Abstract
Tertiary hyperparathyroidism is a complication of kidney transplantation. This complicated condition carries over from the dialysis period and varies according to the function of the transplanted allograft. Treatments include pharmacotherapy (mainly using calcimimetics) and parathyroidectomy, but calcimimetics are currently not covered by the national insurance system in Japan. Two types of parathyroidectomy can be performed: subtotal parathyroidectomy; and total parathyroidectomy with partial autograft. Both types can be expected to improve hypercalcemia. Concerns about the postoperative deterioration of allograft function are influenced by preoperative allograft function, which is even more likely to be affected by early surgery after kidney transplantation. In general, transient deterioration of allograft function after surgery is not expected to affect graft survival rate in the medium to long term. Tertiary hyperparathyroidism in kidney transplant recipients negatively impacts allograft and patient survival rates, and parathyroidectomy can be expected to improve prognosis in both kidney recipients and dialysis patients. However, studies offering high levels of evidence remain lacking.
1. Introduction
The pathophysiology of chronic kidney disease-mineral bone disorders (CKD-MBD) after kidney transplantation includes ‘reversible abnormalities,’ ‘irreversible abnormalities,’ and ‘de novo abnormalities’ [Citation1].
One of the most important clinical conditions in CKD-MBD after kidney transplantation is hyperparathyroidism (HPT), which may include: (1) persistence of pre-transplant renal hyperparathyroidism; and (2) HPT as a complication of impaired allograft function.
The pathogenesis of secondary hyperparathyroidism (SHPT), which develops in dialysis patients, changes with the recovery of allograft function after kidney transplantation, but is complicated by carryover of the pre-transplant pathological state and the effects of reduced allograft function. In addition, although the time of onset varies, a rare case of pathologically mixed adenoma and hyperplasia exists, that is, primary hyperparathyroidism combined with CKD-MBD. The pathophysiology after kidney transplantation is therefore very complicated.
In this State-of-the-Art Review, we refer to a state of SHPT that remains after kidney transplantation as tertiary hyperparathyroidism (THPT), corresponding to 1) above.
A search for articles on the outcome of the treatment of THPT was conducted. Publications were selected from MEDLINE. Original full-text articles (excluding case reports) written in English, published between 1998 and 2022, and describing the adult human population were selected. Articles describing the treatment of THPT, both surgical and medical, and those describing allograft function after the initiation of these therapies were included. The following search terms were used: hyperparathyroidism; parathyroid hormone; kidney/renal transplantation; calcimimetic/cinacalcet; and parathyroidectomy. Although the definition of THPT varies, all definitions used in the literature were included.
The pathophysiology, treatment with a focus on parathyroidectomy (PTx), and outcomes are discussed in detail.
2. Pathophysiology of THPT and the need for therapeutic intervention
2.1. Pathophysiology of THPT
SHPT may improve with the recovery of allograft function after kidney transplantation, but has been reported to persist for more than 1 year in 17–50% of kidney recipients [Citation2]. Risk factors for persistent THPT after kidney transplantation include high levels of parathyroid hormone (PTH), calcium, and phosphorus at the time of transplantation and long-term dialysis [Citation2]. Yamamoto et al. also reported an association with preoperative parathyroid size, along with similar risk factors, although that was a single-center clinical study [Citation3].
In a recent report, patients with SHPT prior to kidney transplantation who were treated with PTx showed significantly lower risks of developing THPT after kidney transplantation than patients treated with pharmacotherapy alone using cinacalcet, and PTx is recommended for patients with SHPT undergoing kidney transplantation after more than 3 years of maintenance dialysis [Citation4].
Histological studies of parathyroid glands removed after kidney transplantation have shown that parathyroid cells in the diffuse hyperplastic stage in the polyclonal form tend to shrink and easily undergo apoptosis, while parathyroid cells that have histologically developed into nodular hyperplasia are less likely to undergo apoptosis even after kidney transplantation [Citation5]. This is attributed to decreased function of various receptors with decreased expression density in nodular hyperplastic glands [Citation5].
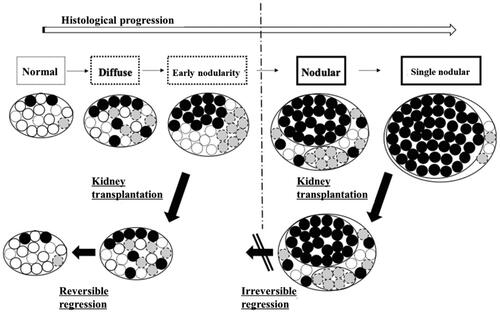
Even if the size of the parathyroid gland is reduced after kidney transplantation, areas within the gland that have developed into nodular hyperplasia may remain, starting the pathogenesis of THPT [Citation6] ().
Figure 1. Hypothesized mechanism of THPT in kidney transplant recipients. Diffuse hyperplastic glands undergo reversible regression after kidney transplantation, while nodular hyperplastic glands show irreversible changes that do not regress. This state in nodular hyperplastic glands is attributed to decreased densities of various parathyroid cell receptors (vitamin D receptors, calcium-sensing receptors, FGF23 receptors, etc.).
2.2. The need for therapeutic intervention
Sporadic reports have described the impact of THPT on graft and patient survival in kidney transplant recipients. Although it is difficult to explain the pathogenic mechanism sufficiently, hypercalcemia associated with THPT and related vascular lesions may be involved. Based on the basic research [Citation7], the glomerular hemodynamic effects of PTH may affect long-term allograft function.
A study comparing survival rates after kidney transplantation in 568 THPT patients and 343 non-THPT patients followed for a mean of 47 months found a significantly poorer survival rate in THPT patients [Citation8].
In a retrospective study of 522 patients with good allograft function in the Norwegian Registry, classified by PTH level at 10 weeks after kidney transplantation and analyzed for composite endpoints (cardiovascular events, graft survival rate, and overall mortality), patients with PTH levels ≥135 pg/ml displayed the worst prognosis [Citation9].
Similarly, comparing allograft survival and mortality in 1840 patients with PTH levels at the time of transplantation classified into quintiles, both outcomes were better in patients whose PTH levels were managed at lower levels [Citation10].
These represent clinically important results that may indicate the negative effects of untreated THPT on allograft and patient survival; in other words, they suggest the importance of therapeutic interventions for THPT after kidney transplantation.
3. Types of therapeutic intervention for THPT
3.1. Pharmacotherapy
Calcimimetics form the mainstay of pharmacotherapy for SHPT, but have yet to gain approval for use in transplant patients in Japan under the national insurance system. The efficacy of calcimimetics against THPT in patients with hypercalcemia has been reported in a prospective overseas study [Citation11]. All reports of administration to kidney transplant recipients in Japan have involved small numbers of cases, and no long-term results have been provided.
On the other hand, active vitamin D analogs act on the parathyroid glands to suppress PTH production, but are difficult to administer to THPT patients with hypercalcemia.
3.2. Surgical treatment
Surgery is performed when pharmacotherapy proves ineffective against THPT. A patient showing resistance to pharmacotherapy would have an advanced THPT complicated by large and/or multiple enlarged glands.
Four possible surgical procedures are currently available to achieve PTx: removal of the enlarged gland only; subtotal PTx; total PTx plus autograft; or total PTx alone.
Kidney transplant recipients often experience difficulty achieving normal renal function and frequently show a renal function equivalent to that in chronic kidney disease stage G3 or G4. Therefore, when considering the pathophysiology of THPT, removal of the enlarged gland alone appears insufficient to improve THPT because of hyperplasia in the remaining gland. Total PTx without autograft is not recommended because of the possibility of severe hypoparathyroidism.
Subtotal PTx is widely performed in Europe and the United States. In Japan, however, considering the possibility of future deterioration of allograft function and the difficulty of re-transplantation, total PTx plus autograft is the most popular procedure and is less likely to cause recurrence.
shows the indications for PTx after kidney transplantation at our institution.
Table 1. Surgical indications for tertiary hyperparathyroidism after kidney transplantation.
4. Outcomes of therapeutic interventions for THPT
Outcomes of therapeutic interventions for THPT involve both short- and long-term outcomes. The former mainly comprise the efficacy and effectiveness of the therapeutic intervention, as exemplified by improved hypercalcemia. However, in the case of kidney recipients, impacts on allograft function should also be carefully considered. The latter may also be considered from a long-term perspective, such as impacts on patient and allograft survival rates, and bone fractures. We review the literature and discuss in detail the merits of medical and surgical interventions, and, with surgical interventions, whether differences in outcomes exist according to the type of procedure and timing of surgery.
4.1. Short-term outcomes
4.1.1. Efficacy of PTx
One review that aimed to analyze the indications and effects of PTx for THPT stated that PTx was effective in achieving normocalcemia in all the reports examined [Citation12]. In addition, only a few complications have been reported to result from PTx. One of these, recurrent nerve palsy, was observed in approximately 2.1% of patients [Citation13].
For THPT, although most reports from Europe and the United States have been based on results with subtotal PTx, total PTx with autograft is also a representative procedure.
Various reports have compared procedures for SHPT [Citation14], but no randomized trials have compared procedures for THPT [Citation15].
Subtotal PTx offers good results in terms of short-term efficacy [Citation16,Citation17]. The advantage of subtotal PTx is that the procedure is less prone to result in hypocalcemia and hypoparathyroidism. A retrospective analysis of PTx (subtotal: 83%) among 30 THPT patients reported that 3 patients (10%) experienced recurrence and required reoperation, all with subtotal or less-than-subtotal resection [Citation18].
No studies have directly compared procedures, but a review by Dulfer [Citation13] stated that the frequencies of persistence and recurrence of hyperparathyroidism with total, subtotal, and limited resection were 4%, 8.9%, and 91%, respectively, and that limited resection should be avoided.
In Japan, the most common procedure for SHPT is total PTx plus autograft, mainly to reduce the risk of recurrent hyperparathyroidism caused by the presence of continued chronic renal failure. Considering that the etiology of SHPT is not resolved after kidney transplantation because the transplanted kidney function is not normal, and that second transplantation after loss of allograft function is unlikely in Japan and continued dialysis therapy will thus be required, we recommend that THPT be treated with total PTx plus autograft, as a procedure less likely to result in recurrence.
4.1.2. Comparison with pharmacotherapies
No reports have compared medical and surgical treatments of THPT in Japan, partly because the use of calcimimetics is not covered by the national insurance system for kidney recipients. Although very few reports have been published from overseas, a retrospective cohort study by Yang et al. reported the efficacy of PTx [Citation19]. In a prospective study comparing 15 patients with cinacalcet and 15 patients with PTx (subtotal PTx) in 30 THPT patients, the rate of achieving normocalcemia at 12 months was 67% in the cinacalcet group and 100% in the PTx group, with the PTx group reporting a greater rate of PTH reduction [Citation17]. Reanalysis was performed with an extended period of 5 years, and a recent addition showed that the recurrence rate (re-elevation of calcium levels) was higher in the cinacalcet group [Citation20].
Other reports comparing PTx and calcimimetics have also shown that PTx is associated with lower calcium and PTH levels and less frequent recurrence [Citation19,Citation21]. On the other hand, results with respect to percutaneous ethanol injection therapy are uncertain and the long-term results appear poor [Citation22]. Although randomized controlled trials of medical versus surgical therapy are difficult to conduct, we believe that the efficacy of reliable PTx for hypercalcemia is indisputable.
4.1.3. Short-term allograft function following PTx
We want to minimize the impact of the allograft function following PTx. One study found that 19 of 69 patients who underwent PTx for THPT experienced a > 20% decrease in estimated glomerular filtration rate (eGFR) and that this decrease in eGFR continued for more than 1 year [Citation23]. On the other hand, some reports have indicated that eGFR is decreased after PTx, but does not remain significantly decreased at 6 months or 1 year [Citation24,Citation25]. The mechanism by which allograft function is impaired after PTx is unclear; however, based on basic research [Citation7], the glomerular hemodynamic effects of PTH may be influential. Additionally, general anesthesia itself may have adverse effects on patients with impaired allograft function.
According to a meta-analysis of impacts on allograft function after PTx [Citation26], many reports have indicated no impact on postoperative allograft function with respect to PTx, along with pharmacotherapy using calcimimetics. However, study reports are not uniform in various items, including the definition of THPT, and these conclusions cannot be considered to represent a high level of evidence.
In discussing these issues, consideration must also be given to the effects on transplanted allograft function of leaving the THPT patient without PTx after kidney transplantation and allowing hypercalcemia to persist.
Transplanted allograft function in kidney transplant recipients is the most important point of concern. Any deleterious impact on allograft function, even in the short term, should be avoided. For this reason, Japanese CKD-MBD guidelines also recommend PTx prior to transplantation in cases where surgery is indicated, so that clinicians do not have to bother with the decision of whether to perform PTx after renal transplantation.
If kidney transplantation is planned for a patient with advanced SHPT who has multiple enlarged glands on imaging and is being treated at or near the maximum drug dosage, PTx should be performed before transplantation.
4.1.4. Perspectives on surgical procedures
In the literature on surgical procedures, among 47 patients who underwent initial PTx for THPT, 35 patients who underwent total PTx plus autograft showed significantly lower postoperative eGFR than those who underwent subtotal resection [Citation23]. The rate of decline appears even more pronounced in cases with low baseline eGFR, and the authors of that report recommended subtotal PTx as the procedure for THPT.
Another study analyzing risk factors for decreased allograft function after PTx reported a low postoperative PTH level as a significant risk factor along with preoperative eGFR [Citation27]. This low postoperative PTH level may be one possible cause of decreased transplanted allograft function after total PTx. None of the study designs provided a high level of evidence due to insufficient numbers of cases, and no single procedure can currently be recommended definitively.
PTH levels reportedly remain persistently high after surgery in 21–46% of cases, even after calcium levels normalize following subtotal or lesser resection [Citation28,Citation29]. An analysis of total PTx plus autograft in Japan reported no effect on allograft function [Citation3]. As discussed later (4.2.1), consideration of allograft prognosis from a long-term perspective is necessary.
4.2. Long-term outcomes
4.2.1. Long-term allograft function following PTx
Sporadic studies have provided long-term follow-up of transplanted allograft function after PTx. We have summarized studies on medium- to long-term allograft function after PTx in . Overall, many studies have reported that PTx performed in kidney transplant recipients results in decreased allograft function in the short postoperative period (e.g., within 1 year), but over the medium to long term, allograft function normalizes and no differences in graft survival rates are apparent [Citation16,Citation24,Citation25,Citation30,Citation31]. However, when PTx is performed within 1 year after kidney transplantation [Citation27,Citation32] or in recipients with low baseline allograft function prior to PTx, recovery of allograft function is reportedly not expected [Citation33]. Patients who must undergo PTx within a year are often severe cases. We speculate that a rapid decrease in PTH levels after PTx in such cases may be associated with an eGFR decline.
Table 2. Summary of studies reporting on allograft function after parathyroidectomy.
PTx should be performed before kidney transplantation in severe cases requiring PTx early after transplantation, and after 1 year in less advanced cases with observation of the clinical course after kidney transplantation. An observational study by Dream et al. [Citation34] reported that delays in PTx after kidney transplantation led to worsened allograft function. If a patient scheduled for kidney transplantation develops moderate or severe SHPT as a complication, we believe that PTx is safest performed prior to kidney transplantation for those cases in which there is uncertainty regarding whether the patient should undergo PTx.
Attention should therefore be paid to parathyroid function prior to kidney transplantation, to avoid missing the optimal timing of PTx prior to transplantation.
When considering the effects of PTx on transplanted allograft function as described above, it should be noted that if hypercalcemia is left untreated with PTx after kidney transplantation, the prognosis in terms of allograft function is likely to prove unfavorable. No reports have compared cases of hypercalcemia left untreated with PTx and those treated with PTx, and such clinical studies are expected to remain difficult to conduct from an ethical standpoint.
4.2.2. Effect of PTx on bone
One outcome of PTx for THPT is the effect on fractures and bone density. Although the harmful effects of THPT on fractures have been reported [Citation35], no reports have clarified the effects of PTx.
On the other hand, the impact of PTx on bone density has been studied and scattered comparisons with pharmacotherapy have been conducted. Calcimimetics are drugs used in the medical treatment for bone in patients with THPT; however, a prospective comparative study showed the superiority of PTx in terms of the bone mineral density of the femoral neck (16). Numerous reports of greater improvements in bone mineral density after PTx compared to calcimimetic administration in THPT patients have indicated that PTx may be more effective than continued pharmacotherapy [Citation17,Citation36]. Osteoporotic drugs may be used for patients with THPT; however, they are not essential.
4.2.3. Effect of PTx on patient survival rate
Many studies of PTx in dialysis patients with SHPT have been reported from various regions of the world, showing improvements in all-cause mortality and cardiovascular morbidity and mortality [Citation37].
One study from Sweden reported on 423 dialysis patients and 156 renal transplant patients who received PTx to clarify the prognostic impact of PTx on respective outcomes. According to that study, dialysis patients showed improved prognosis, supporting previous findings, whereas no significant difference was observed in kidney transplant recipients [Citation38]. In the discussion of limitations, the authors reasoned that the kidney transplant cohort represented a disproportionate population that included a variety of allograft functions, BK virus complications, post-transplant diabetes, and recurrence of the primary disease.
Although various prognostic benefits of PTx have been demonstrated in SHPT, no reports have provided high-level evidence for the prognostic benefits of PTx for THPT. Future clinical studies should therefore focus on this topic. However, as mentioned earlier, given the reports of allograft and patient survival with THPT [Citation8–10], imagining negative effects from a prolonged THPT status is not difficult.
5. Discussion
The pathophysiology and treatment of THPT after kidney transplantation are described, mainly focusing on PTx outcomes in detail.
With regard to concerns about worsening transplanted allograft function after PTx, baseline function would arguably have an effect, and function is more likely to be adversely impacted by PTx early after kidney transplantation. On the other hand, except for cases with poor allograft function or severe THPT requiring early PTx after transplantation, transient decreases in allograft function are not expected to affect survival rates over the medium to long term.
High-level evidence studies have shown that PTx for SHPT improves patient survival. Although clinical studies of THPT are still needed, the outcomes for kidney recipients with severe THPT are poor, and imagining that therapeutic interventions with PTx would prove effective is not difficult.
In conclusion, THPT is a not-uncommon disorder of CKD-MBD after kidney transplantation. Therapeutic interventions, most notably parathyroidectomy, are presumed to improve not only hypercalcemia, but also allograft survival and patient mortality.
In terms of prospects for the future, primary importance should be given to avoiding situations necessitating early PTx after kidney transplantation. To this end, advanced SHPT must be diagnosed before kidney transplantation and not overlooked, so diagnostic imaging modalities such as ultrasonography are indispensable. The treatment strategy should be as follows: 1) For advanced SHPT that is expected to require PTx early after transplantation, PTx should be performed before transplantation, and 2) for other less advanced persistent HPT, the condition should be evaluated approximately 1 year after transplant, when the frequency of post-transplant complications and acute rejection decreases, and if PTx is indicated, it is recommended.
Second, persistent hypercalcemia should not be left untreated. Although the long-term efficacy of pharmacotherapies should be fully explored in the future, PTx by a skilled surgeon should be performed without delay, as this method is sure to be effective in maintaining allograft function for a longer period of time. THPT should not be ignored, for the sake of precious allografts.
Authors contributions
MN contributed to conceptualization, manuscript writing (original draft, review, editing), and supervision. ST, SU, and YT contributed to conceptualization, investigation, and illustration. All authors contributed to manuscript revision, and have read and approved the submitted version.
Acknowledgments
We are grateful to the Tokai University General Research Organization for funding the editing and proofreading of this paper.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Hirukawa T, Kakuta T, Nakamura M, et al. Mineral and bone disorders in kidney transplant recipients: reversible, irreversible, and de novo abnormalityes. Clin Exp Nephrol. 2015;19(4):1–8. doi: 10.1007/s10157-015-1117-z.
- Evenepoel P, Claes K, Kuypers D, et al. Natural history of parathyroid function and calcium metabolism after kidney transplantation: a single-centre study. Nephrol Dial Transplant. 2004;19(5):1281–1287. doi: 10.1093/ndt/gfh128.
- Yamamoto T, Tominaga Y, Okada M, et al. Characteristics of persistent hyperparathyroidism after renal transplantation. World J Surg. 2016;40(3):600–606. doi: 10.1007/s00268-015-3314-z.
- Mathur A, Sutton W, Ahn JB, et al. Association between treatment of secondary hyperparathyroidism and posttransplant outcomes. Transplantation. 2021;105(12):e366–e374. doi: 10.1097/TP.0000000000003653.
- Taniguchi M, Tokumoto M, Matsuo D, et al. Persistent hyperparathyroidism in renal allograft recipients: vitamin D receptor, calcium-sensing receptor, and apoptosis. Kidney Int. 2006;70(2):363–370. doi: 10.1038/sj.ki.5001549.
- Nakamura M, Tanaka K, Marui Y, et al. Clinicapathologicaal analysis of persistent hyperparathyroidism after kidney transplantation in long-term dialysis patients. Ther Apher Dial. 2013;17(5):551–556. doi: 10.1111/1744-9987.12018.
- Massfelder T, Parekh N, Endlich K, et al. Effect of intrarenally infused parathyroid hormone-related protein on renal blood flow and glomerular filtration rate in the anaesthetized rat. Br J Pharmacol. 1996;118(8):1995–2000.
- Araujo MJCLN, Ramalho JAM, Elias RM, et al. Persistent hyperparathyroidism as a risk factor for long-term graft failure: the need to discuss indication for parathyroidectomy. Surgery. 2018;163(5):1144–1150. doi: 10.1016/j.surg.2017.12.010.
- Bleskestad IH, Bergrem H, Leivestad T, et al. Parathyroid hormone and clinical outcome in kidney transplant patients with optimal transplant function. Clin Transplant. 2014;28(4):479–486. doi: 10.1111/ctr.12341.
- Pihlstrøm H, Dahle DO, Mjøen G, et al. Increased risk of all-cause mortality and renal graft loss in stable renal transplant recipients with hyperparathyroidism. Transplantation. 2015;99(2):351–359. doi: 10.1097/TP.0000000000000583.
- Schwarz A, Merkel S, Leitolf H, et al. The effect of cinacalcet on bone remodeling and renal function in transplant patients with persistent hyperparathyroidism. Transplantation. 2011;91(5):560–565. doi: 10.1097/TP.0b013e3182079431.
- Tang JA, Friedman J, Hwang MS, et al. Parathyroidectomy for tertiary hyperparathyroidism: a systematic review. Am J Otolaryngol. 2017;38(5):630–635. doi: 10.1016/j.amjoto.2017.06.009.
- Dulfer RR, Franssen GJH, Hesselink DA, et al. Systematic review of surgical and medical treatment for tertiary hyperparathyroidism. Br J Surg. 2017;104(7):804–813. doi: 10.1002/bjs.10554.
- Yuan Q, Liao Y, Zhou R, et al. Subtotal parathyroidectomy versus total parathyroidectomy with autotransplantation for secondary hyperparathyroidism: an updated systematic review and meta-analysis. Langenbecks Arch Surg. 2019;404(6):669–679. doi: 10.1007/s00423-019-01809-7.
- Triponez F, Clark OH, Vanrenthergem Y, et al. Surgical treatment of persistent hyperparathyroidism after renal transplantation. Ann Surg. 2008;248(1):18–30. doi: 10.1097/SLA.0b013e3181728a2d.
- Evenepoel P, Claes K, Kuypers DR, et al. Parathyroidectomy after successful kidney transplantation: a single center study. Nephrol Dial Transplant. 2007;22(6):1730–1737. doi: 10.1093/ndt/gfm044.
- Cruzado JM, Moreno P, Torregrosa JV, et al. A randomized study comparing parathyroidectomy with cinacalcet for treating hypercalcemia in kidney allograft recipients with hyperparathyroidism. J Am Soc Nephrol. 2016;27(8):2487–2494. doi: 10.1681/ASN.2015060622.
- Dulfer RR, Koh EY, van der Plas WY, et al. Parathyroidectomy versus cinacalcet for tertiary hyperparathyroidism; a retrospective analysis. Langenbecks Arch Surg. 2019;404(1):71–79. doi: 10.1007/s00423-019-01755-4.
- Yang RL, Freeman K, Reinke CE, et al. Tertiary hyperparathyroidism in kidney transplant recipients: characteristic of patients selected for different treatment strategies. Transplantation. 2012;94(1):70–76. doi: 10.1097/TP.0b013e3182530699.
- Moreno P, Coloma A, Torregrosa JV, et al. Long-term results of a randomized study comparing parathyroidectomy with cinacalcet for treating tertiary hyperparathyroidism. Clin Transplant. 2020;34(8):e13988. doi: 10.1111/ctr.13988.
- Lou I, Schneider DF, Leverson G, et al. Parathyroidectomy is underused in patients with tertiary hyperparathyroidism after renal transplantation. Surgery. 2016;159(1):172–179. doi: 10.1016/j.surg.2015.08.039.
- Fletcher S, Kanagasundaram NS, Rayner HC, et al. Assessment of ultrasound guided percutaneous ethanol injection and parathyroidectomy in patients with tertiary hyperparathyroidism. Nephrol Dial Transplant. 1998;13(12):3111–3117. doi: 10.1093/ndt/13.12.3111.
- Schlosser K, Endres N, Celik I, et al. Surgical treatment of tertiary hyperparathyroidism: the choice of procedure matters. World J Surg. 2007;31(10):1947–1953. doi: 10.1007/s00268-007-9187-z.
- Kandil E, Florman S, Alabbas H, et al. Exploring the effect of parathyroidectomy for tertiary hyperparathyroidism after kidney transplantation. Am J Med Sci. 2010;339(5):420–424. doi: 10.1097/MAJ.0b013e3181d8b6ff.
- Dewberry LC, Tata S, Graves S, et al. Predictors of tertiary hyperparathyroidism: who will benefit from parathyroidectomy? Surgery. 2014;156(6):1631–1637. doi: 10.1016/j.surg.2014.08.070.
- Frey S, Goronflot T, Kerleau C, et al. Parathyroidectomy or cinacalcet: do we still not know the best option for graft function in kidney-transplanted patients? A meta-analysis. Surgery. 2021;170(3):727–735. doi: 10.1016/j.surg.2021.02.048.
- Jeon HJ, Kim YJ, Kwon HY, et al. Impact of parathyroidectomy on allograft outcomes in kidney transplantation. Transpl Int. 2012;25(12):1248–1256. doi: 10.1111/j.1432-2277.2012.01564.x.
- Pitt SC, Panneerselvan R, Chen H, et al. Secondary and tertiary hyperparathyroidism: the utility of ioPTH monitoring. World J Surg. 2010;34(6):1343–1349. doi: 10.1007/s00268-010-0575-4.
- Dewberry LK, Weber C, Sharma J. Near total parathyroidectomy is effective therapy for tertiary hyperparathyroidism. Am Surg. 2014;80(7):646–651. doi: 10.1177/000313481408000717.
- Kerby JD, Rue LW, Blair H, et al. Operative treatment or tertiary hyperparathyroidism: a single center experience. Ann Surg. 1998;227(6):878–886. doi: 10.1097/00000658-199806000-00011.
- van der Plas WY, El Moumni M, von Forstner PJ, et al. Timing of parathyroidectomy does not influence renal function after kidney transplantation. World J Surg. 2019;43(8):1972–1980. doi: 10.1007/s00268-019-04952-w.
- Littbarski SA, Kaltenborn A, Gwiasda J, et al. Timing of parathyroidectomy in kidney transplant candidates with secondary hyperparathyroidism: effect of pretransplant versus early or late post-transplant parathyroidectomy. Surgery. 2018;163(2):373–380. doi: 10.1016/j.surg.2017.10.016.
- Triponez F, Dosseh D, Hazzan M, et al. Subtotal parathyroidectomy with thymectomy for autonomous hyperparathyroidism after renal transplantation. Br J Surg. 2005;92(10):1282–1287. doi: 10.1002/bjs.5080.
- Dream S, Chen H, Lindeman B. Tertiary hyperparathyroidism. Why the delay? Ann Surg. 2021;273(3):e120–e122. doi: 10.1097/SLA.0000000000004069.
- Perrin P, Caillard S, Javier RM, et al. Persistent hyperparathyroidism is a major risk factor for fractures in the five years after kidney transplantation. Am J Transplant. 2013;13(10):2653–2663. doi: 10.1111/ajt.12425.
- Sadideen H, Covic A, Goldsmith D. Mineral and bone disorder after renal transplantation: a review. Int Urol Nephrol. 2008;40(1):171–184. doi: 10.1007/s11255-007-9310-y.
- Komaba H, Nakamura M, Fukagawa M. Resurgence of parathyroidectomy: evidence and outcomes. Curr Opin Nephrol Hypertens. 2017;26(4):243–249. doi: 10.1097/MNH.0000000000000326.
- Ivarsson KM, Akaberi S, Isaksson E, et al. The effect of parathyroidectomy on patient survival in secondary hyperparathyroidism. Nephrol Dial Transplant. 2015;30(12):2027–2033. doi: 10.1093/ndt/gfv334.

