Secondary oxalate nephropathy is a highly under-recognized disease, with occupational exposure to organic solvents being one of the rarest causes. This study reports a case of secondary oxalate nephropathy that may have been caused by long-term occupational exposure to paints and thinners containing ethylene glycol.
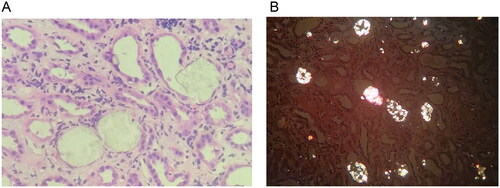
A 50-year-old man was admitted to the hospital after experiencing more than 10 days of abdominal distension and nausea. The attending physician did not identify any physical abnormalities during the physical examination. On admission, laboratory test showed: serum creatinine 888.1 μmol/L, blood urea nitrogen 34 mmol/L, uric acid 684 μmol/L, anion gap 21.1 mmol/L (8–16 mmol/L), carbondioxide combining power 11.8 mmol/L (22–30 mmol/L), calcium 2.11 mmol/L, phosphorus 2.48 mmol/L, parathyroid hormone 203.9 pg/ml, hemoglobin 124 g/L. Urinalysis results showed: specific gravity 1.007, PH 5.0, protein + −, occult blood + −. The ratio of urinary microalbumin to creatinine was 62.09 mg/g. Overall, the patient exhibited renal dysfunction accompanied by mild metabolic acidosis featuring a high anion gap. However, urine analysis showed slight abnormalities. The ultrasound examination revealed a significant enhancement of the patient’s renal echo, surpassing even that of sclerosing nephritis, while the patient’s renal size and cortical thickness remained essentially normal (). Renal pathology revealed a large amount of pale yellow crystalline material in the renal tubular lumen and renal interstitium. Under polarized light, the crystals exhibited polychromatic birefringence and various shapes, proving to be oxalate crystals (). At the same time, moderate to severe acute lesions with chronic changes in the renal tubulointerstitium were found, characterized by infiltration of multiple mononuclear cells in the renal interstitium and focal flattened, brush-like edges or cytoplasmic detachment of multiple renal tubular epithelial cells, accompanied by focal tubular atrophy and basement membrane thickening (about 5%). According to a blood test performed by a professional testing institution (Beijing Fuyou Longhui Genetic Disease Clinic), the blood oxalate concentration is 175.48 μmol/L (< 30 μmol/L), so the patient was diagnosed with oxalate nephropathy.
Figure 1. Kidney under ultrasound. (A) Normal human kidney. (B) The kidney of the patient reported in our case. It showed diffuse echogenic enhancement under ultrasound, with unclear corticomedullary boundary and normal kidney size, abbreviated as ‘large white kidney’. (C) Kidney with acute ischemic injury. (D) End-stage sclerosing kidney.
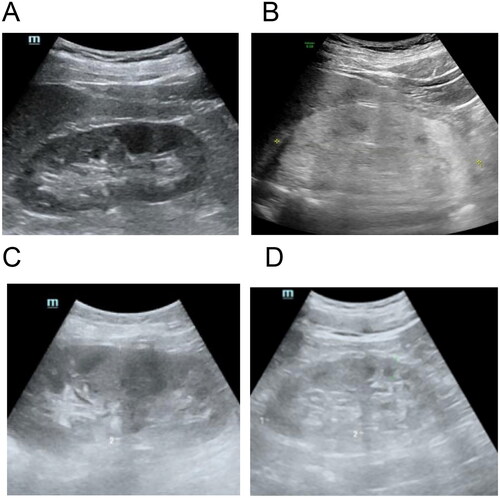
Figure 2. Pathological images of kidney biopsy. (A) In the lumen of the renal tubules, pale yellow crystalline substances are frequently seen and are considered to be oxalate crystals. The epithelial cells of the renal tubules are flattened with brush-like margins or loss of cytoplasm. The renal interstitium is infiltrated by monocytes (HE, ×10). (B) Polarized light microscopy shows polychromatic birefringence and different forms of crystals, confirming that they are oxalate crystals, widely distributed in the tubular lumen and scattered in the interstitium.
Oxalate nephropathy (OxN) is a metabolic kidney disease caused by primary or secondary hyperoxaluria. Most patients with primary hyperoxaluria progress to ESRD before adulthood, accompanied by extrarenal oxalate deposition [Citation1]. The patient in this article has an age of onset of 50 years and no obvious extrarenal manifestations. And the genetic test report ultimately showed no significant genetic changes supporting primary hyperoxaluria, providing strong evidence for the diagnosis of secondary OxN. Secondary OxN is commonly seen in diseases and surgeries associated with excessive use of drugs that are metabolized to oxalic acid, excessive consumption of foods rich in oxalic acid, and increased absorption of oxalic acid, such as inflammatory bowel disease and gastrointestinal surgery for weight loss [Citation2]. OxN caused by organic solvent poisoning is extremely rare and has only been reported in a few cases, such as oral ethylene glycol (EG) [Citation3] and long-term industrial exposure to volatile dimethyl oxalate. EG is a colorless, odorless, slightly sweet liquid with some evaporation, often used as a solvent, antifreeze and for synthesizing polyester fiber. In some paints and thinners, EG can also be detected. Acute renal failure caused by acute EG poisoning is usually associated with high anion gap metabolic acidosis, increased osmotic gap, hypocalcemia, neurological damage, cardiovascular instability and often leads to death [Citation4]. However, the clinical manifestations of chronic low-dose EG poisoning are mild and may present only with acute kidney injury without typical metabolic abnormalities [Citation5–6]. The patient in our case had OxN associated with high anion gap metabolic acidosis with mild clinical manifestations and no evidence of an abnormal pancreas on the images. After excluding the possibility of other pathogenic factors such as steatorrhea and chronic pancreatitis, we thought that patients most likely had OxN caused by EG. We were unable to find out from the manufacturer whether EG was present in the paint thinner, so we tested the EG content of the paint thinner that the patient comed into contact with at work, and the results showed that the concentration of EG was as high as 0.5%.
There may be three main routes of intake of EG by humans: oral intake, skin absorption and inhalation [Citation7]. The most likely route to produce toxic doses is by oral administration, including intentional or unintentional oral ingestion. Desilva et al. reported a case of OxN related to EG caused by ingestion of 1–2 tablespoons of antifreeze 2–3 days per week with a suicidal tendency [Citation6]. After treatment, the patient’s renal function did not recover and ultimately relied on dialysis. In addition, EG can be absorbed through the skin and ingested through nebulized droplets, which has been confirmed by human autopsy and animal experimental studies. In some studies, prolonged skin contact with EG antifreeze has been associated with increased urinary ethylene glycol, oxalic acid and some changes in renal biochemistry in automotive service workers [Citation8]. There have been very few similar reports in recent years, which may be related to the gradual strengthening of occupational protective measures for people to reduce the intake dose of EG. In our case, the patient had been exposed to paints and thinners as a painter for 8 years. According to his daughter’s description, his occupational safety measures were not strict. His hands had been burned several times with paints and thinners, resulting in extensive burn-like symptoms and large peeling of the palms, which may have led to the absorption of EG through the skin. On the other hand, the patient did not wear a mask while working and there was also a possibility of inhalation.
Most of the absorbed EG is metabolized in the liver, where it is converted by ethanol dehydrogenase to glucuronic acid and finally to oxalate crystals. It has been reported that ethanol can be used as an antidote for acute EG poisoning, as it competitively binds to ethanol dehydrogenase with an affinity 100 times that of EG [Citation9]. Interestingly, in our case, the patient’s consumption of ethanol-containing alcohol had decreased significantly over the previous 2 years due to gout attacks. Is it possible that this change is an aggravating factor for the recent accumulation of oxalate crystals in the kidneys of the patient who has been exposed to paints and thinners containing EG for years?
For the patient’s treatment, we recommended that he stop consuming foods and medications high in oxalic acid, such as spinach, purslane, orange, tea, vitamin C, etc. On the other hand, we advised the patient to drink more water (with a daily water intake of over 3000 mL) and to slowly infuse sodium bicarbonate solution intravenously to alkalize the urine in order to accelerate the excretion of oxalate. We also gave him vitamin B6 to reduce the production of oxalic acid and added probiotics to his gut to prevent excessive absorption of oxalate, all of which may be helpful but are still controversial. Of course, we also recommended that the patient undergo renal replacement therapy to help remove oxalate in the circulation, but he strongly refused. we gave the patient oral prednisone acetate at a dose of 30 mg per day since there was also renal tubulointerstitial injury, although steroids are not an established treatments for ON. After 10 days, the creatinine dropped to 522.1 μmol/L. The patient was then followed up in the outpatient clinic, and after one month of treatment, the creatinine was 420 μmol/L. After treatment, the patient’s renal function improved markedly, but it still did not reach normal levels, which was consistent with the pathological manifestations of his kidneys, which were accompanied by chronic lesions.
In conclusion, the oxalate nephropathy reported in our case may have been caused by chronic EG toxicity resulting from long-term occupational exposure to paints and thinners without adequate occupational protection, which is different from the previously reported pattern of toxicity from EG-related renal failure. It serves as a reminder that good occupational protection should always be taken when exposed to organic solvents in an industrial setting to achieve maximum health benefits at the lowest economic cost.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Groothoff JW, Metry E, Deesker L, et al. Clinical practice recommendations for primary hyperoxaluria: an expert consensus statement from ERKNet and OxalEurope. Nat Rev Nephrol. 2023;19(3):1–3. Epub 2023 Jan 5. PMID: 36604599. doi:10.1038/s41581-022-00661-1.
- Liang S, Li L, Chen D, et al. Secondary oxalate nephropathy: causes and clinicopathological characteristics of a case series. Nephron. 2021;145(6):684–691. Epub 2021 Jul 8. PMID: 34237750. doi:10.1159/000517072.
- Guo C, McMartin KE. The cytotoxicity of oxalate, metabolite of ethylene glycol, is due to calcium oxalate monohydrate formation. Toxicology. 2005;208(3):347–355. PMID: 15695020. doi:10.1016/j.tox.2004.11.029.
- Seo JW, Lee JH, Son IS, et al. Acute oxalate nephropathy caused by ethylene glycol poisoning. Kidney Res Clin Pract. 2012;31(4):249–252. Epub 2012 Oct 4. PMID: 26889430; PMCID: PMC4716116. doi:10.1016/j.krcp.2012.09.007.
- Toth-Manikowski SM, Menn-Josephy H, Bhatia J. A case of chronic ethylene glycol intoxication presenting without classic metabolic derangements. Case Rep Nephrol. 2014;2014:128145. Epub 2014 Aug 21. PMID: 25215251; PMCID: PMC4158266. doi:10.1155/2014/128145.
- Desilva MB, Mueller PS. Renal consequences of long-term, low-dose intentional ingestion of ethylene glycol. Ren Fail. 2009;31(7):586–588. PMID: 19839855. doi:10.1080/08860220903003362.
- Palmer RB, Brent J. Derivation of a chemical-specific adjustment factor (CSAF) for use in the assessment of risk from chronic exposure to ethylene glycol: application of International Programme for Chemical Safety guidelines. Toxicol Appl Pharmacol. 2005;207(2 Suppl):576–584. PMID: 15990139. doi:10.1016/j.taap.2005.01.033.
- Laitinen J, Liesivuori J, Savolainen H. Exposure to glycols and their renal effects in motor servicing workers. Occup Med (Lond). 1995;45(5):259–262. PMID: 7579301. doi:10.1093/occmed/45.5.259.
- Beaulieu J, Roberts DM, Gosselin S, et al. Treating ethylene glycol poisoning with alcohol dehydrogenase inhibition, but without extracorporeal treatments: a systematic review. Clin Toxicol (Phila). 2022;60(7):784–797. Epub 2022 Mar 21. Erratum in: Clin Toxicol (Phila). 2022 May 23;:1. PMID: 35311442. doi:10.1080/15563650.2022.2049810.

