients who missed their appointments were followed up by telephone or via the Internet. The medication was then distributed by post to ensure continuous treatment.
Outcome assessment
Throughout the study, participants underwent assessments at regular intervals of 1, 6, 12, 18, 24, and 36 months to evaluate 24-h urinary protein (24hUP), ALB, serum creatinine (SCr), eGFR (calculated using the CKD-EPI equation), total cholesterol, triglycerides, LDL, and aPLA2R. The safety of the MFSD intervention was assessed by routine blood, urine, and feces tests, ALT, AST, CK, CKMB, LDH, and electrocardiogram.
The primary outcome was assessed by the response rate at 12, 24, and 36 months, which included both CR and partial remission (PR). CR was defined as 24hUP < 0.3 g/d, ALB > 35 g/L, and stable renal function (eGFR within the reference range or decreased ≤15% from baseline). PR was defined as 24hUP < 3.5 g/d, a decrease in urine protein of 50% or more, ALB > 35 g/L, and stable renal function, in accordance with the 2012 KDIGO guideline [Citation22]. Any instance of 24hUP > 3.5 g/d following CR or PR was defined as a relapse.
The assessment of secondary outcomes included 24hUP < 3.5 g/L, ALB > 35 g/L, eGFR decline >15%, or aPLA2R conversion to negative (≤20 RU/mL), respectively.
Statistical analysis
According to the literature [Citation7], approximately one-third of patients experience spontaneous remission, clinical remission after treatment, or no remission in MN. In our study, we aimed for a post-treatment remission rate of 50% and set an expected value of 60% to determine efficacy, with α = 0.05 and β = 0.20. We calculated sample size based on objective performance criteria (OPC) and kept the loss rate within 10%. We set the type I error at 0.05, type II error at 0.20, and confidence level at 0.80, with Zα (0.05) = 1.96 and Zβ (0.20) = 0.84. We used the OPC sample size calculation formula to calculate the target power of p0 = 0.5 and the target power of p1 = 0.6. The calculated sample size was n = 196. Accounting for a 10% attrition rate, the total sample size for the study was calculated to be 216 subjects. An independent third party will oversee data monitoring and completion after enrollment commences.
For data analysis, we used SPSS Statistics 25.0 (IBM) software (Armonk, NY). For measurement data that followed a normal distribution, we used mean ± standard deviation, while count data and measurement data that did not follow a normal distribution were expressed as median (interquartile range). We used independent sample t-tests to compare differences between two groups with normal distribution and Mann–Whitney’s U-test to compare differences between two groups with non-normal distribution. For classified variables, we expressed proportions and used the Chi-square test to compare the differences between the two groups. Kaplan–Meier’s survival analysis with a log-rank test was used to compare remission in each group and plot survival curves. To calculate the relative risk of remission, we calculated hazard ratios (HRs) and 95% confidence intervals (CIs) using Cox proportional hazards models. Factors that showed a significant association (p < .05) after univariate analysis or were of clinical importance were entered into multivariate Cox regression analysis.
Results
Enrollment and follow-up
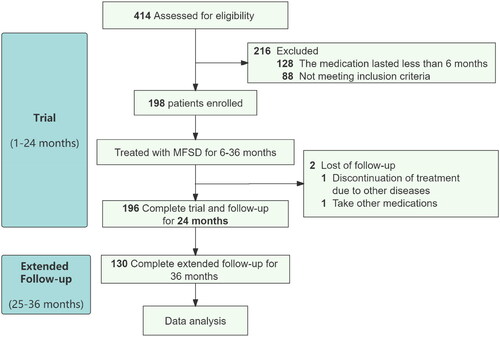
The trial enrollment process is illustrated in . Initially, 414 patients were screened, of which 88 patients were excluded due to failure to meet inclusion criteria, and 128 patients received treatment for less than 6 months. Ultimately, a total of 198 patients met the inclusion criteria and were included in the study. During the course of the study, two participants withdrew from the trial at 7 and 11 months, respectively, due to the development of other diseases and the switch to another treatment regimen by one patient.
Out of the 196 subjects who completed the 24-month trial, a further follow-up of 12 months was conducted on 130 of these participants. MFSD treatment was discontinued for those who achieved CR, while those in PR and non-remission continued receiving MFSD treatment.
Baseline characteristics of participants
Baseline characteristics of study participants are presented in . Of the 198 patients meeting the inclusion criteria, 127 (64.1%) were male and the median age was 49.0 (38.0, 60.0) years. Participants were followed for a median of 36 (31, 36) months. At enrollment, PLA2R antibodies were detected in 102 (51.5%) participants, and of these, 92 (90.2%) were antibody positive (titer > 20 RU/mL). According to the KDIGO risk assessment, 15.7% of participants were at low risk, 28.3% at moderate risk, and 56.1% at high risk, with no patients classified as very high risk. Nearly, half of the participants (44.4%) had a HIST without remission or relapse of MN after remission. The remaining 110 patients had no HIST or had received intermittent immunosuppressive therapy for no more than 6 months. The previous immunosuppressive regimens used by participants included GCs, cyclosporine and/or GCs, tacrolimus and/or GCs, CTX and GCs, RTX, Tripterygium extract, and mycophenolate mofetil. Among these, 47 participants received one regimen, 21 participants received two regimens, and 16 participants received three or more regimens. Medical history revealed that 29 participants had type 2 diabetes mellitus, and 91 had hypertension.
Table 1. Baseline clinical characteristics of participants.
Primary outcome
The primary outcome of the study was assessed at 24 and 36 months. At 24 months, a total of 116 participants were in remission, 43 of whom met the criteria for CR, while 82 participants were not in remission. Therefore, the overall remission rate at 24 months was 58.6% (95% CI: 51.7%, 65.5%), and the CR rate was 21.7%, as reported in . After the extended follow-up period, a total of 130 participants completed the trial for 36 months, of whom 91 achieved remission and 44 met the criteria for CR, while 39 participants remained without remission. The overall remission rate at 36 months was 70.0% (95% CI: 62.0%, 78.0%), and the CR rate was 33.8%.
Table 2. Response rate of participants at 12, 24, and 36 months.
During the 36-month trial and follow-up, 24 individuals experienced a relapse, resulting in a relapse rate of 12.1%. Among those who relapsed, eight individuals returned to remission, including three who achieved CR.
Effect of HIST on participants’ remission rates
Patients with PMN who have a HIST may have a poor outcome on immunosuppressive therapy and switch to mycophenolate sodium (MFSD) treatment. In this trial, we divided the 198 participants into two groups based on the presence or absence of HIST, where HIST was defined as regular 6-month immunosuppressive therapy. Among the participants, 88 had HIST and 110 had no HIST or intermittent immunosuppressive therapy for no more than 6 months. The two groups had no statistical differences in terms of baseline characteristics, including demographic characteristics, laboratory indicators, and medical history, as presented in .
Table 3. Baseline clinical characteristics of participants (grouped according to HIST or age, respectively).
The response rates of the two groups were assessed at 12, 24, and 36 months, as shown in . There was no difference in response rates between the two groups at 12 and 24 months. However, participants without HIST had significantly higher response rates than those with HIST at 36 months (p = .042). Further, Kaplan–Meier’s survival analysis of the response between the two groups of participants showed a statistically significant difference between overall response (HR 1.49, 95% CI 1.05–2.11, p = .024) and complete response (HR 1.68, 95% CI 1.00–2.80, p = .049), as depicted in .
Figure 2. Survival analysis of remission rates. (A, B) The survival analysis of PR + CR or CR rate, grouped according to with or without HIST. The light blue line means with HIST, the dark blue line means without HIST, and the red line means total. (C, D) The survival analysis of PR + CR or CR rate, grouped according to whether the age is over 60 years old. The light blue line means elder, the dark blue line means youth, and the red line means total. Other abbreviations are the same as the previous tables.
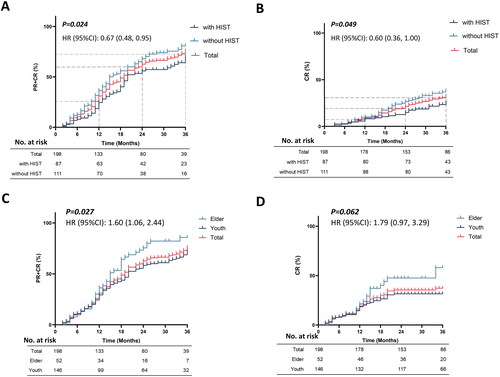
Table 4. Response rate of participants at 12, 24, and 36 months (grouped according to HIST or age, respectively).
Remission rates in elder versus younger participants
In patients with PMN, elderly patients may be more prone to experience side effects of immunosuppressive therapy, making them suitable candidates for MFSD therapy. In this study, we stratified participants into two groups based on their age: a non-elder group (<60 years) and an elder group (≥60 years). We compared the baseline clinical characteristics of the participants in the two groups, and found no statistical difference in remission rates at 24 and 36 months (p > .05) (). However, further Kaplan–Meier’s survival analysis revealed a significantly higher overall remission rate in the elder group compared to the non-elder group over time (HR 1.60, 95% CI 1.06–2.44, p = .027). Nevertheless, there was no statistical difference in the CR rate between the two groups (HR 1.79, 95% CI 0.97–3.29, p = .062) ().
Effect of antibody titers on patient remission rates
We divided the patients into two groups according to whether the PLA2R antibody was >50 RU/mL after excluding seven patients with unspecified specific values among the 92 patients who were antibody-positive. After propensity score matching (PSM), 68 patients with aPLA2R >50 RU/mL and 15 patients with aPLA2R <50 RU/mL were screened for efficacy comparison. There was no difference between the two groups of patients at baseline condition. After comparing remission rates at 12, 24, and 36 months, we found that lower antibody titers implied higher remission rates in patients with longer courses of therapy (p = .043) (refer to Supplementary Data Tables S1 and S2).
Secondary outcomes
To further evaluate the efficacy of MFSD treatment, we conducted an analysis of secondary outcomes, including 24hUP, ALB, eGFR, and PLA2R1 antibody conversion. The change trend of the primary indexes is shown in . After 36 months of MFSD treatment, the median 24hUP of patients decreased from 7.4 g/d to 1.0 g/d (p < .001), a remarkable decrease of 86.5% (refer to Supplementary Data Table S3). Among these participants, 144 (72.7%) achieved a 24hUP level below 3.5 g/d.
Figure 3. The change trend of primary indexes. (A) 24hUP trend for PR + CR patients, non-remission patients, and all patients. The dashed line below indicates that 24hUP is less than 3.5 g/d. (B) ALB trend for PR + CR patients, non-remission patients, and all patients. The dashed line above indicates that ALB is greater than 35 g/L. The dark blue square indicates no remission, the light blue triangle indicates PR + CR, and the red circle indicates all patients.
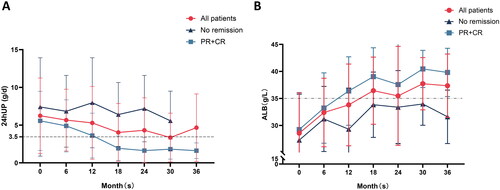
At month 36, the median ALB of participants increased from 27.5 g/L to 40.7 g/L, representing a 148% increase from the baseline (p < .001). Of these participants, 144 (72.7%) achieved an ALB level above 35 g/L (see Supplementary Data Table S3).
Among the 198 participants, 54 had eGFR levels below 90 mL/min/1.73 m2 at baseline. After 36 months of MFSD treatment, eGFR levels increased above 90 mL/min/1.73 m2 in 27 subjects, remained below 90 mL/min/1.73 m2 in 13 subjects, and remained stable in 14 subjects. Additionally, 37 subjects exhibited a decrease in eGFR of more than 15% from baseline after 36 months of treatment, of which 29 remained above 90 mL/min/1.73 m2, while eight had a decrease in eGFR greater than 15% and levels below 90 mL/min/1.73 m2 (refer to Supplementary Data Table S3).
At baseline, 92 subjects tested positive for PLA2R1 antibody. After 36 months of treatment, 40 subjects were retested for PLA2R1 antibody, among whom 32 showed negative conversion, and eight remained positive for PLA2R1 antibody (see Supplementary Data Table S3).
Safety and adverse events
During the course of this trial, adverse events were observed in 12 participants, of which one reported rash and 11 had increased SCr levels. No participant reported adverse events such as upper respiratory tract infections, diarrhea, nausea, vomiting, alopecia, or decreased white blood cells. No participant experienced ESKD or serious adverse events. In the one patient with rash, symptomatic treatment was discontinued after TCM, and the symptoms resolved. The patient continued MFSD treatment without further adverse events.
Elevated SCr levels were observed in 11 subjects during treatment, with three subjects showing SCr levels above the normal range at the first visit. Of the three subjects, one showed a decrease in SCr after treatment, while the other two remained stable or slightly elevated after treatment. The remaining eight subjects showed progressive increases in SCr levels during treatment, with five showing an increase within 24 months of treatment and three showing an increase after 24 months of treatment. The mean pretreatment SCr for these 11 subjects was 98.0 μmol/L, and the mean post-treatment SCr was 127.3 μmol/L. Details of these 11 subjects are provided in Table S4 of the supplementary data.
Furthermore, the MFSD regimen contains herbs such as Ephedra sinica Stapf. and Aconitum carmichaeli Debx, both of which have a reported history of side effects, including palpitations, headache, irritability, excessive sweating, nausea and vomiting, and tingling of the hands and feet. To minimize potential side effects, the dosage of Ephedra sinica Stapf. and Aconitum carmichaeli Debx used in the MFSD regimen was appropriately measured and boiled first. None of the participants in this study reported any of the above side effects.
Analysis of risk factors for efficacy of MFSD regimen
To assess the potential risk factors for the efficacy of the MFSD regimen, we selected several variables, including gender, age, nephrotic syndrome, 24hUP, ALB, eGFR, aPLA2R titers, high risk according to the KDIGO classification, and HIST, for Cox regression analysis (). Univariate Cox regression analysis showed that nephrotic syndrome, 24hUP, ALB, eGFR, and HIST were risk factors for the efficacy of the MFSD regimen (see Table S5 in the supplementary data).
Figure 4. Forest plots of hazard ratios in baseline predictors for remission. The blue squares show no statistical differences and red triangle indicates statistical difference. The dashed line indicates that HR = 1.0. To the left of the dashed line indicates that this factor is a risk factor for remission, to the right of the dashed line indicates a beneficial factor, and crossing the dashed line indicates that this factor has no effect on MN remission.
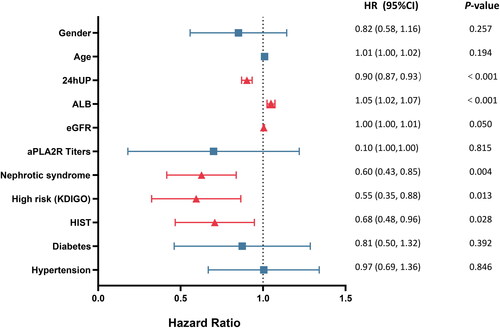
To further evaluate the potential risk factors, we selected factors with a p value <.1 from the univariate Cox regression analysis, including 24hUP, ALB, nephrotic syndrome, eGFR, high risk, HIST, gender, and age, for multivariate Cox regression analysis. Due to the partial overlap of nephrotic syndrome, 24hUP, and ALB, and high risk (KDIGO classification), we constructed three Cox regression models ().
Table 5. Correlation between baseline predictors and mitigation outcomes by multivariate COX regression analysis.
Model A included nephrotic syndrome and showed that nephrotic syndrome (HR 0.59, 95% CI 0.42–0.85, p = .004) and eGFR (HR 1.01, 95% CI 1.00–1.01, p = .047) were independent risk factors for MFSD efficacy. Model B separated nephrotic syndrome into 24hUP and ALB and revealed that 24hUP (HR 0.91, 95% CI 0.86–0.95, p < .001) was the primary risk factor for MFSD efficacy. In model C, we replaced nephrotic syndrome with high risk (KDIGO), which implies more severe 24hUP, lower ALB, or higher aPLA2R titers, and showed that high risk (KDIGO) was a risk factor for MFSD regimens (HR 0.62, 95% CI 0.43–0.88, p = .007). Furthermore, HIST was a risk factor for MFSD treatment in all three models, suggesting that MN patients who chose the MFSD regimen as initial treatment had a better outcome than MN patients who were ineffective or relapsed after immunosuppressive therapy.
Discussion
In this multicenter, prospective clinical study, we aim to further comprehend the therapeutic and side effects of the MFSD regimen. The current KDIGO guidelines recommend immunosuppressive therapies for MN patients with moderate risk or higher, including the modified Italian regimen, CNI ± GC or RTX [Citation8]. However, recent RCTs have indicated that some patients may not respond or may experience relapse after undergoing immunosuppressive therapy [Citation14–16]. Although changing the immunosuppressive regimen may be effective for some patients with refractory PMN who are not in remission or have relapsed, it remains ineffective for some patients.
Among the PMN patients included in this trial, 84.3% belonged to the moderate and high-risk population. Our results demonstrate a remission rate of 58.6% and a CR rate of 21.7% at month 24, with the remission rate increasing to 70.0% and the CR rate to 33.8% at month 36 after treatment with the MFSD regimen. Our findings suggest that the MFSD regimen is effective and that longer treatment durations result in a further increase in the remission rate. Our results are similar to those of our previous retrospective study, with the remission rate being even higher than that observed in the previous retrospective study.
In this trial, 44.4% of the population had HIST, which denotes patients with refractory PMN, while the remaining patients were receiving initial treatment for PMN. Our analysis indicated that there was no significant difference in remission and CR rates between participants with and without HIST after 24 months. However, at 36 months, the without HIST group had a significantly higher remission rate than the with HIST group, with no difference in CR rates. These findings suggest that the MFSD regimen remains effective for PMN patients who do not respond to immunosuppressive therapy or relapse. Moreover, the remission rate is comparable to that of PMN patients on initial treatment, indicating that the MFSD regimen could be a complementary or preferred treatment option for PMN patients.
Further analysis showed that of the 88 participants with HIST, 21 had used two immunosuppressive regimens, and 16 had used three or more immunosuppressive regimens. Their remission rates were 61.9% and 50%, respectively. These results suggest that the MFSD regimen remains an effective option for patients who have undergone multiple immunosuppressive treatment regimens with poor outcomes.
It is noteworthy that most PMN patients have an older age of onset, and for older patients over 60 years of age, the application of immunosuppressive therapy is less effective. This approach is also associated with a high and severe incidence of side effects and a higher rate of progression to ESKD [Citation23]. A Korean RCT study demonstrated that older PMN patients had a significantly lower remission rate than younger patients and a higher risk of progression to ESKD. Similarly, a study by Kim et al. identified age as a risk factor for complications such as infection after immunosuppressive therapy [Citation23]. Another multicenter, retrospective cohort study with a large sample revealed that immunosuppressive therapy significantly increased the risk of infection and worse renal prognosis in elderly PMN patients compared to optimal supportive therapy [Citation24].
Based on our study, we compared the effectiveness of the MFSD regimen in older and younger patients. Our findings showed that there was no significant difference in remission rates between the two groups when treated with the MFSD regimen. Moreover, the elderly group had a trend of superiority over the younger group in terms of remission rate after 24 months of treatment. These findings suggest that the MFSD regimen is suitable for elderly patients and may replace immunosuppressive therapy as the treatment of choice for PMN in the elderly.
Multivariate COX regression showed that 24hUP and high risk (KDIGO) affected the efficacy of MFSD regimens, similar to other studies [Citation25–27]. However, the mechanism by which MFSD works is not fully understood. Previous animal experiments have shown that MFSD treatment improved urinary protein levels and podocyte damage in passive Hyman nephritis rats, and also improved kidney pathology [Citation28]. In our study, we observed increased renal autophagy levels and downregulation of the Wnt/β-catenin pathway in a PHN rat model after MSFD treatment [Citation28]. Additionally, another clinical study demonstrated that serum IL-35 levels increased with remission of PMN in patients with MN treated with MFSD, and the increase of IL-35 was related to the level of iTr35 in peripheral blood, suggesting that MFSD might up-regulate the level of regulatory T cells in peripheral blood and possibly induce immune tolerance of the body [Citation29]. However, further investigations are needed to fully elucidate the therapeutic mechanisms of MFSD treatment.
This trial has several limitations. First, it was a single-arm multicenter study, and thus, lacked data from a control group, which could have provided more insights into the efficacy of the MFSD regimen for PMN. Second, the detection of aPLA2R was lower than expected, which might have limited the ability to comprehensively evaluate the efficacy of the MFSD regimen. Additionally, the study was conducted only in Chinese patients, and further research is needed to determine the efficacy of the MFSD regimen in patients of other ethnicities.
Conclusions
In conclusion, our study confirms that the MFSD regimen is effective in the treatment of PMN with significantly fewer side effects. This finding suggests that the MFSD regimen could be considered as a supplement or alternative to the currently recommended treatment regimen, especially for elderly patients with MN or refractory MN.
Author contributions
BL, HR, and QL were responsible for conception of the study. NZ, SH, QZ, NZ, ZD, YG, XD, YH, and FH are responsible for data analysis and interpretation. NZ was involved in drafting the manuscript. BL, HR, HJ, HD, and WL are responsible for key modifications of important content. BL and HD were responsible for approving the final version to be published and agree to be responsible for all aspects of the work and to ensure that issues relating to the accuracy or completeness of any part of the work are properly investigated and resolved. The authenticity of the original data in this paper has been confirmed by BL and HR, and they are responsible for this.
Supplemental Material
Download PDF (347.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Xu X, Wang G, Chen N, et al. Long-term exposure to air pollution and increased risk of membranous nephropathy in China. J Am Soc Nephrol. 2016;27(12):1–11. doi: 10.1681/ASN.2016010093.
- Qian Q, Nasr SH. Diagnosis and treatment of glomerular diseases in elderly patients. Adv Chronic Kidney Dis. 2014;21(2):228–246. doi: 10.1053/j.ackd.2014.01.004.
- Couser WG. Primary membranous nephropathy. Clin J Am Soc Nephrol. 2017;12(6):983–997. doi: 10.2215/CJN.11761116.
- Beck LHJr., Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361(1):11–21. doi: 10.1056/NEJMoa0810457.
- Meyer-Schwesinger C, Lambeau G, Stahl RA. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med. 2015;372(11):1074–1075. doi: 10.1056/NEJMc1500130.
- Liu W, Huang G, Rui H, et al. Course monitoring of membranous nephropathy: both autoantibodies and podocytes require multidimensional attention. Autoimmun Rev. 2022;21(2):102976. doi: 10.1016/j.autrev.2021.102976.
- Cattran DC. Membranous nephropathy: quo vadis? Kidney Int. 2002;61(1):349–350. doi: 10.1046/j.1523-1755.2002.00125.x.
- Rovin BH, Adler SG, Barratt J, et al. Executive summary of the KDIGO 2021 guideline for the management of glomerular diseases. Kidney Int. 2021;100(4):753–779. doi: 10.1016/j.kint.2021.05.015.
- Dahan K, Debiec H, Plaisier E, et al. Rituximab for severe membranous nephropathy: a 6-month trial with extended follow-up. J Am Soc Nephrol. 2017;28(1):348–358. doi: 10.1681/ASN.2016040449.
- Howman A, Chapman TL, Langdon MM, et al. Immunosuppression for progressive membranous nephropathy: a UK randomised controlled trial. Lancet. 2013;381(9868):744–751. doi: 10.1016/S0140-6736(12)61566-9.
- Wang J, Xie Q, Sun Z, et al. Response to immunosuppressive therapy in PLA(2)R-associated and non-PLA(2)R- associated idiopathic membranous nephropathy: a retrospective, multicenter cohort study. BMC Nephrol. 2017;18(1):227. doi: 10.1186/s12882-017-0636-0.
- Seitz-Polski B, Dahan K, Debiec H, et al. High-dose rituximab and early remission in PLA2R1-related membranous nephropathy. Clin J Am Soc Nephrol. 2019;14(8):1173–1182. doi: 10.2215/CJN.11791018.
- Praga M, Barrio V, Juárez GF, et al. Tacrolimus monotherapy in membranous nephropathy: a randomized controlled trial. Kidney Int. 2007;71(9):924–930. doi: 10.1038/sj.ki.5002215.
- Fervenza FC, Appel GB, Barbour SJ, et al. Rituximab or cyclosporine in the treatment of membranous nephropathy. N Engl J Med. 2019;381(1):36–46. doi: 10.1056/NEJMoa1814427.
- Fernández-Juárez G, Rojas-Rivera J, Logt AV, et al. The STARMEN trial indicates that alternating treatment with corticosteroids and cyclophosphamide is superior to sequential treatment with tacrolimus and rituximab in primary membranous nephropathy. Kidney Int. 2021;99(4):986–998. doi: 10.1016/j.kint.2020.10.014.
- Scolari F, Delbarba E, Santoro D, et al. Rituximab or cyclophosphamide in the treatment of membranous nephropathy: the RI-CYCLO randomized trial. J Am Soc Nephrol. 2021;32(4):972–982. doi: 10.1681/ASN.2020071091.
- Lu Z, Liu W, Gao H, et al. Traditional Chinese medicine as an adjunct therapy in the treatment of idiopathic membranous nephropathy: a systematic review and meta-analysis. PLOS One. 2021;16(5):e0251131. doi: 10.1371/journal.pone.0251131.
- Chen Y, Deng Y, Ni Z, et al. Efficacy and safety of traditional Chinese medicine (Shenqi particle) for patients with idiopathic membranous nephropathy: a multicenter randomized controlled clinical trial. Am J Kidney Dis. 2013;62(6):1068–1076. doi: 10.1053/j.ajkd.2013.05.005.
- Shi B, Zhang RR, Liang Y, et al. Efficacy of traditional Chinese medicine regimen Jian Pi Qu Shi formula for refractory patients with idiopathic membranous nephropathy: a retrospective case-series study. Evid Based Complement Alternat Med. 2018;2018:5854710. doi: 10.1155/2018/5854710.
- Dong Z, Dai H, Gao Y, et al. Effect of Mahuang Fuzi and Shenzhuo Decoction on idiopathic membranous nephropathy: a multicenter, nonrandomized, single-arm clinical trial. Front Pharmacol. 2021;12:724744. doi: 10.3389/fphar.2021.724744.
- Chapter 2: general principles in the management of glomerular disease. Kidney Int Suppl. 2012;2(2):156–162.
- Chapter 7: idiopathic membranous nephropathy. Kidney Int Suppl. 2012;2(2):186–197.
- Kim Y, Yoon HE, Chung BH, et al. Clinical outcomes and effects of treatment in older patients with idiopathic membranous nephropathy. Korean J Intern Med. 2019;34(5):1091–1099. doi: 10.3904/kjim.2018.139.
- Bae E, Lee SW, Park S, et al. Treatment and clinical outcomes of elderly idiopathic membranous nephropathy: a multicenter cohort study in Korea. Arch Gerontol Geriatr. 2018;76:175–181. doi: 10.1016/j.archger.2018.03.002.
- Xiaofan H, Jing X, Chenni G, et al. New risk score for predicting progression of membranous nephropathy. J Transl Med. 2019;17(1):41. doi: 10.1186/s12967-019-1792-8.
- He P, Zha Y, Liu J, et al. Clinical outcomes of patients with primary membranous nephropathy and subnephrotic proteinuria. Front Med. 2021;8:737700. doi: 10.3389/fmed.2021.737700.
- Jurubiță R, Obrișcă B, Sorohan B, et al. Clinical phenotypes and predictors of remission in primary membranous nephropathy. J Clin Med. 2021;10(12):2624. doi: 10.3390/jcm10122624.
- Gao Y, Dai H, Zhang N, et al. The ameliorative effect of Mahuang Fuzi and Shenzhuo Decoction on membranous nephropathy of rodent model is associated with autophagy and Wnt/β-catenin pathway. Front Pharmacol. 2022;13:820130. doi: 10.3389/fphar.2022.820130.
- Zhang N, Dai H, Dong X, et al. Level of interleukin-35 in patients with idiopathic membranous nephropathy and its predictive value for remission time. Front Immunol. 2022;13:926368. doi: 10.3389/fimmu.2022.926368.