Abstract
According to the Global Burden of Disease (GBD) study, chronic kidney disease (CKD) was prevalent in 697.5 million individuals worldwide in 2017. By 2040, it is anticipated that CKD will rank as the fifth most common cause of death. This study aims to examine the epidemiology of CKD in Kazakhstan and to project future trends in CKD prevalence and mortality by 2030. The retrospective analysis was performed on a database acquired from the Unified National Electronic Health System for 703,122 patients with CKD between 2014 and 2020. During the observation period, 444,404 women and 258,718 men were registered with CKD, 459,900 (66%) were Kazakhs and 47% were older than 50. The incidence rate notably decreased: 6365 people per million population (PMP) in 2014 and 4040 people PMP in 2020. The prevalence changed from 10,346 to 38,287 people PMP, and the mortality rate increased dramatically from 279 PMP to 916 PMP. Kazakhstan’s central regions, Turkestan and Kyzylorda were identified as the most burdensome ones. The ARIMA model projected 1,504,694 expected prevalent cases in 2030. The predicted mortality climbed from 17,068 cases in 2020 to 37,305 deaths in 2030. By 2030, the prevalence and mortality of CKD will significantly increase, according to the predicted model. A thorough action plan with effective risk factor management, enhanced screening among risk populations, and prompt treatment are required to lessen the burden of disease in Kazakhstan.
Introduction
Chronic kidney disease (CKD) is a progressive condition characterized by irreversible structural and functional changes in the kidneys. The Global Burden of Disease (GBD) study indicated that in 2017, there were 697.5 million prevalent cases of CKD around the world, and 1.2 million people died from this condition [Citation1]. It is projected that by 2040, CKD will be the fifth leading cause of death [Citation2]. Although the GBD study shows that there were approximately 1.7 million prevalent cases and 1485 deaths from CKD in Kazakhstan, they have used geographical proximities and statistical methods to estimate the epidemiology of disease for countries with no data and lack of sources [Citation1].
Chronic kidney disease does not have a cure; however, the early diagnostics and treatment can prevent disease progression, while kidney replacement therapy in end-stage kidney disease (ESKD) can increase the life expectancy. Moreover, CKD was associated with increased mortality of patients with cardiovascular diseases (CVDs), hypertension, and diabetes [Citation3–5]. The development of nephrology and focus on the treatment of early CKD stages can improve mortality among patients and prevent the progression of ESKD. Therefore, it is important to investigate the burden of CKD and increase the awareness of the general population and healthcare policymakers.
In our previous study [Citation6], we reported the epidemiology of dialysis-treated ESKD in Kazakhstan for the period of 2014–2018 years using big data from the Unified National Electronic Healthcare System (UNEHS) [Citation7]; however, in-depth epidemiology of CKD over the country has never been studied. Forecasting the future trend of CKD for the next 10 years and being prepared for expected CKD cases with appropriate preventive measures are very important. This study aims to investigate the epidemiology of CKD in Kazakhstan, the largest Central Asian country, and to forecast future trends of CKD prevalence and mortality until 2030.
Methods
Study design and population
The data for this retrospective study are extracted from the UNEHS for 2014–2020. The information on UNEHS and its databases are described elsewhere [Citation7]. According to the International Classification of Diseases (ICD) codes, patients who had the ICD-10 code for CKD were included in the study. The list of all ICD-10 codes is presented in Supplementary Table 1. All primary and secondary ICD-10 codes were captured during the cohort set-up. After data cleaning and management, 703,122 people with unique RPN IDs were left in the cohort. Population number was obtained from the Statistics Committee of the Ministry of National Economy of the Republic of Kazakhstan [Citation8].
The study involved secondary data that was derived from the UNEHS. Patients were not involved in the study. Therefore, the requirement for informed consent from study participants was waived by the Nazarbayev University Institutional Review Ethics Committee (NU-IREC 490/18112021).
Exposures and covariates
The information on the date of birth, gender, ethnicity, diagnosis date, and date of death, if occurred, was included in the analysis. Age was categorized as following (1): below 18 years old (y.o.) (2), 18–34 y.o. (3), 35–50 y.o. (4), 51–70 y.o., and (5) above 70 y.o. In Kazakhstan, there are more than 120 nationalities with the major prevalence of Kazakh, followed by Russians and other different minorities; therefore, ethnicity was divided into Kazakhs, Russians, and others. The causes of CKD were categorized as congenital kidney diseases, diabetic nephropathy, glomerular diseases, hypertensive nephropathy, obstructive nephropathy, tubulointerstitial diseases, unknown-CKD, and others. The comorbid conditions of the CKD such as essential hypertension, CVDs, diabetes, chronic obstructive pulmonary disease (COPD) and liver disease based on ICD-10 identification codes were collected by merging the databases using unique RPN IDs. The ICD-10 codes defining abovementioned conditions are presented in Supplementary Table 1.
Outcome assessment
The incidence, prevalence, and all-cause mortality rates in the cohort were evaluated. Death occurring at any time during the observation period was accounted as all-cause death. The rates were calculated by dividing the absolute numbers by the total general population size by the end of each year. The crude mortality rate was calculated as the number of deaths each year divided by the total person-time at risk in the corresponding period. For the survival analysis, the start date was the first day of the first admission, and the follow-up was until 31 December 2020, or until the day of death, if occurred.
Statistical analysis
All characteristics are presented as categorical variables. Incidence, prevalence, and all-cause mortality rates were evaluated as absolute numbers and rates per 1,000,000 population for each year of the observation period. The incidence, prevalence, and mortality for the last year of observation are presented on a map to evaluate the regional disparities within the country. Maps were generated using QGIS 3.16.11 Hannover version (https://www.qgis.org).
Kaplan–Meier’s estimates and Log-rank tests were used to demonstrate crude survival and the significance of difference. After checking the corresponding assumptions, Cox’s regression analyses were performed to demonstrate crude and adjusted hazard ratios. We constructed three sets of multivariable analysis models to test the adjusted effect of variables on mortality. The models were incrementally adjusted for potential confounders depending on theoretical background and their availability in the database. In the first model, only socio-demographic predictors (age, gender, and ethnicity) were included. In the second model, we added the causes of CKD to model 1, and in the third model we added comorbidities to model 2. In all models, the stepwise selection method was used. The fit of the models was evaluated by Akaike information criterion, Bayesian information criterion, and global goodness-of-fit test. The significance level was set at .05. The autoregressive integrated moving average (ARIMA) model was used to forecast the prevalence and mortality for CKD until 2030. All statistical analysis was performed using STATA 16.1 (College Station, TX).
Results
Socio-demographic characteristics
The socio-demographic and medical characteristics of the cohort are presented in . During 2014–2020, 444,404 (63%) women and 258,718 (37%) men had reported CKD in UNEHS. The majority of the cohort (335,267; 47%) were older than 50 years of age, and of Kazakh ethnicity (459,000; 66%). Concurrent hypertension, diabetes, and COPD were presented in 24%, 6%, and 3% of the cohort, respectively. Cardiovascular diseases were found in 21% of the cohort, majority (58,477; 8%) had heart failure (HF).
Table 1. Socio-demographic and medical characteristics of CKD patients between 2014 and 2020.
Prevalence, incidence, and mortality
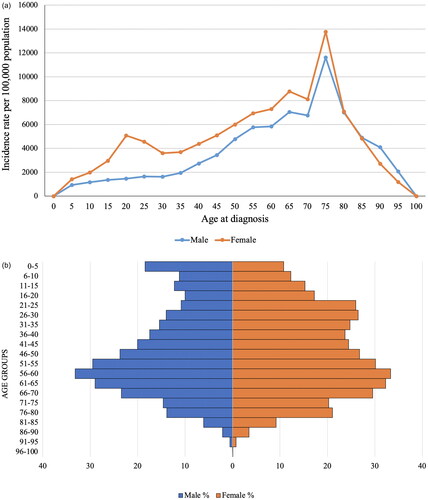
The prevalence of CKD increased dramatically from 10,346 people per million population (PMP) in 2014 to 38,287 people PMP in 2020 (). The incidence dropped from 6365 people PMP to 4040 people PMP over the observation period, while all-cause mortality tripled from 279 people PMP to 916 people PMP. The age- and sex-specific incidence rate (IR) indicates that CKD occurrence gradually rises in both males and females. For females, it notably peaks until the age of 80, with a slight increase between the ages of 20–25 (). The peak of morbidity is 75 years of age for both sexes.
Figure 1. Prevalence, incidence, and all-cause mortality of CKD patients in Kazakhstan in 2014–2020 by years: (a) absolute numbers; (b) rates per million population.
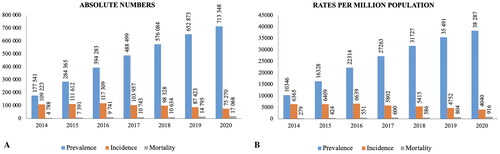
Figure 2. Age- and sex-specific incidence rate of CKD: (a) per 1,000,000 population for the years 2014–2020; (b) age pyramid.
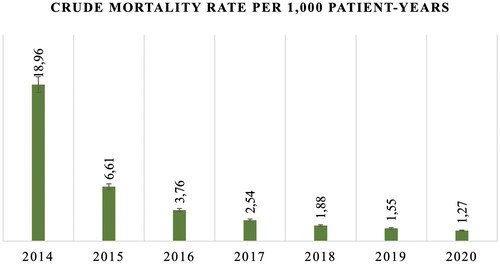
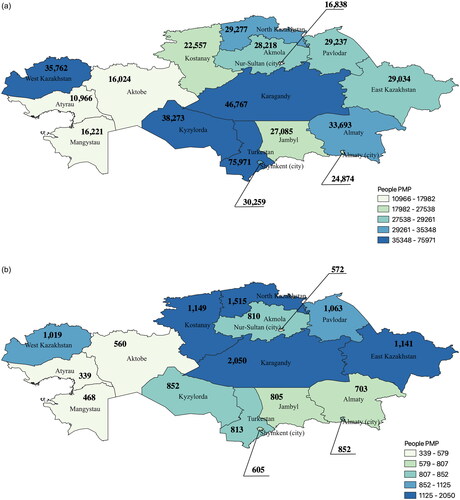
Although the all-cause mortality rate of CKD patients has increased with a reference to the general population, the crude mortality rate within the cohort reduced significantly from 18.96 per 1000 patient-years in 2014 to 1.27 per 1000 patient-years in 2020 (). As for the regional disparities, Turkestan, Karagandy, Kyzylorda, and West Kazakhstan regions had the highest prevalence rate according to . As for the mortality, North Kazakhstan, Kostanay, Karagandy, and East Kazakhstan showed elevated rates ().
Survival of CKD patients
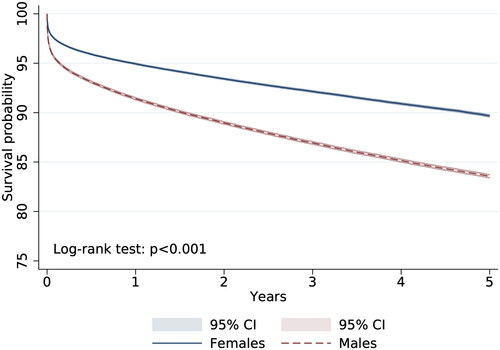
According to survival analysis of CKD patients, females have better survival compared to males: 89.7% vs. 83.6% with Log-rank test p < .001 (). The adjusted Cox’s regression model shows that people of 51–70 years and more than 71 years of age have 13.4- and 34.5-times increased risk of all-cause death compared to underaged patients (). In terms of comorbid conditions, diabetes mellitus increased the risk of death to 35% [HR = 1.39, 95% CI: 1.32–1.39], dysrhythmia for 53% [HR = 1.53, 95% CI: 1.46–1.61], COPD by 29% [HR = 1.29, 95% CI: 1.25–1.33], and liver diseases for 349% [HR = 4.49, 95% CI: 4.35–4.65].
Table 2. Association between socio-demographic and medical parameters and all-cause mortality rates of patients with CKD in 2014–2020.
Forecasting future trends
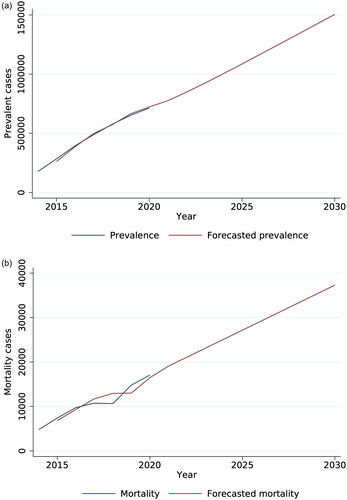
According to the ARIMA model, the number of prevalent cases in 2020 was 713,348, and this observation is forecasted to increase to 1,504,694 prevalent cases by 2030 (). A similar trend was observed for mortality. It is projected to increase from 17,068 mortality cases in 2020 to 37,305 deaths in 2030 ().
Discussion
This is the first study in Kazakhstan, even in Central Asia, that evaluated the increasing burden of CKD and forecasted future trends of prevalence and mortality till 2030. In the cohort, the majority were older than 50 years of age, of Kazakh ethnicity, and female gender. A significant proportion of the patients had tubulointerstitial and glomerular diseases as a cause of CKD, while among comorbid conditions, hypertension and CVDs were common. Although the IR decreased over the observation period, prevalence, and all-cause mortality rates elevated remarkably. However, the crude mortality only among CKD patients’ population notably decreased from 2014 to 2020. The central regions of Kazakhstan had the highest prevalence and all-cause mortality in patients with CKD. The forecasting models show that the burden of disease is going to almost twofold increase in a linear trend by 2030.
Gender, age, and CKD
According to the database of UNEHS, the proportion of females with CKD is significantly higher compared to males. Although there might be differences in ratios within countries, a similar tendency is observed in most regions, for example France, Thailand, Portugal, Turkey, and USA [Citation9,Citation10]. One of the explanations is that women have longer life expectancy [Citation11], and the fact that kidney function decline with aging [Citation12,Citation13], causing a larger population at risk. Another review explains that the high morbidity of CKD in women may be due to the use of glomerular filtration rate (GFR) estimation equations like the Chronic Kidney Disease Epidemiology Collaboration or the Modification of Diet in Renal Disease study, which were not originally developed for diverse populations [Citation9]. This leads to an underestimation of estimated glomerular filtration rate (eGFR) in women in community-based studies, potentially explaining the observed higher prevalence of CKD in females. In addition, the results of this study report that most of the cohort were of elderly age, and age- and sex-specific IRs showed that CKD rates increase dramatically after 50 years for both males and females. Although a higher prevalence is observed for women, they have better survival prognoses compared to men. Low testosterone levels of the elderly men of the observed cohort could be associated with a higher risk of death [Citation14]. Another explanation can be the unhealthier lifestyle choices of males compared to females: poorer dietary habits [Citation15], higher BMI, and plasma glucose [Citation16] can attribute to increased mortality.
Incidence and prevalence of CKD
The results of this study show that the prevalence became approximately four times higher at the end of the observation period compared to the beginning. The nationwide epidemiological studies in USA [Citation17] and China [Citation18] present similar trends of gradual increase in the prevalence of CKD. Although the incidence has been decreasing slowly over the observation period, it is a burdensome public health issue. Diabetes, high blood pressure, CVDs, smoking, and obesity are considered risk factors for kidney diseases. Previous research using the UNEHS databases show that the morbidity of diabetes [Citation19], hypertension [Citation20], and stroke [Citation21] are increasing in Kazakhstan. At the same time, the Global Adult Tobacco Survey shows that the overall prevalence of tobacco in Kazakhstan did not change significantly from 2014 to 2019, 22.9% and 21.5% of the population, respectively [Citation22].
The increasing prevalence and all-cause mortality of CKD patients cannot be fully explained only by comorbid conditions and lifestyle. Researchers indicated that environmental factors such as air pollution can be a risk factor for the development and progression of CKD [Citation23–25]. In 2018, Kazakhstan was ranked 20th in the world by an estimated average of PM2.5 concentration [Citation26]. Some of the regions of the country are more polluted due to industry and factories. The results of this study showed that high prevalence of CKD was observed in central and southern regions. It can be associated with higher values of coal consumption at combined heat and power plants [Citation27], the metallurgical industry, and coal mines that contaminate the air with nitrogen oxide, sulfur, and dust [Citation28]. More profound studies on regional differences in CKD morbidity and mortality are needed.
All-cause mortality of CKD patients
The findings of this research show that the mortality of CKD patients has grown three times during the observation period, which indicates the increasing burden of disease in the country. The introduction of new diagnostic methods and screenings into clinical practice might help to reduce the weight of the disease. According to the treatment protocol in Kazakhstan, diagnostics are carried out only for those people who have complaints that meet the criteria for CKD and have such comorbid diseases as diabetes mellitus, arterial hypertension, obstructive diseases, urinary tract infections, and autoimmune diseases [Citation29]. The National Institute for Health and Care Excellence suggests monitoring the GFR at least annually in adults, children, and youth who are taking medicines that have a negative effect on kidney function [Citation30]. The prevention and delay of progression can lead to a reduction of complications, decrease the development of comorbid conditions, and results in better survival of patients.
Controversially, the mortality of patients within the cohort has dropped significantly during the observation period. The dramatic fall in 2015 can be explained by the fact, that UNEHS started collecting electronic databases only a year earlier [Citation7], and the mortality of the first year indicates the cumulative effect of the period before 2014. Moreover, the development of nephrological services in Kazakhstan was relatively late, and the standard for organizing the provision of nephrological care to the population was approved only in 2014 [Citation31]. In addition, the high mortality of CKD patients in North Kazakhstan, Karagandy, Kostanay, and East Kazakhstan regions points out the most burdensome regions, where some measures can be initiated.
The findings of this study indicate that Russians have significantly higher mortality rates than Kazakhs. Separating the role of the race from other factors that might account for survival differences is challenging. More profound research including social variables such as socioeconomic class, degree of education, work status, and cultural variations in eating and lifestyle patterns is needed.
CKD cause, comorbid conditions, and hazard ratios
Patients with CKD can have different primarily diseases and comorbid conditions that have a negative effect on survival. Among the cohort, tubulointerstitial diseases are leading causes of CKD, followed by hypertensive nephropathy. Tubulointerstitial diseases can damage tubules and interstitium of the kidney, which may result in CKD [Citation32,Citation33]. Hypertensive nephropathy can lead to CKD by causing damage to the nephrons, increasing sodium retention and volume expansion, stimulating sympathetic nervous system activity, activating the renin–angiotensin–aldosterone system, and inducing endothelial dysfunction [Citation34]. The findings of this investigation reveal that diabetes complicated by renal pathology was implicated as the etiological factor in CKD in fewer than 1% of the examined cohort. Additionally, diabetes, when considered as a comorbid ailment, represented merely 6% of the total cohort. These figures markedly contrast with prevailing global literature on the subject. For instance, between 2013 and 2016 in the United States, around 36% of individuals with diabetes experienced the onset of diabetic kidney disease, characterized by sustained albuminuria or diminished eGFR [Citation35]. In a nationwide study of Thailand, the prevalence of CKD was more than 35% in diabetic patients [Citation36], while another one showed 24% [Citation37]. Our research findings may reflect such disparities due to potential coding practices among healthcare providers. Instead of assigning specific ICD-10 codes like E10.2, E11.2, or E13.2, which denote diabetic nephropathy, clinicians might alternatively assign broader diagnostic codes related to tubulointerstitial diseases. This could result in underestimation or misrepresentation of the prevalence of diabetes-related kidney complications in our cohort. Consequently, the discrepancy between our study’s findings and the prevalence rates reported in the global literature might stem from variations in coding practices and diagnostic classification methodologies across different healthcare systems and regions.
The results of this study show that comorbid HF and diabetes increase the risk of death, and these findings are in accordance with other research [Citation38–40]. Diabetes and hypertension are one of the main CKD risk factors [Citation41] and together can fasten the progression of end-stage renal disease [Citation42]. Moreover, it is difficult to determine the etiology of kidney damage in those patients who have both hypertension and diabetes at the advanced stages of CKD. Less than 10% of the cohort had glomerular diseases, which are listed as the leading cause of CKD [Citation43]. In combination with high systolic blood pressure, glomerular diseases are associated with an increased rate of kidney damage [Citation44]. Our study findings underscore the significant impact of comorbid HF on the mortality risk of CKD patients, demonstrating increased likelihood of death. This observation aligns with the recognized interplay between HF and CKD, where the presence of one condition exacerbates the progression and outcomes of the other [Citation45,Citation46]. Similar tendency can be observed for other CVDs such as dysrhythmia, PVD, and stroke [Citation47–49]. Research indicates that when COPD and CKD coexist, patients may experience worse clinical outcomes [Citation50,Citation51]. This could be due to common risk factors they share, such as smoking, or because of systemic inflammation. Our study’s findings support this conclusion, aligning with existing research on the topic. Renal impairment frequently complicates liver cirrhosis, affecting approximately half of patients with liver diseases [Citation52,Citation53] due to hemodynamic changes, fluid retention, and hepatorenal syndrome. The study from the National Center for Health Statistics of Centers for Disease Control and Prevention showed that in individuals with nonalcoholic fatty liver disease, the presence of CKD stages 2–3a was linked to a 2.31-fold increase in overall mortality risk, while stages 3b–5 were associated with a 4.83-fold increase, independent of other factors [Citation54]. Our study yielded similar findings; specifically, in the unadjusted model, the presence of comorbid liver diseases was associated with a 4.62-point increase in mortality risk, while in the adjusted model, this increase was slightly lower at 4.49 points.
Prevalence and mortality forecasting
The forecasting model used in this research shows the linear growth of prevalence and mortality from CKD by 2030. We cannot compare the forecasting trends of CKD epidemiology in Kazakhstan with other countries without proper adjustments on methodology, predictors, risk factors, and time frame. However, the general tendency of an increase in kidney disease prevalence is shown in other research. For example, Hoerger et al. used nationally representative data for 1999 and 2010 to demonstrate the increase in CKD prevalence approximately by 17% in the USA by 2030 [Citation55]. Another study from Singapore determined that the prevalence of CKD will be doubled in the country in the period between 2007 and 2035 [Citation56]. The findings indicate that the healthcare system should pay more attention to disease prevention and early detection to avert high rates of mortality.
Strengths and limitations
Several limitations should be discussed. First of all, the database lacks information on clinical and laboratory data of patients such as albumin to creatinine ratio or GFR. Therefore, we could not assess the stages of CKD by Kidney Disease Improving Global Outcome (KDIGO) classification. There is no information on a family history of kidney malfunction, autoimmune diseases, the stage of CKD, the treatment regime, and the effect of therapy. The causes of CKD provided in the tables are based solely on ICD-10 codes. The database showed that proportion of diabetic nephropathy as a cause of CKD is very low and high for tubulointerstitial diseases. These findings do not correlate with world literature. One of the possible explanations is miscoding and not accurate assignment of diagnoses by healthcare professionals. The database does not include the cause of death, so only all-cause mortality can be calculated.
Despite the mentioned limitations, evaluation of CKD epidemiology and forecasting future trends have several advantages. First, this is the first study analyzing the disease burden in Kazakhstan and making projections for prevalence and mortality by 2030. The database includes information on all CKD cases in the country, which resulted in an explicit description of disease weight for health policymakers. The development of better protocols and management methods for the condition in healthcare settings that consider sociodemographic variables and cultural differences can be aided by these findings. Additionally, it can intensify population awareness programs and amplify worries about leading a healthy lifestyle to prevent early CKD morbidity. Finally, the findings may spur an additional investigation into the affordability of CKD treatment to assess the financial burden of the condition.
Conclusions
This is the first study evaluating the burden of CKD in Kazakhstan, the largest Central Asian country. The retrospective analysis was done using nationwide health system records for 2014–2020. The forecasting model of prevalence and mortality showed that the weight of CKD will be significantly higher in 2030 in the country. The geographical representation of disease weight revealed the most burdensome regions. The results of this study will help health policymakers to establish better CKD management systems and prevention measures.
Author contributions
All authors approved the submitted version of manuscript and agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. GZ analyzed and interpreted data and drafted the article. KM involved in interpretation of data and drafting the article. SY involved in acquisition of data and revision of the manuscript. ArG involved in interpretation of data and drafting the article. YS involved in analysis of data and article drafting. VK involved in acquisition of data and revision of the manuscript. DS involved in interpretation of data and drafting the article. AA involved in interpretation of data and drafting the article. MM, ZK, BB, and DT involved in conception and design of study and revised the article. KY involved in interpretation of data and drafting the article. AbG involved in interpretation of data and revision of the manuscript. All authors read and approved the final version of the manuscript.
Supplemental Material
Download PDF (32.4 KB)Acknowledgements
We thank all staff from the Republican Center of Electronic Healthcare for providing data and consultancy.
The content of this paper was partially presented as a poster at the World Congress of Nephrology’23 (WCN’23). Congress, which was held in 2023 from March 30 to April 2.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the Republican Center for Electronic Health of the Ministry of Health of the Republic of Kazakhstan but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the corresponding author, Gaipov A., upon reasonable request and with permission of the Ministry of Health of the Republic of Kazakhstan.
Additional information
Funding
References
- Bikbov B, Purcell CA, Levey AS, et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):1–12. doi: 10.1016/S0140-6736(20)30045-3.
- Foreman KJ, Marquez N, Dolgert A, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet. 2018;392(10159):2052–2090. doi: 10.1016/S0140-6736(18)31694-5.
- Couser WG, Remuzzi G, Mendis S, et al. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011;80(12):1258–1270. doi: 10.1038/ki.2011.368.
- Matsushita K, Coresh J, Sang Y, et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3(7):514–525. doi: 10.1016/S2213-8587(15)00040-6.
- Chronic Kidney Disease Prognosis Consortium, Matsushita K, van der Velde M, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081.
- Gaipov A, Issanov A, Kadyrzhanuly K, et al. Epidemiology of dialysis-treated end-stage renal disease patients in Kazakhstan: data from Nationwide Large-Scale Registry 2014–2018. BMC Nephrol. 2020;21(1):407. doi: 10.1186/s12882-020-02047-6.
- Gusmanov A, Zhakhina G, Yerdessov S, et al. Review of the research databases on population-based registries of Unified Electronic Healthcare System of Kazakhstan (UNEHS): possibilities and limitations for epidemiological research and real-world evidence. Int J Med Inform. 2022;170:104950. doi: 10.1016/j.ijmedinf.2022.104950.
- Ministry on National economy of the Republic of Kazakhstan Committee on Statistics. Demographic yearbook of Kazakhstan 2022. Astana; 2023.
- Carrero JJ, Hecking M, Chesnaye NC, et al. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol. 2018;14(3):151–164. doi: 10.1038/nrneph.2017.181.
- Goldberg I, Krause I. The role of gender in chronic kidney disease. Eur Med J. 2016;1(2):58–64. doi: 10.33590/emj/10312319.
- Arias E, Tejada-Vera B, Ahmad F, et al. Provisional life expectancy estimates for 2020; 2021. doi: 10.15620/cdc:118999.
- Glassock R, Delanaye P, El Nahas M. An age-calibrated classification of chronic kidney disease. JAMA. 2015;314(6):559–560. doi: 10.1001/jama.2015.6731.
- O’Hare AM, Choi AI, Bertenthal D, et al. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol. 2007;18(10):2758–2765. doi: 10.1681/ASN.2007040422.
- Khurana KK, Navaneethan SD, Arrigain S, et al. Serum testosterone levels and mortality in men with CKD stages 3–4. Am J Kidney Dis. 2014;64(3):367–374. doi: 10.1053/j.ajkd.2014.03.010.
- Crews DC, Kuczmarski MF, Miller Iii ER, et al. Dietary habits, poverty, and chronic kidney disease in an urban population. J Ren Nutr. 2015;25(2):103–110. doi: 10.1053/j.jrn.2014.07.008.
- Verhave JC, Hillege HL, Burgerhof JGM, et al. Cardiovascular risk factors are differently associated with urinary albumin excretion in men and women. J Am Soc Nephrol. 2003;14(5):1330–1335. doi: 10.1097/01.asn.0000060573.77611.73.
- Murphy D, McCulloch CE, Lin F, et al. Trends in prevalence of chronic kidney disease in the United States. Ann Intern Med. 2016;165(7):473–481. doi: 10.7326/M16-0273.
- Yang C, Wang H, Zhao X, et al. CKD in China: evolving spectrum and public health implications. Am J Kidney Dis. 2020;76(2):258–264. doi: 10.1053/j.ajkd.2019.05.032.
- Gaipov A, Galiyeva D, Gusmanov A, et al. Epidemiology of type 1 and type 2 diabetes mellitus in Kazakhstan: data from Unified National Electronic Health System 2014–2019. BMC Endocr Disord. 2022;22(1):275.
- Yerdessov S, Kadyrzhanuly K, Sakko Y, et al. Epidemiology of arterial hypertension in Kazakhstan: data from Unified Nationwide Electronic Healthcare System 2014–2019. J Cardiovasc Dev Dis. 2022;9(2):52. doi: 10.3390/jcdd9020052.
- Zhakhina G, Zhalmagambetov B, Gusmanov A, et al. Incidence and mortality rates of strokes in Kazakhstan in 2014–2019. Sci Rep. 2022;12(1):16041. doi: 10.1038/s41598-022-20302-8.
- The Global Adult Tobacco Survey. The Republic of Kazakhstan 2019. Executive summary. Second round. Astana; 2019.
- Xu X, Nie S, Ding H, et al. Environmental pollution and kidney diseases. Nat Rev Nephrol. 2018;14(5):313–324. doi: 10.1038/nrneph.2018.11.
- Al-Aly Z, Bowe B. Air pollution and kidney disease. Clin J Am Soc Nephrol. 2020;15(3):301–303. doi: 10.2215/CJN.16031219.
- Wu M-Y, Lo W-C, Chao C-T, et al. Association between air pollutants and development of chronic kidney disease: a systematic review and meta-analysis. Sci Total Environ. 2020;706:135522. doi: 10.1016/j.scitotenv.2019.135522.
- IQAir world’s most polluted countries in 2019—PM2.5 ranking. Air Visual; 2022. Available from: https://www.iqair.com/world-most-polluted-countries
- Ormanova G, Karaca F, Kononova N. Analysis of the impacts of atmospheric circulation patterns on the regional air quality over the geographical center of the Eurasian continent. Atmos Res. 2020;237:104858. doi: 10.1016/j.atmosres.2020.104858.
- Assanov D, Zapasnyi V, Kerimray A. Air quality and industrial emissions in the cities of Kazakhstan. Atmosphere. 2021;12(3):314. doi: 10.3390/atmos12030314.
- Ministry of Health of the Republic of Kazakhstan. Clinical protocol for diagnosis and treatment: chronic kidney disease in adults. Protocol 154; 2021. Available from: https://diseases.medelement.com/disease/хроническая-болезнь-почек-у-взрослых-кп-рк-2021/16997
- National Institute for Health and Care Excellence Guideline. Chronic kidney disease: assessment and management; 2021. Available from: https://www.nice.org.uk/guidance/ng203/resources/chronic-kidney-disease-assessment-and-management-pdf-66143713055173
- Ministry of Health of the Republic of Kazakhstan. Order on approval of the standard for organizing the provision of nephrological care to the population in the Republic of Kazakhstan. Astana; 2014.
- Popp B, Ekici AB, Knaup KX, et al. Prevalence of hereditary tubulointerstitial kidney diseases in the German Chronic Kidney Disease Study. Eur J Hum Genet. 2022;30(12):1413–1422. doi: 10.1038/s41431-022-01177-9.
- Chanouzas D, Ball S. Tubulo-interstitial disorders. Medicine. 2019;47(10):649–653. doi: 10.1016/j.mpmed.2019.07.003.
- Ku E, Lee BJ, Wei J, et al. Hypertension in CKD: core curriculum 2019. Am J Kidney Dis. 2019;74(1):120–131. doi: 10.1053/j.ajkd.2018.12.044.
- Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2017 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2018;71(3 Suppl. 1):A7. doi: 10.1053/j.ajkd.2018.01.002.
- Kittiskulnam P, Thokanit NS, Katavetin P, et al. The magnitude of obesity and metabolic syndrome among diabetic chronic kidney disease population: a nationwide study. PLOS One. 2018;13(5):e0196332. doi: 10.1371/journal.pone.0196332.
- Jitraknatee J, Ruengorn C, Nochaiwong S. Prevalence and risk factors of chronic kidney disease among type 2 diabetes patients: a cross-sectional study in primary care practice. Sci Rep. 2020;10(1):6205. doi: 10.1038/s41598-020-63443-4.
- Major RW, Cheng MRI, Grant RA, et al. Cardiovascular disease risk factors in chronic kidney disease: a systematic review and meta-analysis. PLOS One. 2018;13(3):e0192895. doi: 10.1371/journal.pone.0192895.
- Koye DN, Magliano DJ, Nelson RG, et al. The global epidemiology of diabetes and kidney disease. Adv Chronic Kidney Dis. 2018;25(2):121–132. doi: 10.1053/j.ackd.2017.10.011.
- Birkeland KI, Bodegard J, Eriksson JW, et al. Heart failure and chronic kidney disease manifestation and mortality risk associations in type 2 diabetes: a large multinational cohort study. Diabetes Obes Metab. 2020;22(9):1607–1618. doi: 10.1111/dom.14074.
- Pyram R, Kansara A, Banerji MA, et al. Chronic kidney disease and diabetes. Maturitas. 2012;71(2):94–103. doi: 10.1016/j.maturitas.2011.11.009.
- Navaneethan SD, Schold JD, Jolly SE, et al. Diabetes control and the risks of ESRD and mortality in patients with CKD. Am J Kidney Dis. 2017;70(2):191–198. doi: 10.1053/j.ajkd.2016.11.018.
- Turkmen A, Sumnu A, Cebeci E, et al. Epidemiological features of primary glomerular disease in Turkey: a multicenter study by the Turkish Society of Nephrology Glomerular Diseases Working Group. BMC Nephrol. 2020;21(1):481. doi: 10.1186/s12882-020-02134-8.
- Kim HW, Park JT, Joo YS, et al. Systolic blood pressure and chronic kidney disease progression in patients with primary glomerular disease. J Nephrol. 2021;34(4):1057–1067. doi: 10.1007/s40620-020-00930-x.
- House AA, Wanner C, Sarnak MJ, et al. Heart failure in chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019;95(6):1304–1317. doi: 10.1016/j.kint.2019.02.022.
- Tuegel C, Bansal N. Heart failure in patients with kidney disease. Heart. 2017;103(23):1848–1853. doi: 10.1136/heartjnl-2016-310794.
- Kara AV, Gungor O. Screening and diagnosing cardiovascular disease in chronic kidney disease. In: Arıcı M, edtior. Management of chronic kidney disease: a clinician’s guide. Springer; 2023. p. 157–170. doi: 10.1007/978-3-031-42045-0_12.
- Jürgen F. Cardiovascular disease in patients with chronic kidney disease. Herz. 2020;45(2):122–128.
- Ghoshal S, Freedman BI. Mechanisms of stroke in patients with chronic kidney disease. Am J Nephrol. 2019;50(4):229–239. doi: 10.1159/000502446.
- Pelaia C, Pastori D, Armentaro G, et al. Predictors of renal function worsening in patients with chronic obstructive pulmonary disease (COPD): a multicenter observational study. Nutrients. 2021;13(8):2811. doi: 10.3390/nu13082811.
- Trudzinski FC, Alqudrah M, Omlor A, et al. Consequences of chronic kidney disease in chronic obstructive pulmonary disease. Respir Res. 2019;20(1):151. doi: 10.1186/s12931-019-1107-x.
- Wong F, O’Leary JG, Reddy KR, et al. Acute kidney injury in cirrhosis: baseline serum creatinine predicts patient outcomes. Am J Gastroenterol. 2017;112(7):1103–1110. doi: 10.1038/ajg.2017.122.
- Cholongitas E, Ioannidou M, Goulis I, et al. Comparison of creatinine and cystatin formulae with 51Chromium-ethylenediaminetetraacetic acid glomerular filtration rate in patients with decompensated cirrhosis. J Gastroenterol Hepatol. 2017;32(1):191–198. doi: 10.1111/jgh.13446.
- Paik J, Golabi P, Younoszai Z, et al. Chronic kidney disease is independently associated with increased mortality in patients with nonalcoholic fatty liver disease. Liver Int. 2019;39(2):342–352. doi: 10.1111/liv.13992.
- Hoerger TJ, Simpson SA, Yarnoff BO, et al. The future burden of CKD in the United States: a simulation model for the CDC CKD initiative. Am J Kidney Dis. 2015;65(3):403–411. doi: 10.1053/j.ajkd.2014.09.023.
- Wong LY, Liew AST, Weng WT, et al. Projecting the burden of chronic kidney disease in a developed country and its implications on public health. Int J Nephrol. 2018;2018:5196285–5196289. doi: 10.1155/2018/5196285.