Abstract
Background
The systemic inflammatory response index (SIRI), served as a novel inflammatory biomarker, is the synthesis of neutrophils, monocytes and lymphocytes.
Aims
We hypothesized that SIRI has predictive value for contrast-associated acute kidney injury (CA-AKI) and long-term mortality in patients undergoing elective percutaneous coronary intervention (PCI).
Methods
We retrospectively observed 5685 patients undergoing elective PCI from January 2012 to December 2018. Venous blood samples were collected to obtain the experimental data on the day of admission or the morning of the next day. SIRI = neutrophil count × monocyte count/lymphocyte count. CA-AKI was defined as an increase of 50% or 0.3 mg/dl in SCr from baseline within 48 h after contrast exposure.
Results
The incidence of CA-AKI was 6.1% (n = 352). The best cutoff value of SIRI for predicting CA-AKI was 1.39, with a sensitivity of 52.3% and a specificity of 67.3%. [AUC: 0.620, 95% confidence interval (CI): 0.590–0.651, p < 0.001]. After adjusting for potential confounders, multivariate analysis showed that the high SIRI group (SIRI > 1.39) was a strong independent predictor of CA-AKI in patients undergoing elective PCI compared with the low SIRI group (SIRI ≤ 1.39) (odds ratio = 1.642, 95% CI: 1.274–2.116, p < 0.001). Additionally, COX regression analysis showed that SIRI > 1.39 was significantly associated with long-term mortality at a median follow-up of 2.8 years. [Hazard ratio (HR)=1.448, 95%CI: 1.188–1.765; p < 0.001]. Besides, Kaplan–Meier survival curve also indicated that the cumulative rate of mortality was considerably higher in the high SIRI group.
Conclusions
High levels of SIRI are independent predictors of CA-AKI and long-term mortality in patients undergoing elective PCI.
Introduction
Contrast-associated acute kidney injury (CA-AKI) is defined as acute renal impairment associated with the administration of contrast media (CM) and often occurs as a complication of percutaneous coronary intervention (PCI) [Citation1]. With advancements in medical technology, the utilization of contrast media has progressively risen, leading to CA-AKI emerging as a primary cause of iatrogenic renal insufficiency [Citation2]. Current studies have confirmed that CA-AKI is closely related to poor prognosis such as short-term and long-term high mortality after PCI [Citation3,Citation4]. Thus, evaluating CA-AKI in time and making corresponding preventive measures are of great significance.
The specific pathogenesis of CA-AKI is complex and has not been fully elucidated. However, previous studies have found inflammation plays a key role in the pathogenesis of CA-AKI [Citation5]. Some inflammatory markers (e.g. neutrophil, lymphocyte, and platelet counts) as well as other derived parameters [e.g. neutrophil to lymphocyte ratio (NLR) and lymphocyte-to-monocyte ratio (MLR)] have been verified the value to predict the development of CA-AKI [Citation5–8]. The Systemic Inflammatory Response Index (SIRI) is a novel inflammatory marker derived from peripheral blood cell counts, including neutrophils, monocytes, and lymphocytes, specifically designed to reflect the systemic inflammatory response. Recent studies have found that SIRI levels are closely related to the occurrence and development of cardiovascular diseases [Citation9–11]. However, there is a lack of studies on the relationship between SIRI levels and the incidence of CA-AKI in patients undergoing PCI. Consequently, the purpose of the present study was to investigate the predictive value of SIRI in determining the development of CA-AKI in patients undergoing elective PCI.
Method
Study population
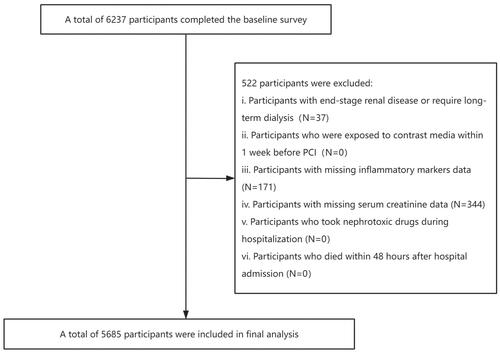
This retrospective study was conducted at Fujian Provincial Hospital from January 2012 to December 2018. Consecutively enrolled were patients undergoing elective percutaneous coronary intervention (PCI), including those with stable angina, chronic stable coronary artery disease, as well as patients diagnosed with acute myocardial infarction beyond the therapeutic window or who did not receive timely emergency PCI. The exclusion criteria were listed as follows: (1) Patients with end-stage renal disease [estimated glomerular filtration rate (eGFR) <15 mL/min/1.73 m2] or those who required for long-term dialysis; (2) Patients who were exposed to contrast media within 1 week prior to PCI; (3) Patients who had missing data on neutrophil, monocyte and lymphocyte counts; (4) Patients who took nephrotoxic drugs during hospitalization; (5) Patients who died within 48 h after hospital admission. Finally, a total of 5685 participants were included in the final analysis (). This study was conducted in accordance with the Declaration of Helsinki and ethical approval was obtained from the Fujian Provincial Hospital ethics committee (Ethical approval number: K2019-07-011). Due to the retrospective nature, the patient’s informed consent is exempt.
Protocol
All demographic, clinical, laboratory, and angiographic data were collected via an electronic medical record database. On the day of admission or the morning of the next day, venous blood samples were collected to obtain the data from blood routine examination, fasting blood lipid, rapid blood glucose, bilirubin, and serum uric acid. The time to measure SCr concentration was at admission and two consecutive days after the procedure. All PCI procedures were performed by experienced interventional cardiologists using nonionic, low-osmolality contrast media (either Iopamiiron or Ultravist, both 370 mgI/mL). Patients received intravenous drip of saline solution at a constant infusion rate of 1 mL/kg/h for 12 h throughout the perioperative period (0.5 mL/kg/h among patients with heart failure). The drug regimens were at the discretion of the treating physician according to the guidelines.
Definition and follow-up
In this study, we set the primary endpoint as the incidence of CA-AKI, defined as a 50% or 0.3 mg/dl increase in SCr baseline within 48 h after contrast exposure [Citation12]. Follow-up data were collected by trained specialists (nurses and associated physicians) through outpatient consultations or telephone interviews. SIRI = neutrophil count × monocyte count/lymphocyte count. The eGFR was calculated as 186.3×(SCr)−1.154×(Age)−0.203×0.742 (if female) [Citation13].
Statistical analysis
Patients were divided into two groups according to the optimal cutoff value of SIRI. All data were presented as mean ± standard deviation or median (interquartile range) for continuous variables and counts (percentage) for categorical variables, respectively. Continuous variables were compared by Student’s t-test (normally distributed) or Mann–Whitney U-test (non-normally distributed). Categorical variables were analyzed using chi-square test or Fisher’s exact test.
Restricted cubic spline curve was used to evaluate the correlation between continuous SIRI and CA-AKI. Area under the curve (AUC) was applied to represent the predictive power of SIRI. Differences in the risks of CA-AKI and long-term mortality between groups were calculated using multivariate logistic regression and Cox regression analysis. We performed 3 multivariate models and variables found to be statistically significant (p < 0.05) or clinically important in the univariate analysis were included in the multivariate analysis: (1) Model 1: adjusted for age > 75 years and gender; (2) Model 2: included the variables from model 1 plus diabetes, hypertension, anemia, history of myocardial infarction, eGFR, contrast volume > 150mL; (3) Model 3: adjusted the variables in model 2 plus acute myocardial infarction. Kaplan–Meier curve was used to visualize the effect of different levels of SIRI on long-term mortality. In addition, subgroup analyses were performed to determine if there were any interactions between the different subgroups.
Statistical analyses were conducted using the R statistical language (version 4.2.1; R Foundation, Vienna, Austria) and SPSS (version 26.0; IBM, Armonk, New York, United States). p < 0.05 (two-tailed) was considered statistically significant.
Result
Patient characteristics
A total of 5685 patients were included in the study. According to the receiver operating characteristic curve (Supplementary Figure 1), the AUC values of SIRI to predict CA-AKI was 0.620 [95% confidence interval (CI):0.590–0.651, p < 0.001]. The best cutoff value of SIRI for predicting CA-AKI was 1.39, with a sensitivity of 52.3% and a specificity of 67.3%. Patients were divided into a low SIRI group (SIRI ≤ 1.39) and a high SIRI group (SIRI > 1.39) according to the optimal cutoff value.
Baseline characteristics of the study population were shown in . The mean age of these patients was 65.31 ± 10.46 years, of which 1235 (21.72%) were female. Patients with high SIRI were older and had a history of smoking. In addition, compared with the low SIRI group, the high SIRI group with a higher proportion of hypertension, atrial fibrillation, congestive heart failure, anemia, hyperuricemia, acute myocardial infarction, and more participants at moderate to high risk of bleeding (Bleeding Academic Research Consortium ≥ 2). Meanwhile, the levels of WBC, PLT, NT-proBNP, ALT, AST, and SCr in the high SIRI group were significantly higher, while the levels of hemoglobin, left ventricular ejection fraction and eGFR were lower. Supplementary Table 1 presented the baseline characteristics of patients with and without CA-AKI. Patients with CA-AKI were older and had higher levels of SIRI.
Table 1. Baseline variables in groups with high or low levels of SIRI.
Predictive value of SIRI for incidence of CA-AKI
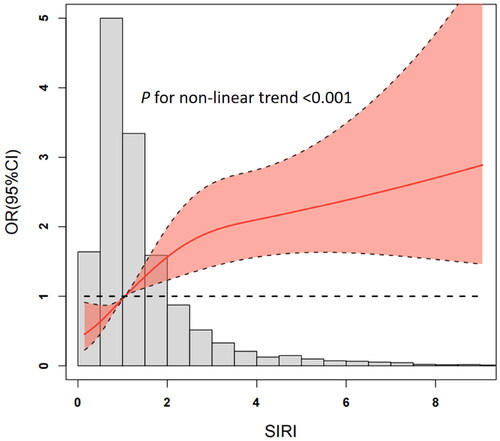
A total of 352 (6.1%) patients developed CA-AKI. Restricted cubic spline curve in logistics regression analysis revealed a nonlinear relationship between SIRI and CA-AKI risk (P for non-linearity < 0.001, ).
Furthermore, to determine the independent effect of SIRI on CA-AKI, we performed univariate and multivariate regression models. In Model 1, high SIRI values were strongly associated with CA-AKI after adjusting for socio-demographic characteristics [high SIRI group VS low SIRI group: Odds ratio (OR)=2.260, 95% CI: 1.813–2.819, p < 0.001]. In Model 2, we further adjusted the comorbidity and contrast dose, the correlation between SIRI and CA-AKI remained significant (high SIRI group VS low SIRI group: OR = 2.147, 95%CI: 1.719–2.681, p < 0.001). Similar results were also observed after adjusting for the acute myocardial infarction in Model 3 (high SIRI VS low SIRI: OR = 1.580, 95% CI: 1.253–1.993, p < 0.001) (). The relationship between SIRI and CA-AKI exhibited a significant interaction with eGFR. Subsequent analyses were stratified based on eGFR levels. The results indicated a notable positive correlation between SIRI and CA-AKI specifically in individuals with eGFR > 60 mL/min/1.73 m2. ().
Figure 3. Subgroup analysis stratified by risk factors for contrast-associated acute kidney injury. CI, confidence interval.
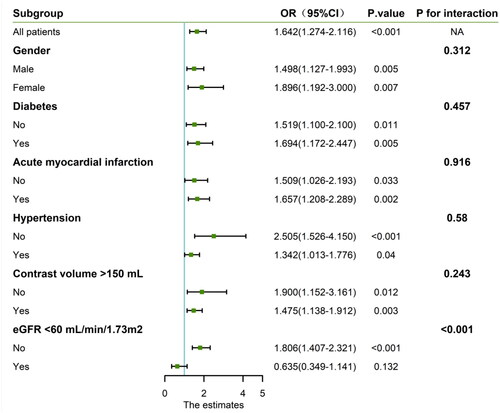
Table 2. Relationship of SIRI with CA-AKI and long-term mortality.
SIRI and long-term mortality
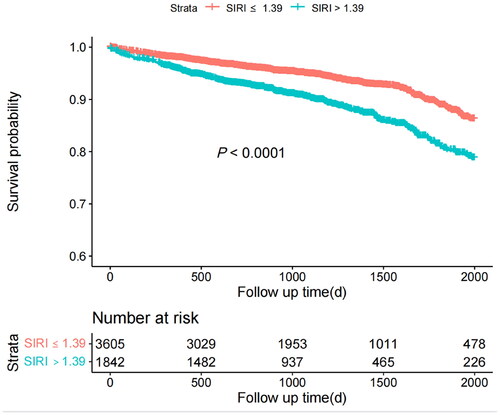
At a median follow-up of 2.8 years, 448(7.8%) deaths were reported. Kaplan-Meier curve showed that higher SIRI levels resulted in higher cumulative mortality (). Besides, we found that higher values of SIRI were strongly associated with long-term mortality according to multivariate Cox regression models adjusted for other risk factors including age > 75 years, gender, diabetes, hypertension, anemia, history of myocardial infarction, eGFR, contrast volume > 150mL and acute myocardial infarction [Hazard ratio (HR)=1.400, 95%CI: 1.164-1.684; p < 0.001] ().
Discussion
In this retrospective observational study, we assessed the association between SIRI values and CA-AKI in patients undergoing elective PCI. We found that high SIRI values were independently associated with an increased risk of developing CA-AKI after adjusting for potential confounders in multivariate models. Moreover, SIRI values were strongly related to long-term mortality. This finding might indicate that SIRI values can be used to assess the risk of developing CA-AKI in patients undergoing elective PCI.
Although the specific pathogenesis of CA-AKI is complex and diverse, current studies have demonstrated that inflammation is one of the main mechanisms leading to CA-AKI [Citation5]. Contrast media can lead to a direct toxic effect on renal tubular epithelial cells, generating interstitial inflammation and cell necrosis [Citation14]. At the same time, the injection of contrast media can also cause a significant increase in inflammatory cytokines (including IL-6 and TNF-α) and cell chemokines, building favorable conditions for leukocytes, including neutrophils and lymphocytes, to infiltrate the injured kidney, thereby exacerbating renal dysfunction and tubular injury [Citation15,Citation16]. Beyond the inflammatory state in connection with underlying coronary lesions, PCI itself can also trigger an acute inflammatory response [Citation17]. A large number of clinical studies have also shown that high levels of inflammatory cytokines before, during and after PCI were relevant to poor prognosis [Citation17]. Therefore, the assessment of inflammation is critical to evaluating the comorbidity and prognosis of patients undergoing PCI.
Neutrophils, monocytes, and lymphocytes are the most common biomarkers of inflammation, and many people have explored their value in diagnosing CA-AKI after PCI. Neutrophil to lymphocyte ratio, as a binding of neutrophils and lymphocytes, was demonstrated to have a high predictive value for the development of CA-AKI in patients undergoing emergency PCI [Citation18]. A study based on 2000 STEMI patients undergoing PCI also confirmed that NLR was a significant independent predictor of CA-AKI [Citation19]. Similarly, the MLR, which combines monocytes and lymphocytes, was also considered to be a predictor of CA-AKI after PCI in acute coronary syndrome (ACS) patient [Citation6]. In addition, the study by Fan et al. also found that the combination of MLR and NLR can improve the prognostic value of predicting long-term major adverse cardiac events (MACE) after PCI [Citation20].
As a combination of NLR and MLR, SIRI is a newly discovered inflammatory biomarker, which plays an independent predictive role in the diagnosis and prognosis assessment of cancer, rheumatoid arthritis and stroke [Citation21–23]. Previous studies have demonstrated that SIRI levels were closely associated with increased cardiovascular disease risk [Citation10,Citation11]. Han et al. also proved that SIRI levels could be used as an independent predictor of MACE occurrence in ACS patients undergoing PCI [Citation9]. Similarly, another retrospective study indicated that SIRI had a high predictive value in predicting MACE in ACS patients undergoing PCI and was superior to NLR and MLR [Citation24]. In patients with acute pancreatitis, SIRI levels can be used as a biomarker to predict the progression of AKI over the course of the disease [Citation25]. To our knowledge, no study has explored the relationship between SIRI and CA-AKI in patients undergoing elective PCI. Our study suggested that high levels of SIRI were independent risk factors for CA-AKI after elective PCI. Following additional stratified analysis based on eGFR levels, a significant positive correlation between SIRI and CA-AKI was observed in individuals with eGFR > 60 mL/min/1.73 m2. This finding suggests that patients without a history of kidney dysfunction should also prioritize the management of their inflammatory status. Furthermore, considering that the restricted cubic spline curve may be limited by overfitting, sensitivity to knot placement, extrapolation limitations, assumption of smoothness, etc, we employed a conservative approach in selecting the number of knots and followed widely accepted guidelines for knot selection to ensure that our approach is both systematic and reproducible. We have examined the fit of the spline model to our data and believe that, in the context of our study, the smoothness assumption is justified. All we mentioned above ensured that we can use restricted cubic spline curve to evaluate the relationship between SIRI and CI-AKI reliably. Moreover, after adjusting for relevant confounders, SIRI values were strongly correlated with long-term mortality. Last but not least, SIRI is an inexpensive and noninvasive parameter that can be easily calculated from a complete blood count. Above all, we have reason to believe that SIRI is expected to be a simple, rapid and effective preoperative inflammatory status assessment tool for predicting the occurrence of CA-AKI and long-term mortality after elective PCI.
Limitation
Several limitations need to be mentioned. First, because our study was retrospective, we could only verify the association between SIRI and CA-AKI, rather than the causal relationship. Likewise, due to the inherent limitations of retrospective studies, our study can only propose a possible association between SIRI and mortality. Therefore, a multicenter prospective study is warranted to validate our results. Second, we did not pay attention to the dynamic changes of neutrophil, monocyte, and other inflammatory cell levels during hospitalization, and only collected data at admission, thus inevitably introducing random errors. Third, we lacked the ability to collect levels of typical inflammatory biomarkers such as hsCRP, so we could not directly compare SIRI values with these inflammatory biomarkers. Fourth, since the patients we selected were those who had received elective PCI, only 2 of them had hypotension and IABP, these two factors were not adjusted. Last but not least, previous research discovered that PCIs performed during off-hours might be associated with a poorer prognosis than procedures conducted during regular working hours [Citation26]. There’s a lack of records in our database about the time of surgery, and we will pay attention to improving the relevant content in our future research. Despite these limitations, this study provides the first insight into the relationship between SIRI and CA-AKI and long-term mortality in patients undergoing elective PCI.
Conclusion
Our study suggests that high preoperative SIRI levels in patients undergoing elective PCI are associated with the development of CA-AKI and long-term mortality. As a simple, inexpensive, and readily available index of inflammation, SIRI may be used as part of risk stratification in high-risk patient groups to predict contrast-related renal complications.
IRB information
The study protocol fulfilled the requirements of the Declaration of Helsinki and was approved by the Ethics Committee of the Fujian Provincial Hospital, China (Ethics Approval no. K2019-07-011). Due to the retrospective nature, the patient’s informed consent is exempt.
Supplemental Material
Download PDF (192.5 KB)Acknowledgement
We would like to acknowledge the presentation of our research at the 34th Great Wall International Congress of Cardiology Asian Heart Society Congress 2023 held during September 7–10 at Beijing, China. The abstract titled ‘The association between systemic inflammatory response index and contrast-associated acute kidney injury in patients undergoing elective percutaneous coronary intervention’ was presented as poster at the Cardiovascular Innovations and Applications. The abstract can be accessed online at https://www.scienceopen.com/hosted-document?doi=10.15212/CVIA.2023.0059. We are grateful for the opportunity to share our work at this prestigious conference.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analyzed in this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Section 4: contrast-induced AKI. Kidney Int Suppl (2011). 2012;2(1):1–9. doi: 10.1038/kisup.2011.34.
- Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930–936. doi: 10.1053/ajkd.2002.32766.
- Skalsky K, Levi A, Bental T, et al. The definition of "acute kidney injury" following percutaneous coronary intervention and cardiovascular outcomes. Am J Cardiol. 2021;156:39–43. doi: 10.1016/j.amjcard.2021.06.033.
- Wi J, Ko YG, Kim JS, et al. Impact of contrast-induced acute kidney injury with transient or persistent renal dysfunction on long-term outcomes of patients with acute myocardial infarction undergoing percutaneous coronary intervention. Heart. 2011;97(21):1753–1757. doi: 10.1136/hrt.2010.218677.
- Zhang F, Lu Z, Wang F. Advances in the pathogenesis and prevention of contrast-induced nephropathy. Life Sci. 2020;259:118379. doi: 10.1016/j.lfs.2020.118379.
- Karauzum I, Karauzum K, Acar B, et al. Predictive value of lymphocyte-to-monocyte ratio in patients with contrast-induced nephropathy after percutaneous coronary intervention for acute coronary syndrome. J Transl Int Med. 2021;9(2):123–130. doi: 10.2478/jtim-2021-0024.
- Wu X, Ma C, Sun D, et al. Inflammatory indicators and hematological indices in contrast-Induced nephropathy among patients receiving coronary intervention: a systematic review and Meta-Analysis. Angiology. 2021;72(9):867–877. doi: 10.1177/00033197211000492.
- Zorlu C, Koseoglu C. Comparison of the relationship between inflammatory markers and contrast-Induced nephropathy in patients with acute coronary syndrome after coronary angiography. Angiology. 2020;71(3):249–255. doi: 10.1177/0003319719892160.
- Han K, Shi D, Yang L, et al. Prognostic value of systemic inflammatory response index in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Ann Med. 2022;54(1):1667–1677. doi: 10.1080/07853890.2022.2083671.
- Jin Z, Wu Q, Chen S, et al. The associations of two novel inflammation indexes, SII and SIRI with the risks for cardiovascular diseases and All-Cause mortality: a Ten-Year follow-up study in 85,154 individuals. J Inflamm Res. 2021;14:131–140. doi: 10.2147/jir.S283835.
- Li J, He D, Yu J, et al. Dynamic status of SII and SIRI alters the risk of cardiovascular diseases: evidence from kailuan cohort study. J Inflamm Res. 2022;15:5945–5957. doi: 10.2147/jir.S378309.
- Mehran R, Dangas GD, Weisbord SD. Contrast-Associated acute kidney injury. N Engl J Med. 2019;380(22):2146–2155. doi: 10.1056/NEJMra1805256.
- Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004.
- Goldenberg I, Matetzky S. Nephropathy induced by contrast media: pathogenesis, risk factors and preventive strategies. CMAJ. 2005;172(11):1461–1471. doi: 10.1503/cmaj.1040847.
- Akcay A, Nguyen Q, Edelstein CL. Mediators of inflammation in acute kidney injury. Mediators Inflamm. 2009;2009:137072–137012. doi: 10.1155/2009/137072.
- Bansal S, Patel RN. Pathophysiology of contrast-Induced acute kidney injury. Interv Cardiol Clin. 2020;9(3):293–298. doi: 10.1016/j.iccl.2020.03.001.
- Tucker B, Vaidya K, Cochran BJ, et al. Inflammation during percutaneous coronary intervention-Prognostic value, mechanisms and therapeutic targets. Cells. 2021;10(6):1391. doi: 10.3390/cells10061391.
- Yuan Y, Qiu H, Hu X, et al. Predictive value of inflammatory factors on contrast-induced acute kidney injury in patients who underwent an emergency percutaneous coronary intervention. Clin Cardiol. 2017;40(9):719–725. doi: 10.1002/clc.22722.
- Tanık VO, Çınar T, Velibey Y, et al. Neutrophil-to-lymphocyte ratio predicts contrast-Induced acute kidney injury in patients with ST-Elevation myocardial infarction treated with primary percutaneous coronary intervention. J Tehran Heart Cent. 2019;14(2):59–66.
- Fan Z, Li Y, Ji H, et al. Prognostic utility of the combination of monocyte-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio in patients with NSTEMI after primary percutaneous coronary intervention: a retrospective cohort study. BMJ Open. 2018;8(10):e023459. doi: 10.1136/bmjopen-2018-023459.
- Chen L, Chen Y, Zhang L, et al. In gastric cancer patients receiving neoadjuvant chemotherapy systemic inflammation response index is a useful prognostic indicator. Pathol Oncol Res. 2021;27:1609811. doi: 10.3389/pore.2021.1609811.
- Xu Y, He H, Zang Y, et al. Systemic inflammation response index (SIRI) as a novel biomarker in patients with rheumatoid arthritis: a multi-center retrospective study. Clin Rheumatol. 2022;41(7):1989–2000. doi: 10.1007/s10067-022-06122-1.
- Zhang Y, Xing Z, Zhou K, et al. The predictive role of systemic inflammation response index (SIRI) in the prognosis of stroke patients. Clin Interv Aging. 2021;16:1997–2007. doi: 10.2147/cia.S339221.
- Li Q, Ma X, Shao Q, et al. Prognostic impact of multiple lymphocyte-based inflammatory indices in acute coronary syndrome patients. Front Cardiovasc Med. 2022;9:811790. doi: 10.3389/fcvm.2022.811790.
- Biyik M, Biyik Z, Asil M, et al. Systemic inflammation response index and systemic immune inflammation index are associated with clinical outcomes in patients with acute pancreatitis? J Invest Surg. 2022;35(8):1613–1620. doi: 10.1080/08941939.2022.2084187.
- Tokarek T, Dziewierz A, Plens K, et al. Percutaneous coronary intervention during on- and off-hours in patients with ST-segment elevation myocardial infarction. Hellenic J Cardiol. 2021;62(3):212–218. doi: 10.1016/j.hjc.2021.01.011.