Abstract
Objective
In China, most of the patients who underwent kidney transplants have unknown causes of end-stage renal disease (uESRD). However, little is known regarding the incidence of graft glomerulonephritis (GN) and graft survival in kidney transplant recipients (KTRs) with uESRD.
Methods
In this retrospective cohort study, 473 of the 565 KTRs who underwent kidney transplantation (KTx) from 2015 to 2020 were included. We mainly observed the occurrence of graft GN between uESRD group and definitively diagnosed GN group, and repeatedly compared after propensity score matching (PSM).
Results
The median follow-up was 50 months in 473 KTRs, and about 75% of KTRs of native kidney disease of unknown etiology. The total cumulative incidence of graft GN was 17%, and no difference was observed between the definitively diagnosed GN group and the uESRD group (p = 0.76). Further, PSM analysis also showed no difference in the incidence of graft GN between the 2 groups. Multivariable analysis disclosed males (p = 0.001), younger age (p = 0.03), and anti-endothelial cell anti-body (AECA) positive pre-KTx (p = 0.001) were independent risk factors for graft GN.
Conclusions
The incidence of graft GN was similar between uESRD and definitively diagnosed GN group. The allograft survival was also similar between two groups.
1. Background
Kidney transplantation (KTx) is the preferred therapy for end-stage renal disease (ESRD) patients, with lower mortality, reduced risk of cardiovascular events, and improved quality of life compared with dialysis treatment [Citation1]. However, various causes still affect the survival of allograft. El-Zoghby et al. [Citation2] concluded that 11.6% of kidney transplant recipients (KTRs) had graft failure during a mean follow-up of 50.3 months, and renal allograft failure due to GN account for 18–36.2% of all cases [Citation2–4]. KTRs with glomerular diseases had an increased risk of graft failure than patients without glomerular diseases [Citation5–7]. About 28% of KTRs had renal failure due to primary or secondary glomerulopathy [Citation8].
In the Chinese population, most KTRs have an unknown etiology of ESRD, and hence lack of comprehensive assessment of primary kidney disease before KTx, which may contribute to difficulty in the postoperative management. Data from our center showed that only 15% of KTRs underwent native kidney biopsies before transplantation. To date, there have been limited studies focused on the KTRs with uESRD, no study reports the incidence of graft GN and graft outcomes of KTRs with uESRD in the Chinese population even if there were majorities of patients with uESRD in China.
2. Method
2.1. Study design
This is a retrospective study to compare the incidence of graft GN in uESRD recipients and definitively diagnosed GN recipients. Recipients who underwent KTx from January 2015 to December 2020 were included in our study. First, we compared the incidence of graft GN and graft survival of KTRs with uESRD and KTRs with definitively diagnosed GN, explored the risk factors of graft GN. Second, uESRD recipients and definitively diagnosed GN recipients were matched 1:1 according to some covariates which affect the primary outcome (graft GN) through the PSM method with a caliper width of 0.01. Third, we explored the occurrence of graft GN in the 2 groups after matching. This study was approved by the Clinical Research Ethics Committee of Jingling Hospital (ID: 2022DZKY-079-02). The requirement for informed consent was waived owing to the observational study design. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the ‘Declaration of Istanbul on Organ Trafficking and Transplant Tourism.’
2.2. Study population
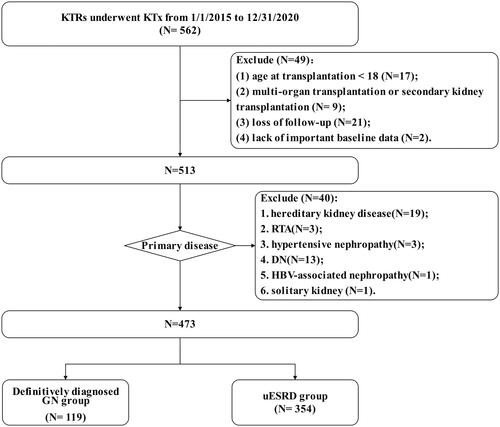
A total of 473 KTRs were included according to the inclusion and exclusion criteria (). All patients received the unified postoperative treatment strategy of our center, including drug regimens (postoperative triple immunosuppressive therapy with calcineurin inhibitors + mycophenolic acid + steroid was applied, and sulfamethoxazole and valganciclovir were applied in the early postoperative period to prevent infection) and uniformed indicators for renal graft biopsy. Protocol biopsy is strongly recommended in KTRs with high immunological risk in renal allograft [Citation9, Citation10]. Indication biopsy of the renal allograft is performed for the presence of delayed graft function [Citation11,Citation12], oliguria (less than 50 mL/24 h), anuria (less than 400 mL/24 h), unexplained Scr elevation of more than 30% of the basal level [Citation13], persistent urinalysis abnormalities (e.g., 24 h proteinuria greater than 1 g/24 h, 100 or more urine red blood cells/high power field) [Citation14,Citation15]. We excluded one patient thought to have hypertensive nephropathy and 13 patients with diabetic nephropathy (DN) due to unclear diagnosis (either not confirmed by rigorous renal biopsy or an atypical presentation of the renal biopsy with a clinical diagnosis of diabetic or hypertensive nephropathy). Most KTRs were followed up regularly in the outpatient department post-KTx, and we also communicated with them over the phone about the latest laboratory test results. The follow-up deadline was in September 2022. Patients were followed-up until the date of the last visit, graft loss or death with graft function.
2.3. Study outcome
The primary outcome was graft GN confirmed by renal graft biopsy. The second outcome included death-censored graft loss. Renal graft loss was defined as return to dialysis, re-transplantation, and graft removal, excluding death with graft function.
2.4. Data collection and definition
The clinical information was collected from electronic medical records by two observers independently, including the baseline data, surgical situation, laboratory data during the follow-up post-KTx (including serum creatinine [Scr], eGFR, urine protein, and urine erythrocyte), patient/graft survival, postoperative adverse events (acute rejection, infection), and hospitalizations. Abnormal urinalysis was defined as the presence of proteinuria ≥ 1+ or hematuria ≥2+ by dipstick urinalysis testing. Graft GN was defined as the presence of immune-mediated endothelial damage to the basement membrane, the mesangium, or the capillary endothelium of the graft as confirmed by renal allograft biopsy. The pathological changes of the renal graft were assessed by a pathologist (M.C.Z).
2.5. Statistical analyses
Numerical results are presented as means (±SD) or medians [interquartile ranges (IQRs)]. Categorical data were compared with the use of either the chi-square or Fisher’s exact test, continuous variables were analyzed by Student’s t-test (parametric data) or a Mann–Whitney U test (nonparametric data). Univariate analysis using binary logistic regression and multivariable logistic regression was used to estimate the correlations between variables and primary study outcome, odd ratio (OR) and 95% confidence intervals (CIs) were calculated from the logistic regression model. Variables with p value <0.25 in the univariate analyses were candidates for the multivariate model, according to the Enter method. We used the Kaplan–Meier method to estimate the cumulative incidence of graft GN and graft survival, and the log-rank test was used as an analysis for comparisons between groups. SPSS Statistics version 22 (SPSS Inc., Chicago, IL) was used for statistical analyses and GraphPad Prism 8.0.2 (GraphPad Software, La Jolla, CA) for mapping. PSM was performed using the SPSS-R plugin. p Value below 0.05 was considered statistically significant.
2.5.1. Propensity score matching
This is a retrospective observational study, the grouping (definitively diagnosed GN group and uESRD group) is naturally formed, and there may be confounding influencing factors between the groups. For example, older recipients may not be able to undergone native kidney diagnostic biopsy due to various factors (such as risk of renal puncture), and younger recipients are more likely to pay attention to the diagnosis and treatment of primary kidney disease. Confounding factor between groups may affect the conclusion of the study. PSM was performed to minimize baseline differences between definitively diagnosed GN group and uESRD group. Patients were matched by propensity score using nearest neighbor matching approach with no replacement. The ratio was paired at 1:1 and the caliper size was 0.01 according to the propensity scores. We evaluated the balance of covariates by estimating standardized differences before and after matching, and the standardized mean difference <0.1 was considered appropriate balance of variables between two groups.
3. Results
3.1. Patients characteristics
The baseline clinical data and clinical information of 473 KTRs are shown in . In the total cohort, most patients were male (70%), and more than 10% of KTRs were positive for AECA pre-KTx. Before transplantation, the predominant subtypes in GN group included IgAN (76/119, 64%), focal segmental glomerulosclerosis (FSGS) (15/119, 13%), mesangial proliferative glomerulonephritis (MesGN) (7/119, 6%) (Supplemental Figure 1). The definitively diagnosed GN group (Hereafter referred to as GN group) and uESRD group had differences in gender, incidence of young onset (<18 years), and type of dialysis. KTRs with definitively diagnosed GN had a higher rate of deceaed donor (DD) transplant (p = 0.04), and a higher rate of infection (within 1-month post-KTx) (p = 0.05).
Table 1. Baseline data for kidney transplant recipients.
3.2. Incidence of graft GN
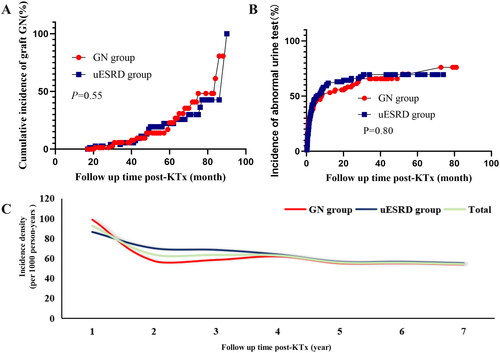
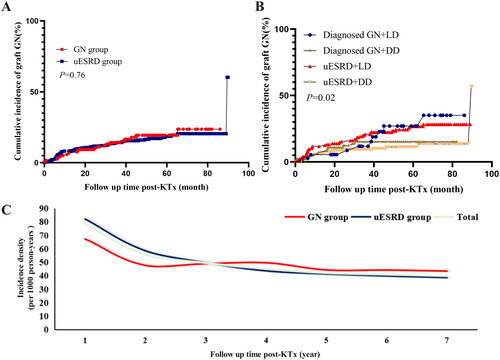
The median follow-up was 50 months (IQR: 34–66 months) post-KTx. During the follow-up period, a total of 80 KTRs (21 KTRs in the GN group and 59 KTRs in the uESRD group) developed biopsy-proved graft GN, accounting for 17% of the total cohort. In the GN group, 13 KTRs (13/21, 62%) had recurrent GN and 8 KTRs (8/21, 38%) developed de novo GN after KTx. The reason for performing allograft biopsy included protocol biopsy (n = 26, 32.5%), abnormal urine test (n = 28, 35%), and elevated Scr level (n = 26, 32.5%). Significantly, there was no difference in cumulative incidence of graft GN between the two groups (21/119 vs. 59/354, p = 0.76, ). We further conducted a subgroup analysis of KTRs according to the primary disease (definitively diagnosed GN or uESRD) and donor type (living donor [LD] or DD), which showed that there were statistical differences in cumulative incidence of graft GN among groups (, p = 0.02).
Figure 2. The incidence of graft GN among groups. (A) the cumulative incidence of graft GN between GN group and uESRD group; (B) the cumulative incidence of graft GN after receiving LD or DD transplant in recipients with diagnosed GN or uESRD; (C) Incidence density of graft GN in KTRs among groups. GN: glomerulonephritis; uESRD: unknown causes of end-stage renal disease; LD: living donor; DD: deceased donor.
In this study, follow-up was terminated due to loss of graft function (N = 13) or death (N = 4) in some patients during follow-up, and the duration of follow-up was not consistent across patients, we therefore assessed the incidence density of graft GN at each follow-up time point (postoperative years 1, 2, 3, 4, 5, 6, and 7) (, Supplemental Table 1). Interestingly, we found that the incidence density was higher in the uESRD group than in the GN group within 3 years, whereas the incidence densities of the two groups were roughly the same after 2 years. Three years post-KTx, the annual incidence density for both groups was around 43 per 1000 person-years (Supplemental Table 1).
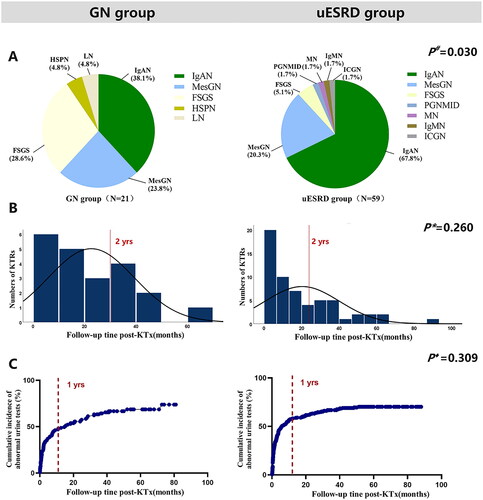
The distribution, diagnostic time from KTx and occurrence time of abnormal urine test were summarized in . In the GN group, the main GN subtypes of graft GN included IgAN (38%) and FSGS (29%), followed by MesGN (24%), Henoch–Schonlein purpura nephritis (HSPN) (5%), and lupus nephritis (LN) (5%); In the uESRD group, up to 68% of graft GN was IgAN, followed by MesGN (20%). A significant difference was observed in the graft GN subtypes among the two groups (, p = 0.03). The median diagnostic time from KTx in the GN group was 17 and 13 months in the uESRD group, and no difference was found between the two groups (, p = 0.26). One year post-KTx, the cumulative incidence of abnormal urine test of KTRs in the GN group was more than 48%, and that in the uESRD group was more than 58%, and there was no difference in the occurrence of abnormal urine test between the two groups (, p = 0.31).
Figure 3. The distribution of GN subtypes (A), diagnosis time (B), and incidence of abnormal urine test (C) in GN group and uESRD group. #Distribution of glomerular disease subtypes in GN group and uESRD group after transplantation; *Time of GN diagnosis in GN group and ESRD group; ♦ Occurrence of postoperative abnormal urine test in graft GN KTRs in GN group and uESRD group. LN, lupus nephritis; HSPN: Henoch–Schonlein purpura nephritis; MesGN, mesangial glomerulonephritis; FSGS, focal segmental glomerulosclerosis; PGNMID, proliferative glomerulonephritis with monoclonal IgG deposits; MN, membranous nephropathy; IgMN, IgM nephropathy; ICGN, Immune complex glomerulonephritis; GN, glomerulonephritis; uESRD, unknown causes of end-stage renal disease.
3.3. Graft survival
The 5-year death-censored graft survival was 96%. There were 13 KTRs had graft loss during the follow-up (3%) and 4 KTRs died with graft function. There was no difference in terms of death-censored graft survival (Supplemental Figure 2). Seven out of 80 patients diagnosed with graft GN had lost the graft, while 6 out of 393 patients without graft GN had graft loss. The graft survival of the no graft GN group was better than that graft GN group (7/80 vs. 6/393, p = 0.001) (Supplemental Figure 3).
3.4. Risk factors of graft GN
Univariate analysis showed that LD (p < 0.001), male (p = 0.001), age at transplantation (p = 0.001), positive AECA pre-KTx (p = 0.002), shorter cold ischemia time (p = 0.002) were risk factors for graft GN (). Multivariate analysis revealed that males, younger age at transplantation, and positive ACEA were independent risk factors for graft GN (). The risk of graft GN in male KTRs was 3.113 times higher than that in female KTRs (OR3.114, 95%CI 1.562–6.206, p = 0.001); KTRs with positive ACEA pre-KTx had 2.942 times higher risk of graft GN than ACEA-negative recipients (OR 2.942, 95%CI 1.545–5.600, p = 0.001); Age was a protective factor for graft GN, the risk of graft GN decreased by 3% for each increase of the age of 1 year at transplantation (OR 0.968, 95%CI 0.939–0.997, p = 0.03).
Table 2. Analysis of risk factors of graft GN.
3.5. After matching
3.5.1. Patients characteristics
In order to further explore the effects of primary kidney disease (definitively diagnosed GN or uESRD) on graft GN, we matched GN group and uESRD group 1:1 according to age at transplantation, gender, donor type (LD or DD), AECA, and cold ischemia time through PSM. shows the comparison of baseline data between the two groups after matching. A total of 81 pairs of KTRs were successfully matched and covariates that were not balanced between the two groups were balanced after matching. The balance of each covariate across the two comparison groups was significantly improved and the standardized differences were controlled within 10% after matching (Supplemental Figure 4).
Table 3. Comparison of clinical characteristics after propensity score matching.
3.5.2. Incidence of graft GN
A total of 36 KTRs developed graft GN (18 in the GN group and 18 in the uESRD group). After matching, the cumulative incidence of graft GN showed no correlation with primary kidney disease (definitively diagnosed GN or uESRD) (Log-rank, p = 0.55, ). Similarly, the incidence of abnormal postoperative urinalysis did not differ between the GN group and uESRD group after matching (Log-rank, p = 0.80, ). We also assessed the incidence density of graft GN in each stage of KTRs (postoperative years 1, 2, 3, 4, 5, 6, and 7) in both groups after propensity score matching (PSM). Again, we found that the incidence density of graft GN was higher in the uESRD group than in the GN group within three years post-KTx, whereas it was nearly the same (55 per 1000 person-years) in the two groups as the follow-up time increased (, Supplemental Table 2).
Discussion
Several large cohort studies have shown that a significant proportion of KTRs has an unknown cause of ESRD. About 27–58% of KTRs received KTx for an unidentified cause of ESRD [Citation16–19]. In China, KTRs with uESRD are very common. These patients usually present to the hospital with acute symptoms (e.g., dizziness, nausea, and vomiting) and are found to have high creatinine levels and reduced kidney volume on renal ultrasound. Most of the patients initiate maintenance dialysis treatment in a short period. Given a few studies on KTRs with uESRD, it is necessary to explore the incidence of graft GN which is closely related to graft outcomes to benefit clinical management.
It has been estimated that 45–82% of patients were primary glomerular diseases, which was the most common renal disease [Citation20–22]. The prevalence of allograft GN increases with the duration of follow-up [Citation4], the cumulative incidence was 5.2%, 18.2%, 21.7%, 35.8%, and 42.3% of recipients at 1, 3,5, 8, and 10 years post-KTx [Citation23]. Kim et al. reported that the cumulative incidence of graft GN among KTRs with uESRD was significantly lower than KTRs with primary IgAN and was similar to that of primary DN, and IgAN was the most common subtype in KTRs with uESRD post-KTx [Citation16]. In this study, a total of 119 patients had GN as the primary disease, among which IgAN accounted for 64%, as the most common subtype of GN. Of note, in the Chinese population, we observed a similar incidence of cumulative graft GN in KTRs with definitively diagnosed GN and KTRs with uESRD (18% vs. 17%), which was also discovered after matching. Those two groups had a significant difference in graft GN subtype: about 68% of KTRs with uESRD were diagnosed with IgAN post-KTx, which may be related to the high incidence of IgAN in the Chinese population. In this study, some patients did not undergo graft biopsy (n = 332, 70.2%), 176 of which (176/332, 53.0%) were unwilling to perform biopsy because of stable condition (stable Scr levels and a normal urinalysis), and 131 of patients (131/332, 39.5%) with abnormal urinalysis were relieved by regimen adjustment on an outpatients basis. For this reason, we compared the cumulative incidence of abnormal urine test between the GN group and uESRD group, and no difference was seen before (p = 0.31) and after matching (p = 0.80).
We also found that the cumulative incidence of graft GN was statistically different among four groups (p = 0.02) after grouping the KTRs according to the primary disease (definitively diagnosed GN or uESRD) and donor type (LD or DD), among which the incidence of recipients receiving LD was higher. The recurrent rates of IgAN [Citation24–27] were significantly higher in LD KTRs compared to deceased donor KTRs. More than 50% of graft GN in the uESRD group was IgAN, some of these patients may have IgAN as a primary disease and develop recurrent IgAN post-transplant. Significantly, the relative risk of developing IgA was 16 times higher in first-degree relatives and above two times higher in second-degree relatives in patients with familial IgAN [Citation28]. This may increase the risk of post-transplant IgAN in KTRs receiving LD transplants.
This study further explored the risk factors for graft GN and showed that younger age, male was independent risk factors for graft GN, which is in agreement with the previous studies [Citation3]. This may be due to the higher immunoreactivity in male and younger KTRs, as well as insufficient doses of immunosuppressive regimens based on body weight alone. Also, younger recipients imply earlier onset, more rapid disease progression or ineffectiveness of conventional immunotherapy regimens, which suggests more aggressive course of the disease pre-KTx, and therefore may be at higher risk of graft GN post-KTx. However, no study has confirmed the association between AECA positivity and the incidence of graft GN. Initially, Gobel et al. found that in patients with Wegener’s granulomatosis, the level of AECAs was associated with the activity and the lapse to of the disease [Citation29]. The AECA-IgG titer was significantly higher in patients with lupus enteritis or lupus erythematosus than in healthy controls [Citation30]. The discovery that positive AECA pre-KTx may be related to the graft GN needs to be confirmed by further large-scale and prospective studies.
We did not observe the difference in graft survival between KTRs with definitively diagnosed GN and KTRs with uESRD (p = 0.93) when there was no difference in postoperative adverse events and follow-up time. Previous studies have reported that the five-year graft survival of KTRs with uESRD was not inferior to KTRs with primary IgAN and was better than KTRs with primary DN [Citation16]. It is important to point out that compared with KTRs with diagnosed GN, KTRs with uESRD had a significantly lower incidence of GN-related death-censored graft failure (1% vs. 5%), lower overall 5- and 10-year graft failure (79% and 63% vs. 84% and 67%), and lower death-censored graft loss [Citation17]. In terms of acute rejection post-KTx, there was no difference between patients with uESRD and patients with primary IgAN or primary DN [Citation16] (22.9% vs. 20.0% vs. 24.4%). In our study, there was no difference in the incidence of acute rejection between the GN group and uESRD group, nor did the graft survival rate, which was consistent with previous studies. IgAN was the main subtype in KTRs with definitively diagnosed GN, and IgAN also accounted for more than half of the graft GN in KTRs with uESRD. To our knowledge, the influence of graft GN on graft survival is closely related to the subtype of GN, and KTRs with IgAN have the highest graft survival rate among all the subtypes of GN [Citation6,Citation31]. Therefore, the high graft survival rate of the two groups may be related to the highest prevalence of IgAN in the Chinese population.
This study has some limitations. First, this was a single-center retrospective study. Second, some patients may miss the diagnosis due to the lack of protocol biopsy and the incidence of graft GN may be underestimated. From this, we also compared the occurrence of urinalysis abnormalities in the two groups. Third, considering that some GN subtypes have an insidious onset or late onset (such as IgAN), the follow-up time in this study was somewhat short (median follow-up time 50 months), and we will continue to follow up in the future. Fourth, the geographically restrictive nature of the included population may affect the reference value of the study results, and further validation by national multi-center study is needed in the future.
In conclusion, the cumulative incidence of graft GN and death-censored graft failure of KTRs with uESRD were similar to that of KTRs with diagnosed GN. Male, younger age at KTx, and positive AECA pre-KTx were independent risk factors for graft GN. For KTRs with uESRD, IgAN was the most common subtype of graft GN.
Author contributions
All authors contributed to the conception of the study. WJQ and WQQ participated in research design. NXF, WQQ, CJS, CDR, XKN, LX, and WJQ participated in patient management and data collection. The manuscript was drafted and written by WQQ and NXF. ZMC was involved in biopsy interpretation. All authors read and approved the final manuscript.
Supplemental Material
Download PDF (466 KB)Disclosure statement
All the authors declared no competing interests.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Additional information
Funding
References
- Tonelli M, Wiebe N, Knoll G, et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant. 2011;11(10):1–10. doi: 10.1111/j.1600-6143.2011.03686.x.
- El-Zoghby ZM, Stegall MD, Lager DJ, et al. Identifying specific causes of kidney allograft loss. Am J Transplant. 2009;9(3):527–535. doi: 10.1111/j.1600-6143.2008.02519.x.
- Chailimpamontree W, Dmitrienko S, Li G, et al. Probability, predictors, and prognosis of posttransplantation glomerulonephritis. J Am Soc Nephrol. 2009;20(4):843–851. doi: 10.1681/ASN.2008050454.
- Sellarés J, de Freitas DG, Mengel M, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12(2):388–399. doi: 10.1111/j.1600-6143.2011.03840.x.
- Hariharan S, Adams MB, Brennan DC, et al. Recurrent and de novo glomerular disease after renal transplantation: a report from renal allograft disease registry (RADR). Transplantation. 1999;68(5):635–641. doi: 10.1097/00007890-199909150-00007.
- O’Shaughnessy MM, Liu S, Montez-Rath ME, et al. Kidney transplantation outcomes across GN subtypes in the United States. J Am Soc Nephrol. 2017;28(2):632–644. doi: 10.1681/ASN.2016020126.
- Park H, Park WY, Kang SS, et al. Clinical outcomes of kidney transplantation in patients with biopsy-Proven glomerulonephritis. Transplant Proc. 2018;50(4):1009–1012. doi: 10.1016/j.transproceed.2018.02.039.
- Dabade TS, Grande JP, Norby SM, et al. Recurrent idiopathic membranous nephropathy after kidney transplantation: a surveillance biopsy study. Am J Transplant. 2008;8(6):1318–1322. doi: 10.1111/j.1600-6143.2008.02237.x.
- Nankivell BJ, Chapman JR. The significance of subclinical rejection and the value of protocol biopsies. Am J Transplant. 2006;6(9):2006–2012. doi: 10.1111/j.1600-6143.2006.01436.x.
- Huang Y, Farkash E. Protocol biopsies: utility and limitations. Adv Chronic Kidney Dis. 2016;23(5):326–331. doi: 10.1053/j.ackd.2016.09.002.
- Kasiske BL, Zeier MG, Chapman JR, et al. KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney Int. 2010;77(4):299–311. doi: 10.1038/ki.2009.377.
- Bia M, Adey DB, Bloom RD, et al. KDOQI US commentary on the 2009 KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Kidney Dis. 2010;56(2):189–218. doi: 10.1053/j.ajkd.2010.04.010.
- Kasiske BL, Andany MA, Danielson B. A thirty percent chronic decline in inverse serum creatinine is an excellent predictor of late renal allograft failure. Am J Kidney Dis. 2002;39(4):762–768. doi: 10.1053/ajkd.2002.31996.
- Halimi JM. Low-grade proteinuria and microalbuminuria in renal transplantation. Transplantation. 2013;96(2):121–130. doi: 10.1097/TP.0b013e31828719fb.
- Parajuli S, Swanson KJ, Alstott J, et al. Transplant kidney biopsy for proteinuria with stable creatinine: findings and outcomes. Clin Transplant. 2021;35(10):e14436.
- Kim HJ, Kim H, Cho HS, et al. Outcomes of kidney allograft in recipients with kidney disease of unknown etiology. Clin Transplant. 2013;27(6):866–874. doi: 10.1111/ctr.12247.
- Lim WH, Wong G, McDonald SP, et al. Long-term outcomes of kidney transplant recipients with end-stage kidney disease attributed to presumed/advanced glomerulonephritis or unknown cause. Sci Rep. 2018;8(1):9021. doi: 10.1038/s41598-018-27151-4.
- Foroncewicz B, Mucha K, Florczak M, et al. Long-term outcome of renal transplantation: a 10-year follow-up of 765 recipients. Pol Arch Intern Med. 2019;129(7–8):476–483.
- Szymańska A, Mucha K, Kosieradzki M, et al. Organization of post-transplant care and the 5-year outcomes of kidney transplantation. Int J Environ Res Public Health. 2022;19(4):2010. doi: 10.3390/ijerph19042010.
- Zuo L, Wang M. Chinese association of blood purification management of chinese hospital A. Current burden and probable increasing incidence of ESRD in China. Clin Nephrol. 2010;74(1):S20–S22.
- Xu X, Ning Y, Shang W, et al. Analysis of 4931 renal biopsy data in Central China from 1994 to 2014. Ren Fail. 2016;38(7):1021–1030. doi: 10.1080/0886022X.2016.1183443.
- Hou JH, Zhu HX, Zhou ML, et al. Changes in the spectrum of kidney diseases: an analysis of 40,759 biopsy-Proven cases from 2003 to 2014 in China. Kidney Dis (Basel). 2018;4(1):10–19. doi: 10.1159/000484717.
- Cosio FG, Cattran DC. Recent advances in our understanding of recurrent primary glomerulonephritis after kidney transplantation. Kidney Int. 2017;91(2):304–314. doi: 10.1016/j.kint.2016.08.030.
- Andresdottir MB, Hoitsma AJ, Assmann KJ, et al. Favorable outcome of renal transplantation in patients with IgA nephropathy. Clin Nephrol. 2001;56(4):279–288.
- Kennard AL, Jiang SH, Walters GD. Increased glomerulonephritis recurrence after living related donation. BMC Nephrol. 2017;18(1):25. doi: 10.1186/s12882-016-0435-z.
- Wang AY, Lai FM, Yu AW, et al. Recurrent IgA nephropathy in renal transplant allografts. Am J Kidney Dis. 2001;38(3):588–596. doi: 10.1053/ajkd.2001.26885.
- Han SS, Huh W, Park SK, et al. Impact of recurrent disease and chronic allograft nephropathy on the long-term allograft outcome in patients with IgA nephropathy. Transpl Int. 2010;23(2):169–175. doi: 10.1111/j.1432-2277.2009.00966.x.
- Schena FP, Cerullo G, Rossini M, et al. Increased risk of end-stage renal disease in familial IgA nephropathy. J Am Soc Nephrol. 2002;13(2):453–460. doi: 10.1681/ASN.V132453.
- Göbel U, Eichhorn J, Kettritz R, et al. Disease activity and autoantibodies to endothelial cells in patients with wegener’s granulomatosis. Am J Kidney Dis. 1996;28(2):186–194. doi: 10.1016/s0272-6386(96)90300-5.
- Kwok SK, Seo SH, Ju JH, et al. Lupus enteritis: clinical characteristics, risk factor for relapse and association with anti-endothelial cell antibody. Lupus. 2007;16(10):803–809. doi: 10.1177/0961203307082383.
- Pippias M, Stel VS, Aresté-Fosalba N, et al. Long-term kidney transplant outcomes in primary glomerulonephritis: analysis from the ERA-EDTA registry. Transplantation. 2016;100(9):1955–1962. doi: 10.1097/TP.0000000000000962.