Abstract
Anti-glomerular basement membrane (GBM) disease is a rare autoimmune condition characterized by the presence of positive anti-GBM autoantibodies, linear deposition of immunoglobulin G (IgG) along the GBM and severe kidney injury. In a limited number of cases, the association of anti-GBM disease with other glomerulonephritis has been reported. Herein, we present the case of a 66-year-old female patient with progressive worsen kidney function and decreased urine output. A renal biopsy revealed crescent glomerulonephritis with lineal IgG deposition along the GBM and mesangial IgA deposition, which supported the diagnosis of concurrent anti-GBM disease and IgA nephropathy (IgAN). In an extensive literature review, we identified a total of thirty-nine patients were reported anti-GBM disease combined with IgAN. The clinical characteristics of these patients demonstrate that the anti-GBM disease combined with IgAN tends to be milder with a more indolent course and a better prognosis than the classic anti-GBM disease, and its potential pathogenesis deserves to be further explored.
Introduction
Anti-glomerular basement membrane (anti-GBM) disease is a rare autoimmune disorder characterized by the presence of positive anti-GBM autoantibodies and the linear deposition of immunoglobulin G (IgG) along specific basement membranes, such as the GBM and alveolar basement membrane [Citation1,Citation2]. Histologically, it is characterized by extensive crescent formation and clinically, it is associated with rapidly progressive glomerulonephritis (RPGN). Anti-GBM disease is mediated by serum circulating autoantibodies and its main autoantigen is the non-collagen domain of the α3 chain of type IV collagen [α3(IV)NC1)] [Citation3–5]. Two conformational epitopes have been identified on α3(IV)NC1, specified as EA and EB [Citation1,Citation6,Citation7].
In recent years, a growing number of atypical manifestations of anti-GBM disease have been reported, suggesting that it might be composed of heterogeneous subgroups [Citation1, Citation8]. Concurrent presentations of two types of renal pathology are not rare findings in kidney biopsy. With progress in diagnostic technology, anti-GBM diseases with antineutrophil cytoplasmic antibody (ANCA) vasculitis and membranous nephropathy (MN) have been frequently reported [Citation1,Citation9,Citation10]. However, cases of anti-GBM disease complicated by IgA nephropathy (IgAN) are uncommon. IgAN is the most prevalent glomerulonephritis worldwide, characterized by dominant IgA deposition in the glomerular mesangium [Citation11]. The combination of anti-GBM disease with IgAN is usually a milder disease with a less aggressive pattern than typical anti-GBM disease. The underlying pathogenesis needs to be further elucidated [Citation8].
Herein, we describe a case of concurrent anti-GBM disease and IgAN in our institute. In an extensive literature review, we identified a total of thirty-nine patients reporting anti-GBM glomerulonephritis combined with IgAN to better understand this disease.
Methods
We reviewed the literature for all papers on anti-GBM combined with IgAN published until September 30, 2023, searching the PubMed database of the National Center for Biotechnology Information. The following key words were used: ‘anti-GBM disease’ OR ‘anti-GBM nephritis’ OR ‘anti-GBM glomerulonephritis’ in combination with ‘IgA nephropathy’. Combined anti-GBM disease and IgAN were diagnosed using the following criteria. (i) Circulating anti-GBM antibodies were detected by a commercially available enzyme-linked immunosorbent assay with bovine α(IV)NC1 as solid-phase antigen. (ii) On kidney specimens, IgA staining (strength ≥2+) was identified in glomerular mesangium on immunofluorescence, and an electron-dense deposit was observed in the glomerular mesangial area on electron microscopy. (iii) Patients with combined other glomerular diseases or tumors, and patients with unavailable blood samples and missing data were excluded. Two readers (a nephrologist GX and a nephrology resident CZW) assessed the full text separately to extract the following data: gender, age, country, oliguria/anuria, hemoglobin, proteinuria, serum creatinine (SCr) on diagnosis, level of anti-GBM antibody, crescent ratio, immunofluorescence on GBM, hemodialysis on diagnosis, immunosuppressive therapy, follow-up time, and outcome. All variables are unified: (i) SCr (1 mg/dL =88.4 µmol/L). (ii) Hemoglobin (1 g/dL = 10 g/L). (iii) Proteinuria (24-h proteinuria quantification: ‘<0.15 g’ = ‘–’, ‘0.15–0.30g’ = ‘±’, ‘0.3–1.5 g’ = ‘1+’, ‘1.5–3 g’ = ‘2+’, ‘3–6 g’ = ‘3+’, ‘> 6 g’ = ‘4+’). Chi-square test was used to estimate the relationships between selected variables and renal revival in patients (SPSS 26.0 software). p < 0.05 was considered statistically significant.
Results
Case report
History of present illness
A 68-year-old female was admitted to the Kidney Institute of PLA (Changzheng Hospital, Shanghai, China) in October 2021 with complaints of fever and increasing SCr for one month. One month before this hospitalization, she developed symptoms such as fever, cough, fatigue, and nausea. Laboratory tests revealed a neutrophil rate of 80.9% with C-reactive protein (CRP) of 76.8 mg/L, as well as a Scr level of 116.1 µmol/L. Computed tomography (CT) scan showed normal signs of the lungs, with the exception of multiple small nodules. Then she received anti-infection treatment for two weeks. However, no improvement was detected in clinical symptoms, instead of decreased urine output, and a repeat laboratory test indicated progressive decreased kidney function with a SCr level of 496 µmol/L. Moreover, the anti-GBM antibody was positive, and urinalysis revealed urinary occult blood 3+ and urinary protein 3+. Subsequently, the patient was immediately transferred to our institute for further treatment.
Personal and family history
The patient denied having a family history and previous medical history of illness, such as hypertension, diabetes mellitus, arthritis, or chronic kidney disease.
Physical examination
On admission, the physical examination was unremarkable except for pallor, moderate bilateral pitting pedal edema and an elevated blood pressure of 153/82 mmHg.
Laboratory and imaging examinations
Urinalysis showed an erythrocyte level of 496.8/µL and 24-h urine protein quantification of 1.916 g. The SCr level had risen from 496 µmol/L to 1040 µmol/L during one week. In addition, the hemoglobin (Hb) level was 82 g/L and CRP was 162.92 mg/L. Serum immunoglobulin A (IgA) (5.08 g/L) and immunoglobulin G (IgG) (19.70 g/L) were increased, while complement C3 (1.110 g/L) and C4 (0.368 g/L) was normal. The level of serum anti-GBM antibody was 184.94 EU/ml (cat no. EA 1251-9601 G, EUROIMMUN, Medical Laboratory Diagnostics Co., Ltd, Luebeck, Germany). The antibody profile was negative for p-ANCA, c-ANCA and anti-double stranded DNA. Ultrasonogram showed normal signs of the kidneys.
Renal biopsy
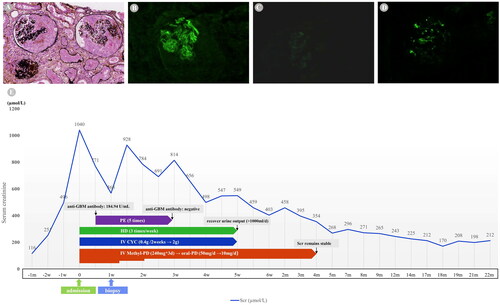
Based on the above findings, rapidly progressive glomerulonephritis was diagnosed and an ultrasound-guided renal biopsy was immediately performed. Light microscopy showed 13 glomeruli, including 2 all-nodular sclerosing glomeruli, 7 spherical cellular crescents, 1 spherical fibrocellular crescent, and 1 segmental cellular crescent (). The glomerular mesangial cells and matrix were slightly proliferative. In addition, diffuse necrotic tubular epithelial and infiltration or inflammatory cells showed severe injury to the tubulointerstitium. Immunofluorescence staining revealed linear deposition of IgG (++) along the capillary wall, and the IgG subclass showed capillary lineal deposition of IgG3 (+) (). Granular and bolus-type deposits of IgA (++) were observed in the mesangial area (). Furthermore, electron microscopy revealed electron-dense mass deposits in the mesangial area.
Figure 1. Pathological results of kidney biopsy and clinical course. (A) Light microscopy showing the formation of large cellular crescents (PASM staining, × 200). (B) Immunofluorescence showing linear deposition of IgG along the GBM. (C) IgG subclass shows capillary lineal deposition of IgG3. (D) Immunofluorescence showing lumpy deposition of IgA in the mesangium. (E) Clinical course of patient. GBM, glomerular basement membrane; Ig, immunoglobulin; Scr creatinine; HD, hemodialysis; PE, plasma exchange; MethyI-PD, Methylprednisolone; IV, intravenous; CYC, cyclophosphamide; w. week; m, month.
Final diagnosis
A diagnosis of anti-GBM disease combined with IgAN was confirmed.
Treatment
After admission, the patient firstly received a pulse dose of intravenous methylprednisolone (IV Methyl-PD; Pfizer Ltd., Kent, UK) of 240 mg/day for 3 days followed by oral prednisolone acetate (Shanghai Xinyi Pharmaceutical Co., Ltd, Shanghai, China) of 50 mg/day, which was gradually tapered to 10 mg/day with a total of four months treatment duration. Intravenous cyclophosphamide (Baxter Oncology GmbH, German) of 400 mg/2 weeks was administered, with a cumulative dose of 2 g (end up with lung infection). In addition, she received plasmapheresis for 5 sessions during hospitalization, until the anti-GBM antibody was negative. Hemodialysis was performed simultaneously until urine output was restored ().
Outcome and follow-up
Throughout the 20-month follow-up, the anti-GBM antibody titer was persistently negative, the level of SCr was stabilized (170–212 µmol/L), and urinalysis showed no abnormalities. The patient was generally with a stable health condition.
Literature review
Patient characteristics
Thirty-nine cases of anti-GBM disease combined with IgAN were identified (including the present case) from 2005 to 2023 with an average age of 42.5 ± 17.1 years old, and 19/39 (48.7%) males (). A majority of patients were from China (29/39, 74.4%), flowed by India (4/39, 10.2%), Japan (3/39, 7.7%), Asia (2/39, 5.1%) and Korean (1/39, 2.6%). Oliguria or anuria was detected in 13/31 (41.9%) cases. The number of patients with proteinuria 1+, 2+, 3+, 4+ was 9 (23.7%), 11 (28.9%), 12 (31.6%), and 6 (15.8%), respectively. The average levels of hemoglobin and SCr were 9.4 ± 1.5 g/dL and 6.3 (3.3, 8.6) mg/dL, respectively. As expected, circulating anti-GBM antibodies were detected in all patients. The mean overall crescent ratio was 0.71 ± 0.22. As presented in , 34/38 (89.5%) patients were treated with IV Methyl-PD, and 12/38 (31.6%) patients required hemodialysis at presentation. A majority of patients (81.6%) received plasma exchange therapies. During the according follow-up, 25/39 (64.1%) patients had a significant renal revival, and 14/39 (35.9%) patients progressed to ESKD or remained on maintenance hemodialysis (MHD).
Table 1. Clinical characteristics of anti-GBM disease combined with IgAN (2005–2023).
Correlated factors with renal survival of patients
To explore the potential clinical factors associated with the renal survival, we divided the patients into ESKD group and non-ESKD group based on the clinical outcomes (). Statistical analysis shows that the SCr level above 4 mg/dL (p < 0.001) and hemodialysis at diagnosis (p = 0.002) were significantly associated with ESKD. However, the factors of age, country, oliguria/anuria, anemia, proteinuria, and crescent ratio were not associated with ESKD.
Table 2. Factors associated with renal survival of patients.
Discussion
In our present study, a rare case of concurrent anti-GBM disease and IgAN was reported. Since the first report of a recurrent anti-GBM disease with IgAN in 1998 [Citation12], the coexistence of anti-GBM disease and IgAN has been documented sporadically in several studies. A literature review for Anti-GBM disease and IgAN was performed resulting in a total of thirty-nine cases () [Citation2, Citation8–10, Citation13–33]. The current therapy involves a combination of plasmapheresis and immunosuppressive therapy to quench active inflammation and weaken cellular reaction and antibody production. Based on the analysis of the collected cases, we discovered that most of patients were treated with a combination of steroids, cytotoxic immunosuppressive therapy and plasmapheresis. Among 39 cases, we found that more than half (64.1%) of patients had favorable renal recovery. However, the clinical presentations of anti-GBM diseases with ANCA-associated vasculitis were often severe, and the majority manifested acute renal failure with low renal survival rates [Citation34]. Moreover, a latest article reported on 28 patients who were diagnosed with concurrent anti-GBM disease and MN, and their prognosis was very poor despite prompt plasmapheresis and immunosuppression [Citation35]. The combination of anti-GBM disease with IgAN tends to be a milder disease with a less aggressive pattern than typical anti-GBM disease.
Anti-GBM disease presents with severe and abrupt symptoms, with about 90% of patients having rapidly progressive glomerulonephritis. A study found that patients with 100% crescents on renal biopsy who receive dialysis and show renal failure at presentation have a terrible kidney survival rate of 8% at 1 year of follow-up [Citation1]. Previous studies revealed that advancing age, low glomerular filtration rate (GFR), low hemoglobin, high crescent ratio, and high SCr level were significant negative risk factors associated with the prognosis of typical anti-GBM disease [Citation36–39]. In our study, the chi-square test showed that ESKD was related to high SCr level (p < 0.001) and hemodialysis on admission (p = 0.002). A prospective study reported that in-hospital dialysis was an indicative factor of short-term renal survival [Citation38]. However, the limited number of cases and incomplete clinical information prevented us from conducting a multivariate Cox regression analysis to further explore the risk factors in our patients.
In addition to the above clinical characteristics, an increasing number of studies have focused on the pathogenesis of the disease. In accordance with previous case reports, IgAN was diagnosed prior to or synchronously with anti-GBM disease, rather than as a subsequent event. Pathogenesis of IgAN is characterized by IgA-containing immune complexes passing through the glomerular capillary walls and depositing extensively in the mesangium [Citation11]. It has been suggested that epitopes are isolated under normal circumstances and exposed following some physicochemical disruption, which then allows the initiation of immune response [Citation17]. There was one hypothesis that IgA-related immune complexes might promote immunologic and inflammatory activities, leading to conformational changes and exposure of GBM antigens resulting in the development of anti-GBM autoantibodies [Citation22]. In addition, abnormalities in IgA molecules might be another factor. Deposits of IgA1 with aberrant polysaccharide chains along GBM and anti-GBM IgG detected in patients with anti-GBM disease combined with IgAN led us to speculate that the deposition of abnormal IgA1 along GBM might induce novel antigen formation and consequently cause the production of anti-GBM antibodies [Citation32]. Takahiro Matsuno [Citation2] has reported a male patient who developed anti-GBM disease after being diagnosed with IgAN. Despite this, it is challenging to determine whether anti-GBM disease is an incidental complication of IgAN or a secondary manifestation, since there is no established marker that may differentiate primary from secondary anti-GBM disease.
There is one hypothesis concerning epitopes on GBM. In natural conditions, the type IV collagen of human GBM is composed of five subclasses of α chains (α1, α2, α3, α4 and α5), and the main target antigen of anti-GBM antibodies recognizes conformational epitopes within the α3 chain of type IV collagen. Dissociation of the α3 monomer from the hexamer structure leads to exposure of cryptic epitopes (Ea and Eb) to the host immune system [Citation5, Citation40]. Juan Zhao et al. [Citation41] found that the level of autoantibodies against α3 (IV) NC1 was a significant independent risk factor for a higher SCr level on diagnosis by regression analysis. It suggested that autoantibodies against α3 (IV) NC1 were vital in the pathogenesis of renal damage in anti-GBM disease, and autoantibodies against α1, α2, α4 and α5 chains caused less kidney damage than α3 chain. A similar study also reported that the level of antibodies against α3 (IV) NC1 is an independent risk factor for renal damage. A new study in IgAN revealed that α5 (IV) NC1 was characterized by a strong linear pattern on the vertical cut surface of GBM, and in the glomeruli with cellular crescents, the α5 (IV) expression in the GBM was disrupted, indicating rupture of the GBM [Citation42]. The above information suggests that different epitopes of α chains might explain partly the better prognosis of anti-GBM disease combined with IgAN.
Another popular explanation suggested that the distribution of anti-GBM autoantibody IgG subclasses was associated with disease severity. IgG is categorized into four subclasses (IgG1, IgG2, IgG3, and IgG4) according to their heavy chains, which elicit different immunological and inflammatory responses. Anti-GBM antibodies subclasses detected in the normal human serum belong to IgG2 and IgG4 [Citation43]. IgG1 and IgG3 are the most dominant IgG subclass in typical anti-GBM disease, while the proportion of IgG2 and IgG4 seem to be greater than IgG1 and IgG3 in anti-GBM disease combined with IgAN [Citation8]. In our case, we found that IgG3 was deposited along the GBM, which might explain a severe renal dysfunction on admission. It suggested that during the progression of anti-GBM disease, IgG1 and IgG3 subclasses against target GBM were also the most crucial contributors to the severity of renal function [Citation43–45]. It should be mentioned that autoantibodies of the IgG1 and IgG3 subclasses might have had an evident role in pathogenesis, as these two subclasses were known to activate the complement. Once activated, complement chemotactic factors may then attract effector cells such as macrophages and neutrophils, which are known to contribute to glomerulonephritis [Citation46]. Complement activation has been shown to have a pathogenic role in accelerated nephrotoxic nephritis models and involving the pathogenesis of anti-GBM disease [Citation47, Citation48]. In addition, both IgG2 and IgG4 are weak activators of the complement system, likely explaining their less aggressive process. Another mechanism for IgG including tissue damage lies in its high affinity binding to Fc receptors (FcR) on phagocytic cells to mediate opsonization. The IgG-FcR interaction in glomeruli could also mediate macrophage accumulation to induce renal injury [Citation49, Citation50]. Weak classical complement pathway activation and weak binding to IgG Fc receptors on mononuclear cells by IgG4 make it less likely to cause an aggressive inflammatory reaction [Citation51, Citation52]. Further research is needed to determine whether the IgG subclass contributes to a better prognosis in patients with anti-GBM glomerulonephritis in combination with IgAN.
In the case of anti-GBM disease complicated with IgAN, whether GBM damage is mediated by IgG or IgA also raised our attention. Literature review revealed linear IgG deposition on the glomerular basement membrane by renal biopsy in most cases. The high diagnostic accuracy of commercial assays to detect only IgG antibodies against anti-GBM antibodies could be limited to typical anti-GBM disease, and generate a negative result for serum containing non-IgG anti-GBM antibodies. Previous studies found undetectable circulating anti-GBM antibodies such as IgA and IgM on enzyme linked immunosorbent assay (ELISA), indirect immunofluorescence (IF), and western blot (WB) in patients with non-typical anti-GBM disease [Citation1]. Some patients have high affinity antibodies trapped in the kidneys with a low circulation titer. The low titer is not detectable in conventional detection assays, resulting in false negative results. Intriguingly, while Marilina Antonelou [Citation53] described a case of anti-GBM disease combined with IgA nephropathy mediated by IgA anti-GBM antibodies not detected by standard serological tests, however, whether the IgA deposited along GBM or the IgA in mesangium in patients with combined diseases are homologous merits further exploration.
In conclusion, we describe a case of concurrent anti-GBM disease and IgAN, and review the cases published in the literature to better understand the disease. An in-depth examination of the underlying pathogenic relationship between anti-GBM disease and IgAN needs to be conducted in more cases.
Authors’ contributions
Zewei Chen, Dechao Xu, and Fangzheng Cui were involved in the patient case, collected the necessary data, and drafted and finalized the manuscript. Xiang Gao and Huihui Hou was involved in the patient case, delivered the necessary data, and critically revised the manuscript for intellectual content. Xiang Gao and Zhiguo Mao were accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved. All authors contributed to the article and approved the submitted version.
Acknowledgements
The authors appreciate the patient for her patience and cooperation in the diagnostic work-up and follow-up.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bharati J, Yang Y, Sharma P, et al. Atypical anti-glomerular basement membrane disease. Kidney Int Rep. 2023;8(6):1–9. doi: 10.1016/j.ekir.2023.03.010.
- Matsuno T, Okumura T. Anti-glomerular basement membrane disease after diagnosis of immunoglobulin a nephropathy: a case report. Cureus. 2023;15(5):e39737. doi: 10.7759/cureus.39737.
- Zhao J, Yan Y, Cui Z, et al. The immunoglobulin G subclass distribution of anti-GBM autoantibodies against rHalpha3(IV)NC1 is associated with disease severity. Hum Immunol. 2009;70(6):425–429. doi: 10.1016/j.humimm.2009.04.004.
- Borza DB, Chedid MF, Colon S, et al. Recurrent goodpasture’s disease secondary to a monoclonal IgA1-kappa antibody autoreactive with the alpha1/alpha2 chains of type IV collagen. Am J Kidney Dis. 2005;45(2):397–406. doi: 10.1053/j.ajkd.2004.09.029.
- Revert F, Merino R, Monteagudo C, et al. Increased goodpasture antigen-binding protein expression induces type IV collagen disorganization and deposit of immunoglobulin a in glomerular basement membrane. Am J Pathol. 2007;171(5):1419–1430. doi: 10.2353/ajpath.2007.070205.
- Horita S, Nitta K, Honda K, et al. A case of IgA nephropathy with split reduction of alpha5(V) chain of type IV collagen in glomerular basement membrane. Nephron. 1998;80(4):482–483. doi: 10.1159/000045228.
- Ghohestani RF, Rotunda SL, Hudson B, et al. Crescentic glomerulonephritis and subepidermal blisters with autoantibodies to alpha5 and alpha6 chains of type IV collagen. Lab Invest. 2003;83(5):605–611. doi: 10.1097/01.lab.0000067497.86646.4d.
- Shen CR, Jia XY, Cui Z, et al. Clinical and immunological characteristics of patients with combined anti-glomerular basement membrane disease and IgA nephropathy. Clin Kidney J. 2023;16(9):1480–1488. doi: 10.1093/ckj/sfad068.
- Qu W, Liu N, Xu T, et al. Case report: coexistence of anti-glomerular basement membrane disease, membranous nephropathy, and IgA nephropathy in a female patient with preserved renal function. Front Pharmacol. 2022;13(13):e876512. doi: 10.3389/fphar.2022.876512.
- Longano A. Concurrent anti-GBM disease and IgA glomerulonephritis. Pathology. 2019;51(3):336–338. doi: 10.1016/j.pathol.2018.09.065.
- Noor SM, Abuazzam F, Mathew R, et al. IgA nephropathy: a review of existing and emerging therapies. Front Nephrol. 2023;3:1175088. doi: 10.3389/fneph.2023.1175088.
- Trpkov K, Abdulkareem F, Jim K, et al. Recurrence of anti-GBM antibody disease twelve years after transplantation associated with de novo IgA nephropathy. Clin Nephrol. 1998;49(2):124–128.
- Yu C, Yanbei S, Hua Z, et al. A case of anti-glomerular basement membrane disease accompanied with IgA nephropathy. Chin Clin Res. 2023;36(05):758–759.
- Guo C, Ye M, Li S, et al. Anti-glomerular basement membrane disease with IgA nephropathy: a case report. World J Clin Cases. 2022;10(12):3916–3922. doi: 10.12998/wjcc.v10.i12.3916.
- Chen H, Jin J, Cheng MJ, et al. High-frequency plasma exchange therapy for immunocompromised, type I crescentic glomerulonephritis complicated with IgA nephropathy: a case report and literature review. Medicine (Baltimore). 2023;102(3):e32698. doi: 10.1097/MD.0000000000032698.
- Shaojie F, Sensen S, Jingda H, et al. Great prognosis of concurrent anti-GBM disease and IgA nephropathy in a young woman: a case report. Medicine (Baltimore). 2022;101(37):e30686. doi: 10.1097/MD.0000000000030686.
- Bajaj V, Thakur S, Barwad A, et al. IgA nephropathy and atypical anti-GBM disease: a rare dual pathology in a pediatric rapidly progressive glomerulonephritis. Glomerular Dis. 2021;2(1):54–57. doi: 10.1159/000521582.
- Zhang M, Yang D, Wang W, et al. Pneumocystis pneumonia secondary to intensive immunosuppression treatment for anti-GBM disease complicated with IgA nephropathy: a case report and literature review. Medicine (Baltimore). 2021;100(45):e27728. doi: 10.1097/MD.0000000000027728.
- Khor C, Wong MG, Reagh J. Anti-glomerular basement membrane disease and IgA nephropathy in a patient with previous renal cell carcinoma. BMJ Case Rep. 2021;14(7):e236555. doi: 10.1136/bcr-2020-236555.
- Malviya PB, Modigonda S, Maitra S, et al. Anti-glomerular basement membrane disease with atypical associations. Saudi J Kidney Dis Transpl. 2021;32(1):227–231. doi: 10.4103/1319-2442.318529.
- Suh KS, Choi SY, Bae GE, et al. Concurrent anti-glomerular basement membrane nephritis and IgA nephropathy. J Pathol Transl Med. 2019;53(6):399–402. doi: 10.4132/jptm.2019.08.05.
- Kojima T, Hirose G, Komatsu S, et al. Development of anti-glomerular basement membrane glomerulonephritis during the course of IgA nephropathy: a case report. BMC Nephrol. 2019;20(1):25. doi: 10.1186/s12882-019-1207-3.
- Gupta Y, Swain M, Gowrishankar S. Anti-glomerular basement membrane disease combined with IgA nephropathy. Indian J Nephrol. 2019;29(5):375–377. doi: 10.4103/ijn.IJN_309_18.
- Jing S, Ruihai H, Zhifang H, et al. A case of anti-glomerular basement membrane disease accompanied with IgA nephropathy with abdominal distension as the first symptom. People’s Military Surgeon. 2018;61(03):265–266.
- Annamalai I, Chandramohan G, Srinivasa Prasad ND, et al. Rapidly progressive glomerulonephritis due to anti-glomerular basement membrane disease accompanied by IgA nephropathy: an unusual association. Saudi J Kidney Dis Transpl. 2017;28(6):1404–1407. doi: 10.4103/1319-2442.220866.
- Xu D, Wu J, Wu J, et al. Novel therapy for anti-glomerular basement membrane disease with IgA nephropathy: a case report. Exp Ther Med. 2016;11(5):1889–1892. doi: 10.3892/etm.2016.3149.
- Ge YT, Liao JL, Liang W, et al. Anti-glomerular basement membrane disease combined with IgA nephropathy complicated with reversible posterior leukoencephalopathy syndrome: an unusual case. Am J Case Rep. 2015;16:849–853. doi: 10.12659/ajcr.894619.
- Weifeng S, Di W, Shuwang G, et al. One case report of IgA nephropathy complicated with anti-glomerular basement membrane disease and literature review. J Internal Intensive Med. 2015;21(01):40–43.
- Yamaguchi H, Takizawa H, Ogawa Y, et al. A case report of the anti-glomerular basement membrane glomerulonephritis with mesangial IgA deposition. CEN Case Rep. 2013;2(1):6–10. doi: 10.1007/s13730-012-0029-y.
- Wang A, Wang Y, Wang G, et al. Mesangial IgA deposits indicate pathogenesis of anti-glomerular basement membrane disease. Mol Med Rep. 2012;5(5):1212–1214.
- Bixia G, Mingxi L, Wenli X, et al. Anti-glomerular basement membrane disease accompanied with IgA nephropathy: a case report and literature. J Beijing Med. 2009;31(03):185–187.
- Cui Z, Zhao MH, Wang SX, et al. Concurrent anti-glomerular basement membrane disease and immune complex glomerulonephritis. Ren Fail. 2006;28(1):7–14. doi: 10.1080/08860220500461195.
- Yan W, Liping Z, Lin Y, et al. A case of antiglomerular basement membrane disease accompanied with IgA nephropathy. Chin J Nephrol. 2005;09:501.
- Philip R, Dumont A, Martin Silva N, et al. ANCA and anti-glomerular basement membrane double-positive patients: a systematic review of the literature. Autoimmun Rev. 2021;20(9):102885. doi: 10.1016/j.autrev.2021.102885.
- Bu L, Said SM, Herrera Hernandez L, et al. The characteristics of concurrent anti-glomerular basement membrane nephritis and membranous nephropathy. Kidney Int Rep. 2023;8(10):2164–2167. doi: 10.1016/j.ekir.2023.07.031.
- Kantauskaitė M, Laučytė-Cibulskienė A, Miglinas M. Histopathological classification – a prognostic tool for rapidly progressive glomerulonephritis. Medicina (Kaunas). 2018;54(2):17. doi: 10.3390/medicina54020017.
- Maliakkal JG, Hicks MJ, Michael M, et al. Renal survival in children with glomerulonephritis with crescents: a pediatric nephrology research consortium cohort study. J Clin Med. 2020;9(8):2385. doi: 10.3390/jcm9082385.
- Chen Z, Xu J, Wu J, et al. Prognostic analysis of crescentic glomerulonephritis with acute kidney injury: a single-center cohort with 5-year follow-up. Int Urol Nephrol. 2022;54(9):2375–2383. doi: 10.1007/s11255-022-03111-w.
- Cui Z, Zhao J, Jia XY, et al. Anti-glomerular basement membrane disease: outcomes of different therapeutic regimens in a large single-center Chinese cohort study. Medicine (Baltimore). 2011;90(5):303–311. doi: 10.1097/MD.0b013e31822f6f68.
- Yang R, Hellmark T, Zhao J, et al. Levels of epitope-specific autoantibodies correlate with renal damage in anti-GBM disease. Nephrol Dial Transplant. 2009;24(6):1838–1844. doi: 10.1093/ndt/gfn761.
- Zhao J, Cui Z, Yang R, et al. Anti-glomerular basement membrane autoantibodies against different target antigens are associated with disease severity. Kidney Int. 2009;76(10):1108–1115. doi: 10.1038/ki.2009.348.
- Masuda Y, Yamanaka N, Ishikawa A, et al. Glomerular basement membrane injuries in IgA nephropathy evaluated by double immunostaining for α5(IV) and α2(IV) chains of type IV collagen and low-vacuum scanning electron microscopy. Clin Exp Nephrol. 2015;19(3):427–435. doi: 10.1007/s10157-014-1008-8.
- Cui Z, Wang HY, Zhao MH. Natural autoantibodies against glomerular basement membrane exist in normal human sera. Kidney Int. 2006;69(5):894–899. doi: 10.1038/sj.ki.5000135.
- Bowman C, Ambrus K, Lockwood CM. Restriction of human IgG subclass expression in the population of auto-antibodies to glomerular basement membrane. Clin Exp Immunol. 1987;69(2):341–349.
- Qu Z, Cui Z, Liu G, et al. The distribution of IgG subclass deposition on renal tissues from patients with anti-glomerular basement membrane disease. BMC Immunol. 2013;14(1):19. doi: 10.1186/1471-2172-14-19.
- Couser WG, Baker PJ, Adler S. Complement and the direct mediation of immune glomerular injury: a new perspective. Kidney Int. 1985;28(6):879–890. doi: 10.1038/ki.1985.214.
- Otten MA, Groeneveld TW, Flierman R, et al. Both complement and IgG fc receptors are required for development of attenuated anti-glomerular basement membrane nephritis in mice. J Immunol. 2009;183(6):3980–3988. doi: 10.4049/jimmunol.0901301.
- Ma R, Cui Z, Liao YH, et al. Complement activation contributes to the injury and outcome of kidney in human anti-glomerular basement membrane disease. J Clin Immunol. 2013;33(1):172–178. doi: 10.1007/s10875-012-9772-2.
- Hart SP, Jackson C, Kremmel LM, et al. Specific binding of an antigen-antibody complex to apoptotic human neutrophils. Am J Pathol. 2003;162(3):1011–1018. doi: 10.1016/S0002-9440(10)63895-3.
- Kovalenko P, Fujinaka H, Yoshida Y, et al. Fc receptor-mediated accumulation of macrophages in crescentic glomerulonephritis induced by anti-glomerular basement membrane antibody administration in WKY rats. Int Immunol. 2004;16(5):625–634. doi: 10.1093/intimm/dxh058.
- Ohlsson S, Herlitz H, Lundberg S, et al. Circulating anti-glomerular basement membrane antibodies with predominance of subclass IgG4 and false-negative immunoassay test results in anti-glomerular basement membrane disease. Am J Kidney Dis. 2014;63(2):289–293. doi: 10.1053/j.ajkd.2013.08.032.
- Cui Z, Zhao MH, Singh AK, et al. Antiglomerular basement membrane disease with normal renal function. Kidney Int. 2007;72(11):1403–1408. doi: 10.1038/sj.ki.5002525.
- Antonelou M, Henderson SR, Bhangal G, et al. Binding truths: atypical anti-glomerular basement membrane disease mediated by IgA anti-glomerular basement membrane antibodies targeting the α1 chain of type IV collagen. Kidney Int Rep. 2018;4(1):163–167. doi: 10.1016/j.ekir.2018.08.005.

