Abstract
Background
Monocyte to high-density lipoprotein cholesterol ratio (MHR) was confirmed as a novel inflammatory marker and strongly associated with the risk of several diseases. This study aimed to investigate the relationship between MHR and chronic kidney disease (CKD) in a Chinese adult population.
Methods
In this cross-sectional study, 232,775 community-dwelling adults in Binhai who completed health checkups in 2021 were enrolled. Participants were categorized based on the MHR quartiles. Clinical characteristics of participants across different groups were compared using one-way ANOVA, Kruskal-Wallis h-test, and Chi-squared test as appropriate. Univariate and multivariable logistic regression analyses were taken to assess the relationship between MHR and the presence of CKD, as well as its association with low estimated glomerular filtration rate (eGFR) and proteinuria. Subgroup analyses were further executed to confirm the reliability of this relationship.
Results
A total of 21,014 (9.0%) individuals were diagnosed with CKD. Characteristic indicators including waist circumference, body mass index (BMI), blood pressure (BP), serum uric acid (SUA), triglyceride, and fasting blood glucose (FBG) showed a gradual increase with higher MHR quartiles, whereas parameters such as age, total cholesterol, high-density lipoprotein cholesterol (HDL-C), and eGFR decreased (p < .001). In the multivariable logistic regression analysis, we observed independent associations between MHR (per 1 SD increase) and CKD, as well as low eGFR and proteinuria, with odds ratio (ORs) and 95% confidence intervals (95%CIs) of 1.206 (1.186–1.225), 1.289 (1.260–1.319), and 1.150 (1.129–1.171), respectively (p < .001). Similar conclusions were confirmed in subgroup analysis stratified by gender, age, BMI, central obesity, hypertension, and diabetes mellitus, after justification for confounding factors.
Conclusion
Elevated MHR level was independently associated with the presence of CKD, suggesting that it might serve as a useful clinical tool for risk stratification, offering valuable insights to inform preventive and therapeutic approaches for clinicians in their routine medical practice.
Introduction
Chronic kidney disease (CKD) is acknowledged as a critical threat to public health worldwide. According to the Global Burden of Disease Study, CKD and its impact on cardiovascular disease have a major effect on global health. It is responsible for approximately 35.8 million disability-adjusted life years (DALYs) and 2.6 million deaths every year all over the world [Citation1]. Patients with CKD are more likely to develop atherosclerosis as the disease often accompanies inflammation, oxidative stress, and disturbed lipid metabolism [Citation2].
Inflammation is typically accompanied by several alterations in lipid metabolism, including elevated triglyceride levels and reductions in high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C). Moreover, it also affects the functionality of lipoproteins, which could lead to an increased risk of atherosclerosis.[Citation3]. As a proinflammatory state, CKD is also expected to have changes in lipids, lipoproteins, and lipoprotein-associated proteins. Indeed, the lipidome in CKD patients differs from that of healthy individuals with increased levels of triglycerides and N-acyltaurines, and decreased levels of phosphatidylcholines, plasmenyl ethanolamines, sulfatides, ceramides, and cholesterol sulfate [Citation4,Citation5]. Animal model studies have shown that CKD promotes lipid accumulation in the artery wall and kidney, leading to atherosclerosis, glomerulosclerosis, and tubulointerstitial fibrosis [Citation6,Citation7].
Monocyte to high-density lipoprotein cholesterol ratio (MHR) is a composite biomarker reflecting the balance between inflammation, oxidative stress, and lipid metabolism [Citation8,Citation9]. Its level has been revealed to be related to the risk of diabetes mellitus (DM) [Citation10], hypertension (HTN)[Citation11], coronary heart disease [Citation12], atrial fibrillation [Citation13,Citation14], fatty liver disease [Citation15,Citation16], and cardiovascular mortality [Citation17,Citation18]. Although high MHR level has been known as a prognostic marker for diabetic nephropathy (DN), acute kidney injury, and renal dysfunction [Citation10,Citation19–21], limited data has been presented on the relationship between MHR and CKD. Our research aimed to investigate the association between MHR and CKD in a Chinese community population.
Materials and methods
Study population
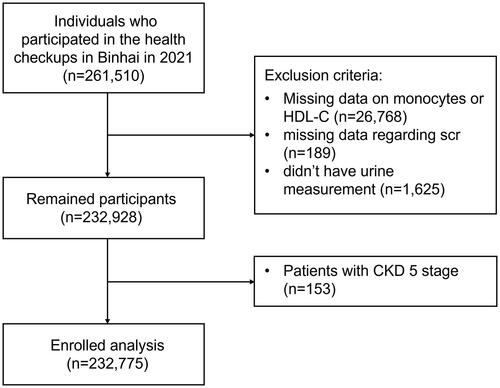
A retrospective cross-sectional study was conducted in Binhai, Jiangsu Province, China. The prevalence of CKD in this region, located in Eastern China, was 8.1% (7.2%–8.9%) from 2018 to 2019[Citation22]. Binhai was selected because of its universal coverage of free primary care and an integrated electronic health information system. The annual health examination program conducted by the local government was free of charge for all the residents there. Its information was disseminated through various media channels such as the Internet, television, newspapers, and others before the health examination took place. All the community residents in Binhai were eligible to participate in the health examination freely by presenting their identification cards. Finally, a total of 261,510 individuals participated in the health examination project from 1st January 2021 to 31st December 2021 in Binhai. We excluded participants who lacked urine measurement data (n = 1,625), monocyte or HDL-C count (n = 26,768), or serum creatinine (Scr) measurements (n = 189). Additionally, Considering the impact of inflammation-malnutrition syndrome and the potential effects of dialysis technique on lipid homeostasis, patients with CKD stage G5 (n = 153) were excluded due to their distinct pathophysiology [Citation23]. A total of 232,775 participants aged ≥18 years were finally enrolled in this study. The flow chart in shows the inclusion and exclusion criteria for participants. Informed consent was obtained from each participant before data collection.
Data collection
All data were collected by trained nurses using questionnaires and anthropometric measures. Height, weight, and waist circumference were all measured twice and averaged for analysis. The average of three blood pressure (BP) measurements made by the nurses was documented. Blood samples were obtained after overnight fasting and examined with automatic analyzers (Beckman Coulter AU5800 and Sysmex XN-350). MHR was calculated by the count of circulating monocytes divided by serum HDL-C level. A random spot urine sample was collected and assessed using a urine dipstick test to detect urinary protein. The results were shown as negative, trace, +1, +2, or +3.
Definition of metabolic variables
Systolic BP (SBP) ≥140 mmHg or diastolic BP (DBP) ≥90 mmHg, or the present antihypertensive treatment, was used as the diagnostic criteria for HTN. Pre-HTN was defined as either SBP 120–139 mmHg or DBP 80-89 mmHg [Citation24]. Fasting blood glucose (FBG) level was classified into three groups: <5.6 mmol/L (no-DM), 5.6–6.9 mmol/L (pre-DM), and ≥7.0 mmol/L (DM) [Citation25]. Participants with a waist circumference of ≥80 cm for females and ≥90 cm for males were identified to have central obesity [Citation26]. The criteria set by the Working Group on Obesity in China were as follows: overweight with a body mass index (BMI) of 24.0–27.9 kg/m2, and obesity with a BMI of ≥28.0 kg/m2 [Citation27].
Definition of CKD and CKD stages
The CKD epidemiology collaboration creatinine equation (CKD-EPI) was used to report the estimated glomerular filtration rate (eGFR) in this study [Citation28]. Despite widespread awareness of urinary albumin/creatinine ratio (UACR) as one of the gold standards for assessing CKD, the urine dipstick test remains widely used as a screening tool for proteinuria evaluation, especially in large-scale population health screenings, due to its low cost, simplicity, and ability to provide rapid point-of-care information [Citation29]. The proteinuria was evaluated with a urine dipstick test in this study, which was classified as normal (negative or trace), mild (+1), or heavy (≥ +2) [Citation30]. Referencing previous epidemiological research methods and the Kidney Disease Improving Global Outcomes (KDIGO) 2012 Clinical Practice Guideline, CKD was diagnosed based on low eGFR [<60 mL·min−1·(1.73 m2)−1] and/or proteinuria (≥ +1)[Citation31–33]. CKD stages are defined as follows: Stage G1, proteinuria with eGFR ≥ 90 mL·min−1·(1.73 m2)−1; Stage G2, proteinuria with eGFR of 60–89 mL·min−1·(1.73 m2)−1; Stage G3, an eGFR of 30–59 mL·min−1·(1.73 m2)−1; Stage G4, an eGFR of 15–29 mL·min−1·(1.73 m2)−1; Stage G5, eGFR < 15 mL·min−1·(1.73 m2)−1[Citation33].
Statistical analysis
Statistical analyses were performed with SPSS version 23 (SPSS Inc, Chicago, Illinois). Continuous variables were described using mean ± standard deviation (SD) or interquartile range (IQR) and analyzed with one-way ANOVA or the Kruskal–Wallis H-test, where applicable. Categorical variables were expressed as numbers with percentages, and the difference was determined by the Chi-squared test. The missing rates for all variables were below 0.25%, and regression estimation was used to deal with the missing data. The quantitative variable MHR was chosen to be divided into quartiles to explore potential non-linear relationships between MHR and baseline clinical features more flexibly within this population. We examined the unadjusted association between latent variables and CKD in a univariate logistic regression analysis. The association between MHR (per 1 SD increase) and the presence of CKD as well as low eGFR and proteinuria was measured using multivariate logistic regression analysis. Variables in model 1 were unadjusted. Gender and age were adjusted in model 2. In addition, various clinical variables such as BMI, FBG, SBP, DBP, WBC, platelet, hemoglobin, total cholesterol, serum uric acid (SUA), triglyceride, waist circumference, and LDL-C were further adjusted in model 3. Moreover, subgroup analyses were further conducted to examine the robustness of this relationship. The Bonferroni correction was used to adjust p values for interaction in the subgroup analyses. The formula for a Bonferroni Correction is as follows: αnew = αoriginal/n, where αoriginal=0.05, and n represents the total number of comparisons or tests being performed.
Results
Clinical characteristics of the population
The clinical characteristics of the study population grouped by quartiles of MHR are shown in . A total of 232,775 individuals were included in the study, with an average age of 58.39 ± 14.35 years, 44.9% were male. Participants were divided into four subgroups according to MHR quartiles using the following cutoff values (Q1: <0.1769, Q2: 0.1769–0.2452, Q3: 0.2453–0.3382, Q4: ≥0.3383). The proportion of male participants increased as MHR quartiles increased. The levels of waist circumference, BMI, SBP, DBP, SUA, triglyceride, FBG, white blood cell (WBC), hemoglobin, platelet, and Scr increased significantly across the increasing quartiles, whereas age, total cholesterol, and HDL-C decreased with higher MHR in this study. Notably, eGFR decreased gradually from Q1 to Q4, which were 94.91 ± 16.76, 94.17 ± 17.38, 93.21 ± 18.25, and 92.11 ± 19.78 mL·min−1·(1.73 m2)−1 (p < .001), respectively. The prevalence and severity of proteinuria also increased across the increasing quartiles (p < .001).
Table 1. Clinical characteristics of the population by MHR quartiles.
The prevalence of CKD across different quartiles of MHR groups
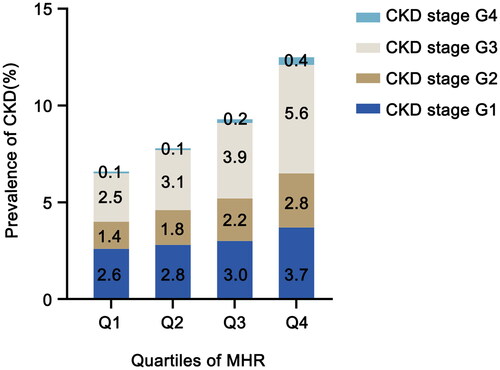
The prevalence of CKD and the distribution of its components across the four MHR quartiles are illustrated in . Overall, 21,014 (9.0%) individuals were diagnosed with CKD, which is consistent with previous epidemiological findings in this region. In groups from Q1 to Q4, the prevalence of CKD was 6.6%, 7.8%, 9.2%, and 12.5%, respectively. With the increase of MHR quartiles, the proportion of advanced CKD also gradually increased, especially the CKD stage G3 and stage G4. In Q3 and Q4 groups, the proportions of patients with CKD stage G3 were 3.9% and 5.6%, respectively, while the proportions of patients with CKD stage G4 were 0.2% and 0.4%, which were significantly higher than those of the lower quartile groups (p < .001).
Figure 2. Prevalence of CKD according to the quartile of MHR. The prevalence of CKD according to the quartiles of MHR was shown in different colored bars (dark blue: CKD stage G1, brown: CKD stage G2, gray: CKD stage G3, light blue: CKD stage G4). MHR: monocyte to high-density lipoprotein cholesterol ratio; CKD: chronic kidney disease.
The association between CKD and clinical characteristics
We performed a univariate analysis of the association between CKD and the relevant covariates (). The development of CKD in this study was positively correlated with age, BMI, SBP, DBP, SUA, FBG, total cholesterol, triglyceride, WBC, monocytes, and MHR. No significant difference was observed between males and females. Individuals with central obesity had a higher risk of CKD. Furthermore, we discovered a substantial negative correlation between CKD and relevant variables such as hemoglobin, platelet, HDL-C, and LDL-C.
Table 2. Univariate logistic analysis of the relationships between the other variables and CKD.
The association between MHR and CKD
Multivariate regression analysis was further performed to clarify the association between MHR and the presence of CKD (). In model 2, after adjusting for age and gender, a significant association was observed between higher MHR levels and the risk of CKD, as well as low eGFR and proteinuria (p < .001). In model 3, confounding factors such as waist circumference, BMI, FBS, SBP, DBP, total cholesterol, SUA, triglyceride, WBC, platelet, hemoglobin, and LDL-C were further adjusted. Independent associations were observed between MHR(per 1 SD increase) and CKD, as well as low eGFR and proteinuria. The odds ratio (ORs) and 95% confidence intervals (95%CIs) were 1.206(1.186–1.225), 1.289(1.260–1.319), and 1.150 (1.129–1.171) in model 3, respectively (p < .001).
Table 3. Multivariate logistic analysis of the association between MHR (per-1 SD increase) and the presence of CKD, low eGFR, and proteinuria.
Higher levels of MHR were associated with the risk of CKD in subgroup analysis
Subgroup analysis was further performed to explore the robustness of the association between MHR (per 1 SD increase) and CKD using multivariate analysis (). In this analysis, the population was stratified based on demographic and clinical characteristics and further adjusted for age, gender, platelet, hemoglobin, WBC, FBG, SUA, BMI, BP, total cholesterol, triglycerides, central obesity, and LDL-C.
Figure 3. The odds ratio (95% CI) of MHR (per 1 SD increase) for CKD in subgroups. The odds ratio (95% CI) of per 1 SD increase of MHR for CKD, were adjusted for age, gender, BMI, FBS, SBP, DBP, total cholesterol, SUA, triglyceride, central obesity (yes or no), Hb, WBC, PLT, and LDL-C. *represents statistical significance after the Bonferroni Correction.
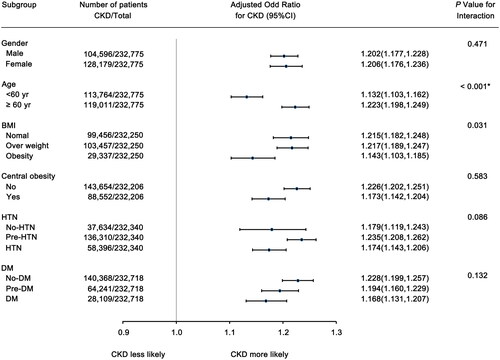
The risk of CKD increased by 20.6% (95%CI, 17.6%–23.6%) per 1 SD increase in MHR in females while 20.2% (95%CI, 17.7%–22.8%) in males. The association between MHR and CKD was not significantly different between the two groups (p = .471 for interaction). Increased MHR was linked to a greater risk of CKD in the elderly (OR = 1.223, per 1 SD increase in MHR, 95%CI, 1.198–1.249), compared to those younger than 60 years (OR = 1.132, per 1 SD increase in MHR, 95%CI, 1.103–1.162) (p < .001 for interaction).
After stratification according to BMI, the ORs of CKD for the overweight population was 1.217 (per 1 SD increase in MHR, 95%CI, 1.189–1.247), while the ORs for the normal group was 1.215 (per 1 SD increase in MHR, 95%CI, 1.182–1.248), which were slightly higher than that for the obese population (OR = 1.143, per 1 SD increase in MHR, 95%CI, 1.103–1.185) (p = .031 for interaction). However, after Bonferroni correction, there was no significant statistical significance observed in the p for interaction among these three subgroups. In addition, a higher level of MHR was consistently and independently associated with the risk of CKD regardless of whether individuals were comorbid with central obesity, HTN, or abnormal FBG, with no statistically significant difference in ORs between these subgroups. Positive correlations were further observed between MHR (per 1 SD increase) and the presence of low eGFR and proteinuria in individuals in different subgroups (Supplement Figure 1).
Discussion
Previous studies suggest that the development of CKD is associated with inflammation and disturbed lipid metabolism [Citation34–37]. Patients even with mild renal dysfunction tend to have elevated monocyte counts and reduced serum HDL-C concentration [Citation38,Citation39]. MHR has been recognized as an emerging marker of inflammation with abnormal lipid metabolism and the prognostic predictor of numerous diseases. To date, it is strongly associated with the risk of several metabolic diseases such as diabetes [Citation10], HTN [Citation11], obesity [Citation40], and target organ damage [Citation12,Citation18,Citation19,Citation41].
In this study, we observed a significant decrease in eGFR as MHR levels increased, while a notable increase in the prevalence and severity of proteinuria within a large general population. Importantly, a higher level of MHR was consistently and independently associated with the risk of CKD, low eGFR, and proteinuria. This association remained robust even after adjusting for confounding variables, regardless of whether individuals were comorbid with HTN, diabetes, obesity, or central obesity, as shown in the subgroup analysis.
It is well known that inflammation can lead to various alterations in lipid and lipoprotein metabolism. Elevated triglyceride levels and reductions in HDL-C are the most common changes associated with the inflammatory state. Moreover, a sustained increase in small dense LDL and Lp(a) levels, along with decreased LDL-C levels, can be observed. Additionally, inflammation affects the functionality of lipoproteins, such as diminishing the anti-inflammatory, antioxidant, and anti-atherosclerotic capacity of HDL-C. This dysfunctional HDL-C leads to an increase in oxidized LDL-C and loses its ability to promote cholesterol efflux from cells [Citation3].
As a proinflammatory state, CKD is expected to have a similar adverse impact on lipid balance. In recent years, the relationship between MHR and the risk of CKD has received increasing attention. Previous studies confirmed that elevated MHR levels were associated with the presence and severity of proteinuria and renal insufficiency, which is consistent with our conclusion [Citation20,Citation42]. Moreover, MHR was found to be independently linked with urine albumin excretion in patients with diabetic nephropathy, suggesting that it may serve as a biomarker for diabetic renal injury [Citation10,Citation19]. In our study, a positive correlation was observed between MHR and the presence of proteinuria and low eGFR regardless of whether individuals have diabetes. Furthermore, studies have shown that MHR was independently related to several comorbidities and the outcome in patients with CKD. Kanbay et al. showed that higher MHR values were related to both composite cardiovascular events [hazard ratio (HR)=4.91, 95%CI, 2.88-6.94] and higher mortality rate (HR = 2.24, 95%CI, 1.85-2.39) in patients with CKD [Citation43]. MHR may also be associated with multiple anti-HTN treatments and refractory HTN in CKD patients [Citation44]. Furthermore, two clinical studies suggested that patients undergoing peritoneal dialysis (PD) had an increased risk of cardiovascular disease and death from all causes when their MHR was elevated [Citation45,Citation46].
As well known, peripheral monocyte count has been demonstrated as a powerful predictor of atherosclerosis, especially in individuals with CKD [Citation47,Citation48]. The elevated number of circulating monocytes and their differentiation into lipid-laden macrophages are known to be critical in plaque formation. Monocytes also play key roles in immune surveillance and the maintenance of kidney homeostasis [Citation49]. It has been proven that interfering with monocyte recruitment prevented renal injury and subsequent scarring in most settings [Citation50,Citation51]. The ability of HDL-C to reverse cholesterol transport and its antioxidant and anti-inflammatory activities are essential protective factors in atherosclerosis formation. However, compared to healthy individuals, patients with CKD exhibit decreased levels and impaired maturation of HDL-C. This leads to decreased antioxidative and anti-inflammatory activities of HDL-C in patients [Citation52]. HDL-C abnormalities contribute to lipid accumulation in the artery wall and kidney tissue, which subsequently cause glomerulosclerosis, tubulointerstitial, and atherosclerosis injury in CKD [Citation53,Citation54].
In addition, a potential association between abnormal HDL-C metabolism and monocyte counts in patients with CKD has been noted. Anjali Ganda et al. illustrated that low HDL-C was independently associated with monocytosis in individuals with mild renal dysfunction [Citation39]. Studies indicated that monocytes were more likely to develop an inflammatory state in individuals with perturbed lipid profiles, and their cytokine production such as IL-1β was negatively correlated with HDL-C [Citation55]. HDL-C showed an anti-atherosclerotic effect by compromising the pro-oxidative and pro-inflammatory effects of monocytes by suppressing macrophage migration and LDL-C oxidation, as well as cholesterol efflux from these cells. In addition, HDL-C can also decrease the proliferation and differentiation of monocyte progenitor cells [Citation56].
It should be noted that patients with end-stage renal disease (ESRD) were excluded from this study because of their distinct pathophysiology. Differing from the general population, higher cholesterol concentrations are associated with improved clinical outcomes in patients with ESRD. This phenomenon might be explained by the interaction of inflammation and malnutrition [Citation57]. Moreover, the dialysis technique, selection of dialysate, and repeated use of heparin may contribute to additional disruptions in lipid homeostasis among patients undergoing maintenance hemodialysis. Additionally, substantial protein loss and glucose loading from the peritoneal dialysate (PD) both have an impact on lipid metabolism in patients undergoing PD [Citation23]. A specialized clinical cohort may be more suitable for the assessment of this subset of patients.
Our study confirmed the relationship between MHR and CKD risk in a general community population. More importantly, the association remained statistically significant after adjusting for confounding variables. These results were consistent with previous studies. However, previous studies have their limitations due to the small clinical sample sizes and different ethnic groups. According to our knowledge, this is the first study to explore the relationship between MHR and the prevalence of CKD in a large general Chinese community population. This will help us identify high-risk groups of CKD early and then follow up.
We acknowledge several limitations in our research. Firstly, the definition of CKD was based on a single measurement in this study. Existing epidemiological studies aiming to investigate the prevalence of CKD usually assess the eGFR and proteinuria of each participant once due to practical considerations, as taking repeated measurements is time-consuming and expensive [Citation31,Citation32,Citation58]. It’s important to note that a single measurement of eGFR in CKD diagnosis has a false positive rate of approximately 30%, which is even higher for albuminuria [Citation59]. Secondly, we assessed the proteinuria using a urine dipstick test in the study. However, many factors can influence the excretion of proteinuria including obesity, age, posture, and others, leading to wide fluctuations, hence false positivity of proteinuria [Citation59]. Previous researches have substantiated that using a threshold of urine protein ≥1+ could significantly decrease the chances of false positives [Citation60]. Thirdly, as a retrospective study, this research lacked information on certain potential confounding factors, such as acute infections, history of kidney transplantation, the presence of inflammatory diseases such as lupus or vasculitis, and inflammatory markers (C-reactive protein, etc.). Further investigations with additional data support are needed to assess the generalizability of our findings to other CKD populations. To address these shortcomings in this research, we have established a cohort to enhance the credibility and persuasiveness of the study’s findings. The results from this validation cohort support our study’s conclusions, with details available in the supplementary materials section.
In conclusion, we demonstrated that MHR values were independently associated with CKD prevalence in the general population of the Chinese community. Since MHR combines monocytes and HDL-C, an increased MHR can represent an enhanced inflammatory response and dyslipidemia response in CKD and may predict poor outcomes of CKD. As MHR measurement is cheap and convenient, it might serve as a useful clinical tool for risk stratification, offering valuable insights to inform preventive and therapeutic approaches for clinicians in their routine medical practice. Further prospective clinical research is necessary to verify the causal relationship and potential pathophysiological mechanisms between MHR and the progression of CKD in the future.
Authors contributions
JY guaranteed the complete integrity of this research. JY, YZ, and PW designed this study. LX, DL, JL, and ZS were responsible for carrying out the clinical studies. LX, JL, DL, and ZS all contributed to gathering the data. Data analysis and manuscript writing were performed by LX and PW. The paper was revised by JY after being edited by YZ. All authors have reviewed and given their approval to the final manuscript. LX and DL contributed to this work equally.
Consent form
All coauthors and participants have approved this paper for publishing in Renal Failure.
Ethical approval
The Medical Ethics Committee of the Second Affiliated Hospital of Nanjing Medical University approved this study (ID: [2019]KY105).
Supplemental Material
Download MS Word (33.4 KB)Acknowledgments
The researchers appreciate the dedication of each participant and their families to this research. Special thanks to Shen Tai Web Health Technology (Nanjing) Co., Ltd. for providing data technical support for this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used in this work are accessible upon reasonable request from the corresponding author.
Additional information
Funding
References
- Bikbov B, Purcell CA, Levey AS, et al. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2020;395(10225):1–10. doi: 10.1016/S0140-6736(20)30045-3.
- Vaziri ND, Navab M, Fogelman AM. HDL metabolism and activity in chronic kidney disease. Nat Rev Nephrol. 2010;6(5):287–296. doi: 10.1038/nrneph.2010.36.
- Feingold KR, Grunfeld C, et al. The effect of inflammation and infection on lipids and lipoproteins. In Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, editors. Endotext. South Dartmouth (MA): MDText.com, Inc.; 2000.
- Reis A, Rudnitskaya A, Chariyavilaskul P, et al. Top-down lipidomics of low density lipoprotein reveal altered lipid profiles in advanced chronic kidney disease. J Lipid Res. 2015;56(2):413–422. doi: 10.1194/jlr.M055624.
- Chen H, Chen L, Liu D, et al. Combined clinical phenotype and lipidomic analysis reveals the impact of chronic kidney disease on lipid metabolism. J Proteome Res. 2017;16(4):1566–1578. doi: 10.1021/acs.jproteome.6b00956.
- Kim HJ, Moradi H, Yuan J, et al. Renal mass reduction results in accumulation of lipids and dysregulation of lipid regulatory proteins in the remnant kidney. Am J Physiol Renal Physiol. 2009;296(6):F1297–F1306. doi: 10.1152/ajprenal.90761.2008.
- Apostolov EO, Ray D, Savenka AV, et al. Chronic uremia stimulates LDL carbamylation and atherosclerosis. J Am Soc Nephrol. 2010;21(11):1852–1857. doi: 10.1681/ASN.2010040365.
- Demirbaş A, Elmas ÖF, Atasoy M, et al. Can monocyte to HDL cholesterol ratio and monocyte to lymphocyte ratio be markers for inflammation and oxidative stress in patients with vitiligo? A preliminary study. Arch Dermatol Res. 2021;313(6):491–498. doi: 10.1007/s00403-020-02129-3.
- Canpolat U, Cetin EH, Cetin S, et al. Association of monocyte-to-HDL cholesterol ratio with slow coronary flow is linked to systemic inflammation. Clin Appl Thromb Hemost. 2016;22(5):476–482. doi: 10.1177/1076029615594002.
- Karatas A, Turkmen E, Erdem E, et al. Monocyte to high-density lipoprotein cholesterol ratio in patients with diabetes mellitus and diabetic nephropathy. Biomark Med. 2018;12(9):953–959. doi: 10.2217/bmm-2018-0048.
- Zhou Y, Dan H, Bai L, et al. Continuous positive linear association between the monocyte to high-density lipoprotein cholesterol ratio and hypertension: a cross-sectional study. Int J Hypertens. 2022;2022:8501726–8501729. doi: 10.1155/2022/8501726.
- Liu HT, Jiang ZH, Yang ZB, et al. Monocyte to high-density lipoprotein ratio predict long-term clinical outcomes in patients with coronary heart disease: a meta-analysis of 9 studies. Medicine (Baltimore). 2022;101(33):e30109. doi: 10.1097/MD.0000000000030109.
- Chen SA, Zhang MM, Zheng M, et al. The preablation monocyte/high density lipoprotein ratio predicts the late recurrence of paroxysmal atrial fibrillation after radiofrequency ablation. BMC Cardiovasc Disord. 2020;20(1):401. doi: 10.1186/s12872-020-01670-3.
- Canpolat U. Monocyte-to-HDL-cholesterol ratio and left atrial remodelling in atrial fibrillation. Europace. 2017;19(8):1409–1409. doi: 10.1093/europace/euw195.
- Huang H, Wang Q, Shi X, et al. Association between monocyte to high-density lipoprotein cholesterol ratio and nonalcoholic fatty liver disease: a cross-sectional study. Mediators Inflamm. 2021;2021:6642246–6642247. doi: 10.1155/2021/6642246.
- Wang L, Dong J, Xu M, et al. Association between monocyte to high-density lipoprotein cholesterol ratio and risk of non-alcoholic fatty liver disease: a cross-sectional study. Front Med (Lausanne). 2022;9:898931. doi: 10.3389/fmed.2022.898931.
- Ekizler FA, Cay S, Açar B, et al. Monocyte to high-density lipoprotein cholesterol ratio predicts adverse cardiac events in patients with hypertrophic cardiomyopathy. Biomark Med. 2019;13(14):1175–1186. doi: 10.2217/bmm-2019-0089.
- Jiang M, Yang J, Zou H, et al. Monocyte-to-high-density lipoprotein-cholesterol ratio (MHR) and the risk of all-cause and cardiovascular mortality: a nationwide cohort study in the United States. Lipids Health Dis. 2022;21(1):30. doi: 10.1186/s12944-022-01638-6.
- Onalan E. The relationship between monocyte to high-density lipoprotein cholesterol ratio and diabetic nephropathy. Pak J Med Sci. 2019;35(4):1081–1086. doi: 10.12669/pjms.35.4.534.
- Shi WR, Wang HY, Chen S, et al. The impact of monocyte to high-density lipoprotein ratio on reduced renal function: insights from a large population. Biomark Med. 2019;13(9):773–783. doi: 10.2217/bmm-2018-0406.
- Saskin H. Is pre-operative monocyte count-high-density lipoprotein ratio associated with postoperative acute kidney injury in isolated coronary artery bypass grafting? Cardiovasc J Afr. 2022;33:1–7. doi: 10.5830/CVJA-2022-055.
- Wang L, Xu X, Zhang M, et al. Prevalence of chronic kidney disease in China: results from the sixth China chronic disease and risk factor surveillance. JAMA Intern Med. 2023;183(4):298–310. doi: 10.1001/jamainternmed.2022.6817.
- Lacquaniti A, Bolignano D, Donato V, et al. Alterations of lipid metabolism in chronic nephropathies: mechanisms, diagnosis and treatment. Kidney Blood Press Res. 2010;33(2):100–110. doi: 10.1159/000302712.
- Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community a statement by the American society of hypertension and the international society of hypertension. J Hypertens. 2014;32(1):3–15. doi: 10.1097/HJH.0000000000000065.
- American Diabetes Association Professional Practice Committee. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S17–S38. doi: 10.2337/dc22-S002.
- Alberti KG, Zimmet P, Shaw J. The metabolic syndrome–a new worldwide definition. Lancet. 2005;366(9491):1059–1062. doi: 10.1016/S0140-6736(05)67402-8.
- Zhou BF. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15(1):83–96.
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006.
- Brück K, Jager KJ, Dounousi E, et al. Methodology used in studies reporting chronic kidney disease prevalence: a systematic literature review. Nephrol Dial Transplant. 2015;30(Suppl 4):iv6–16. doi: 10.1093/ndt/gfv131.
- Lamb EJ, MacKenzie F, Stevens PE. How should proteinuria be detected and measured?. Ann Clin Biochem. 2009;46(Pt 3):205–217. doi: 10.1258/acb.2009.009007.
- Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379(9818):815–822. doi: 10.1016/S0140-6736(12)60033-6.
- Glassock RJ, Warnock DG, Delanaye P. The global burden of chronic kidney disease: estimates, variability and pitfalls. Nat Rev Nephrol. 2017;13(2):104–114. doi: 10.1038/nrneph.2016.163.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150.
- Oberg BP, McMenamin E, Lucas FL, et al. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004;65(3):1009–1016. doi: 10.1111/j.1523-1755.2004.00465.x.
- Xu G, Luo K, Liu H, et al. The progress of inflammation and oxidative stress in patients with chronic kidney disease. Ren Fail. 2015;37(1):45–49. doi: 10.3109/0886022X.2014.964141.
- Kwan BC, Kronenberg F, Beddhu S, et al. Lipoprotein metabolism and lipid management in chronic kidney disease. J Am Soc Nephrol. 2007;18(4):1246–1261. doi: 10.1681/ASN.2006091006.
- Xu L, Liu J, Li D, et al. Association between metabolic syndrome components and chronic kidney disease among 37,533 old Chinese individuals. Int Urol Nephrol. 2022;54(6):1445–1454. doi: 10.1007/s11255-021-03013-3.
- Evangelopoulos AA, Vallianou NG, Bountziouka V, et al. Association between serum cystatin C, monocytes and other inflammatory markers. Intern Med J. 2012;42(5):517–522. doi: 10.1111/j.1445-5994.2011.02500.x.
- Ganda A, Magnusson M, Yvan-Charvet L, et al. Mild renal dysfunction and metabolites tied to low HDL cholesterol are associated with monocytosis and atherosclerosis. Circulation. 2013;127(9):988–996. doi: 10.1161/CIRCULATIONAHA.112.000682.
- Serin SO, Sisik A, Basak F. Relationship between monocyte-to-high-density lipoprotein cholesterol ratio and excess weight loss in laparoscopic sleeve gastrectomy. Biomark Med. 2021;15(15):1367–1375. doi: 10.2217/bmm-2021-0244.
- Tang X, Tan Y, Yang Y, et al. Association of the monocyte-to-high-density lipoprotein cholesterol ratio with diabetic retinopathy. Front Cardiovasc Med. 2021;8:707008. doi: 10.3389/fcvm.2021.707008.
- Yılmaz Aydın F, Eynel E, Oruç İ, et al. The role of monocyte to high-density lipoprotein cholesterol ratio in predicting the severity of proteinuria and renal dysfunction in primary nephrotic syndrome. Cureus. 2021;13(12):e20345. doi: 10.7759/cureus.20345.
- Kanbay M, Solak Y, Unal HU, et al. Monocyte count/HDL cholesterol ratio and cardiovascular events in patients with chronic kidney disease. Int Urol Nephrol. 2014;46(8):1619–1625. doi: 10.1007/s11255-014-0730-1.
- Gembillo G, Siligato R, Cernaro V, et al. Monocyte to HDL ratio: a novel marker of resistant hypertension in CKD patients. Int Urol Nephrol. 2022;54(2):395–403. doi: 10.1007/s11255-021-02904-9.
- Chen J, Zhong Z, Shi D, et al. Association between monocyte count to high-density lipoprotein cholesterol ratio and mortality in patients undergoing peritoneal dialysis. Nutr Metab Cardiovasc Dis. 2021;31(7):2081–2088. doi: 10.1016/j.numecd.2021.03.014.
- Zhan X, Pan D, Wei X, et al. Monocyte to high-density lipoprotein ratio and cardiovascular events in patients on peritoneal dialysis. Nutr Metab Cardiovasc Dis. 2020;30(7):1130–1136. doi: 10.1016/j.numecd.2020.03.011.
- Björkegren JLM, Lusis AJ. Atherosclerosis: recent developments. Cell. 2022;185(10):1630–1645. doi: 10.1016/j.cell.2022.04.004.
- Rogacev KS, Ulrich C, Blömer L, et al. Monocyte heterogeneity in obesity and subclinical atherosclerosis. Eur Heart J. 2010;31(3):369–376. doi: 10.1093/eurheartj/ehp308.
- Tang PM, Nikolic-Paterson DJ, Lan HY. Macrophages: versatile players in renal inflammation and fibrosis. Nat Rev Nephrol. 2019;15(3):144–158. doi: 10.1038/s41581-019-0110-2.
- Meng XM, Mak TS, Lan HY. Macrophages in renal fibrosis. Adv Exp Med Biol. 2019;1165:285–303. doi: 10.1007/978-981-13-8871-2_13.
- Bell RMB, Conway BR. Macrophages in the kidney in health, injury and repair. Int Rev Cell Mol Biol. 2022;367:101–147. doi: 10.1016/bs.ircmb.2022.01.005.
- Noels H, Lehrke M, Vanholder R, et al. Lipoproteins and fatty acids in chronic kidney disease: molecular and metabolic alterations. Nat Rev Nephrol. 2021;17(8):528–542. doi: 10.1038/s41581-021-00423-5.
- Vaziri ND. HDL abnormalities in nephrotic syndrome and chronic kidney disease. Nat Rev Nephrol. 2016;12(1):37–47. doi: 10.1038/nrneph.2015.180.
- Bowe B, Xie Y, Xian H, et al. Low levels of high-density lipoprotein cholesterol increase the risk of incident kidney disease and its progression. Kidney Int. 2016;89(4):886–896. doi: 10.1016/j.kint.2015.12.034.
- Patel VK, Williams H, Li SCH, et al. Monocyte inflammatory profile is specific for individuals and associated with altered blood lipid levels. Atherosclerosis. 2017;263:15–23. doi: 10.1016/j.atherosclerosis.2017.05.026. PMID: 28570862.
- Ganjali S, Gotto AM, Ruscica M, et al. Monocyte-to-HDL-cholesterol ratio as a prognostic marker in cardiovascular diseases. J Cell Physiol. 2018;233(12):9237–9246. doi: 10.1002/jcp.27028.PMID: 30076716.
- Liu Y, Coresh J, Eustace JA, et al. Association between cholesterol level and mortality in dialysis patients: role of inflammation and malnutrition. JAMA. 2004;291(4):451–459. doi: 10.1001/jama.291.4.451.
- Mills KT, Xu Y, Zhang W, et al. A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int. 2015;88(5):950–957. doi: 10.1038/ki.2015.230.
- Benghanem Gharbi M, Elseviers M, Zamd M, et al. Chronic kidney disease, hypertension, diabetes, and obesity in the adult population of Morocco: how to avoid "over"- and "under"-diagnosis of CKD. Kidney Int. 2016;89(6):1363–1371. doi: 10.1016/j.kint.2016.02.019.
- White SL, Yu R, Craig JC, et al. Diagnostic accuracy of urine dipsticks for detection of albuminuria in the general community. Am J Kidney Dis. 2011;58(1):19–28. doi: 10.1053/j.ajkd.2010.12.026.