In children, atypical hemolytic uremic syndrome mostly presents with a hypertensive crisis [Citation1–4]. In a retrospective study of 45 children with atypical hemolytic uremic syndrome, 71% of patients presented with severe hypertension [Citation4]. In another observational study, 8% of patients with atypical hemolytic uremic syndrome had malignant hypertension [Citation3]. We report a child who presented to us with a hypertensive crisis and thrombotic microangiopathy (TMA), and on evaluation, it was found that severe hypertension led to TMA, and not vice-versa which is common in children.
Mostly in children, atypical hemolytic uremic syndrome would be thought to occur first leading to a hypertensive crisis. However, this is rare to find thrombotic microangiopathy secondary to an uncontrolled hypertension due to an underlying reflux nephropathy and chronic kidney disease in a child, as in our current case.
A 7-year-old girl with no known comorbidities was admitted to the hospital with complaints of abdominal pain, nausea, vomiting, and generalized weakness for 15 days. The patient had no fever, loose motion, or urinary complaints. On examination, she was conscious and oriented, had severe pallor, weight 23 kg (50th percentile for age and sex) and height 123cms (50th percentile for age and sex), had a blood pressure of 204/118 mmHg. The rest of the physical examination results were unremarkable. Fundus examination revealed grade 4 hypertensive retinopathy with optic disk edema and exudates. The patient had a no disc history of hypertension from external medical records. The patient was on routine pediatric out-patient care for vaccinations. Given her symptoms and presence of severe hypertension, the possibility of a hypertensive crisis, with end-organ damage was considered.
Given the accelerated hypertension, she underwent further workup, as shown in . As evident from her clinical and laboratory evaluations, she had acute thrombotic microangiopathy with kidney dysfunction. The workup for common tropical illnesses, such as dengue, malaria, and typhoid, was negative. As evident from her short history and laboratory evaluations showing high reticulocytosis, low haptoglobin, increased LDH value, presence of schistocytes on peripheral smear with thrombocytopenia and acute renal dysfunction, she had the classic triad pointing toward thrombotic microangiopathy. Intravenous eculizumab was administered at 600 mg twice weekly and then 600 mg after 2 weeks. She was initiated on intravenous labetalol infusion with oral antihypertensive agents (amlodipine, prazosin, and clonidine) along with supportive care for acute kidney injury and anemia. The patient was closely monitored for a reduction in blood pressure, and the blood pressure was gradually reduced over the next 48 hours.
Table 1. Laboratory investigations.
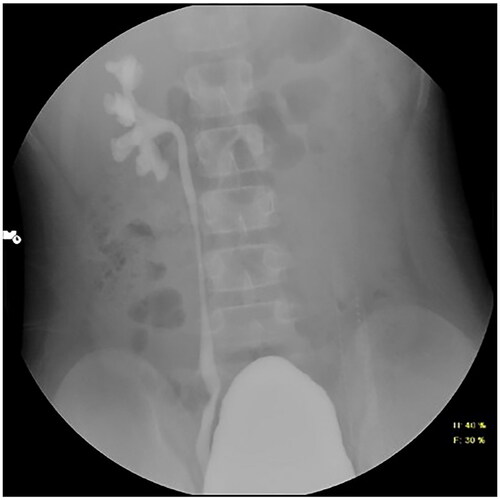
On evaluation for hypertension, fundus examination showed grade 4 hypertensive retinopathy, echocardiography showed moderate concentric left ventricular hypertrophy with proteinuria (3+ protein dipstick; urine protein creatinine ratio 4.1). A DMSA nuclear scan showed bilateral scars in the upper pole of both kidneys (). A contrast enhanced MRI angiography revealed a normal aorta from the arch to the renal vessels. Micturating cystourethrography showed bilateral grade III vesicoureteric reflux (VUR) (). Whole exome sequencing and multiplex ligand probe amplification assays to check for underlying complement abnormalities showed normal results.
Figure 1. DMSA nuclear scan showing mildly lobulated contour with cortical scarring in upper poles of both kidneys.
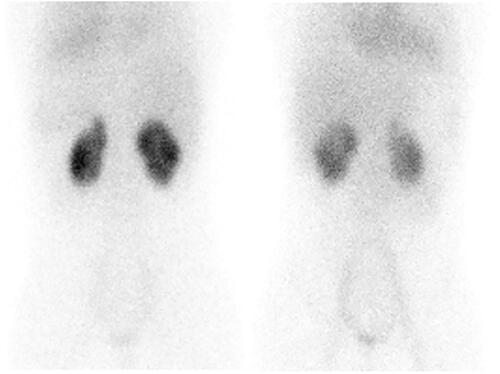
Figure 2. Micturating cystourethrogram image showing right grade-III vesicoureteric reflux. Mild bladder wall irregularities are present. No reflux is seen on left side.
The possibility of malignant hypertension-induced thrombotic microangiopathy was considered in our patient because of marked hypertension with preexisting left ventricular hypertrophy, grade 4 hypertensive retinopathy, loss of corticomedullary differentiation of the kidneys on ultrasound, and the presence of bilateral VUR. To summarize, this patient had acute kidney injury (short history, thrombotic microangiopathy) with a background of chronic kidney disease (presence of end-organ changes, marked hypertension with concentric left ventricular hypertrophy, grade 4 hypertensive retinopathy proteinuria, and loss of corticomedullary differentiation of both kidneys).
Hemolysis and thrombocytopenia markedly improved after the first dose of eculizumab and her serum creatinine level decreased to a nadir of 1.4 mg/dl. Intravenous eculizumab was stopped after three doses after finding no apparent underlying complement abnormalities on genetics. The child currently has chronic kidney disease with a serum creatinine 1.4 mg/dl, urine protein-to-creatinine ratio 1.4 and is currently on of 3 oral antihypertensive agents.
Our patient had no prior history of hypertension but had signs of hypertensive end-organ changes (including concentric LVH and retinopathy suggestive of a preexisting untreated hypertension), leading to malignant hypertension-associated thrombotic microangiopathy (TMA).
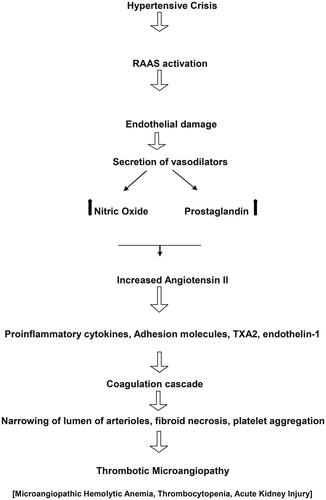
The differentiation between thrombotic microangiopathy (TMA) associated with malignant hypertension and TMA causing malignant hypertension is essential, as the latter may indicate atypical hemolytic uremic syndrome (aHUS) (especially in children) and respond well to eculizumab. The pathogenesis of TMA in malignant hypertension is shown in . Hypertensive crisis is common in children with primary atypical hemolytic uremic syndrome [Citation5]. While the absence of retinal changes typically aligns with TMA causing malignant hypertension, it is not solely definitive [Citation3–5]. TMA induced by malignant hypertension (common in adults) is classified as secondary TMA, because hypertension itself can cause acute endothelial damage and lead to aHUS like illness. [Citation5]. Hypertension-associated microangiopathy and atypical hemolytic uremic syndrome share a similar ultrastructural appearance with light microscopy. In our case, severe uncontrollable hypertension appeared to trigger complement activation and endothelial damage leading to TMA [Citation6,Citation7]. Moreover excellent response to eculizumab further confirmed this.
Figure 3. Cascade of events in hypertensive crisis that can lead to thrombotic microangiopathy. RAAS: renin-angiotensin-aldosterone system; NO: nitric oxide; TXA2: Thromboxane A2.
It is important to note, that complement abnormalities may be seen in almost 50–60% of adults with hypertension-associated microangiopathy and TMA can recur despite aggressive usage of anti-hypertensive medications [Citation4–7]. Therefore, it is essential to evaluate all children and adults with hypertension-associated TMA for complement defects. Patients with complement defects and malignant hypertensive require long-term anti-complement therapy, along with antihypertensive agents. Our patient showed no evidence of an underlying complement abnormality with a normal anti-factor H assay, whole exome sequencing, and multiplex ligand probe amplification. She improved after hypertension control and TMA did not recur after stopping anti-complement therapy, further suggesting that the implicating agent of TMA was uncontrolled hypertension and not vice-a-versa.
The uniqueness of this case lies in the atypical presentation of thrombotic microangiopathy (TMA) secondary to uncontrolled hypertension in a child rather than the more commonly expected scenario of atypical hemolytic uremic syndrome (aHUS) leading to a hypertensive crisis. This case was challenging to diagnose because standard tests for complement abnormalities did not show abnormalities despite symptoms resembling atypical hemolytic uremic syndrome (aHUS). The successful management through anti-complement therapy with intravenous eculizumab, blood pressure control, and supportive care led to rapid improvement in hemolysis, resolution of thrombocytopenia, and a significant reduction in serum creatinine levels. The cessation of eculizumab without TMA recurrence further suggested that uncontrolled hypertension was the primary cause rather than the reverse. The significance of this case lies in shedding light on the potential association between severe hypertension and TMA, triggering a cascade of events resembling aHUS, and emphasizing the importance to understand that severe hypertension may itself lead to complement activation, even in the absence of genetic complement abnormalities. These patients may also benefit from anti-complement agents, since complement activation is an integral part of the pathogenesis [Citation4–7].
To summarize, it is essential to evaluate hypertension-associated TMA for complement defects even in children. Aggressive hypertension control and specific targeted therapies should be promptly initiated for better outcomes. Severe hypertension may itself lead to complement activation leading to TMA and aHUS like picture, and anti-complement agents may also help in the management of these patients.
Consent
A signed, parental consent was taken for publishing this report
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Brain MC. Microangiopathic hemolytic anemia. N Engl J Med. 1969;281(15):1–4. doi: 10.1056/NEJM196910092811507.
- Neuhaus TJ, Calonder S, Leumann EP. Heterogeneity of atypical haemolytic uraemic syndromes. Arch Dis Child. 1997;76(6):518–521. doi: 10.1136/adc.76.6.518.
- Noris M, Caprioli J, Bresin E, et al. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol. 2010;5(10):1844–1859. doi: 10.2215/CJN.02210310.
- Geerdink LM, Westra D, van Wijk JA, et al. Atypical hemolytic uremic syndrome in children: complement mutations and clinical characteristics. Pediatr Nephrol. 2012;27(8):1283–1291. doi: 10.1007/s00467-012-2131-y.
- El Karoui K, Boudhabhay I, Petitprez F, Vieira-Martins P, Fakhouri F, Zuber J, Aulagnon F, Matignon M, Rondeau E, Mesnard L, Halimi JM, Frémeaux-Bacchi V. Impact of hypertensive emergency and rare complement variants on the presentation and outcome of atypical hemolytic uremic syndrome. Haematologica 2019;104(12):2501–2511.
- Bandaru SS, Anaji SC, Stowe IT. A case of thrombotic microangiopathy secondary to hypertensive emergency. Cureus. 2022;14(4):e24237. doi: 10.3324/haematol.2019.216903.
- Palma LMP, Sridharan M, Sethi S. Complement in secondary thrombotic microangiopathy. Kidney Int Rep. 2021;6(1):11–23. doi: 10.1016/j.ekir.2020.10.009.

