Abstract
Background
We aimed to compare the cardiovascular events and mortality in patients who underwent either physician-oriented or patient-oriented kidney replacement therapy (KRT) conversion due to discontinuation of peritoneal dialysis (PD).
Methods
Patients with end-stage kidney disease who were receiving PD and required a switch to an alternative KRT were included. They were divided into physician-oriented group or patient-oriented group based on the decision-making process. Logistic regression analysis was used to explore the influencing factors related to KRT conversion in PD patients. The association of physician-oriented or patient-oriented KRT conversion with outcomes after the conversion was assessed by using Cox proportional hazards models.
Results
A total of 257 PD patients were included in the study. The median age at catheterization was 35 years. 69.6% of the participants were male. The median duration of PD was 20 months. 162 participants had patient-oriented KRT conversion, while 95 had physician-oriented KRT conversion. Younger patients, those with higher education levels, higher income, and no diabetes were more likely to have patient-oriented KRT conversion. Over a median follow-up of 39 months, 40 patients experienced cardiovascular events and 16 patients died. Physician-oriented KRT conversion increased nearly 3.8-fold and 4.0-fold risk of cardiovascular events and death, respectively. After adjusting for confounders, physician-oriented KRT conversion remained about a 3-fold risk of cardiovascular events.
Conclusion
Compared to patient-oriented KRT conversion, PD patients who underwent physician-oriented conversion had higher risks of cardiovascular events and all-cause mortality. Factors included age at catheterization, education level, annual household income, and history of diabetes mellitus.
1. Introduction
Chronic kidney disease (CKD) has become a global public health concern in recent years [Citation1]. The prevalence of CKD in Chinese adults was 8.2% from 2018 to 2019, with an estimated 82 million adults with CKD [Citation2]. Patients with CKD will eventually develop end-stage kidney disease (ESKD), even with favorable treatment. Kidney replacement therapy (KRT) is a crucial treatment for patients with ESKD. It mainly consists of hemodialysis (HD), peritoneal dialysis (PD), and kidney transplantation (KTx). These interventions play a significant role in improving clinical symptoms and extending the life expectancy of patients [Citation1]. PD is an important home-based treatment and accounts for 11% of all dialysis and 9% of all KRT globally [Citation3]. Compared to in-center HD, PD has several advantages. These include equal or better survival rates, preservation of residual kidney function and potential vascular accesses, greater autonomy and freedom (especially for remote areas), and higher cost-effectiveness [Citation3–6]. PD is also considered a better pretransplant dialysis option during the transition to KTx [Citation7–9]. As a result, PD is widely recognized and preferred as a treatment [Citation10,Citation11]. Especially, PD is now largely increasingly used for KRT in China [Citation12].
In clinical practice, most patients who initially choose HD rather than PD are actually suitable for both types of dialysis, and there are many factors contributing to this phenomenon, including pre-dialysis education of patients, clinician’s familiarity with HD and PD, and patient-related factors [Citation13,Citation14]. A study from the United States showed that nearly half of HD patients felt that they had no choice, that their decision to undergo HD was largely not their own choice, and that the higher degree of shared decision-making was associated with a higher proportion of choosing PD [Citation15]. In fact, it should be noted that the choice of treatment mode is not a one-way decision. As PD treatment progresses, residual kidney function is progressively lost and detrimental changes occur in the structure and function of the peritoneum after exposure to dialysate, leading to inadequate solute clearance and ultrafiltration [Citation12,Citation16,Citation17]. In addition, there are a number of problems such as infection mainly peritonitis and catheter dysfunction [Citation18]. Alternatively, some patients encounter a suitable kidney donor. These factors can cause patients to interrupt PD and switch to other KRT. Withdrawal from PD is inevitable, so PD patients often need to be reevaluated regularly to decide whether to continue with the current mode or switch to another mode [Citation11]. It has been reported that up to 35% of patients transition from PD to in-center HD per year [Citation19], and the high dropout rate of PD cannot be ignored. A retrospective study from Taiwan highlighted the importance of non-medical factors in PD withdrawal [Citation20]. Of note, due to the complexity of the actual PD process, the reasons for discontinuation of PD treatment at different points of follow-up are various, with many potential risk factors, and the relative contribution of these factors also varies with the duration of PD [Citation21]. We found that the conversion of KRT mode was sometimes initiated by patients, or was recommended by healthcare providers because they could not continue PD. Previous studies have only focused on the observation of cardiovascular events and mortality after PD. However, no studies have reported clinical outcomes after such a transition and the effect of a physician-oriented or patient-oriented conversion on the outcomes.
The transition from ESKD to PD and among modalities is a time when patients may be particularly vulnerable, so multiple factors must be considered when choosing initial and subsequent treatment modalities [Citation5]. Therefore, we aimed to compare the incidence of cardiovascular events and mortality in patients who underwent either physician-oriented or patient-oriented KRT conversion due to discontinuation of PD and to discuss the potential factors influencing the occurrence of such a conversion. In the end, it was to get closer to the real world and to provide more detailed information to guide patient decision-making regarding the choice of KRT in the future.
2. Materials and methods
2.1. Study design and population
This was a single-center retrospective cohort study. Patients with maintenance PD were selected from the Department of Nephrology, Xinqiao Hospital of Army Medical University during January 1, 2007, and April 30, 2021. Then, these patients who switched to another mode of KRT (HD or KTx) before January 31, 2022, were enrolled. The patients aged < 18 years at the initiation of PD, treated with PD therapy for less than 3 months, and lost to contact were excluded. This study was conducted in compliance with the ethical principles of the Declaration of Helsinki and was approved by the local ethics committee of The Second Affiliated Hospital Of Army Medical University (No. 2023-150-01). All participants provided verbal informed consent.
The included patients who switched to another mode of KRT were classified either into the physician-oriented KRT conversion group or the patient-oriented KRT conversion group. Physician-oriented conversion was defined as PD could not be unable to satisfy the needs of solute removal and fluid filtration in the body, or the occurrence of serious complications preventing the PD from continuing, or physical conditions could not continue to support PD, and therefore the patients removed the PD tube and changed PD to HD or KTx at the recommendation of nephrologists. The patient-oriented conversion was referred to patients voluntarily withdrawing from PD to convert to other KRT treatments for their own subjective reasons. In this part, We first consulted the medical files of PD patients established and maintained by our PD center, recorded the specific reasons for patients withdrawing from PD, and determined whether it was a physician-oriented or patient-oriented conversion through previous registered information and relevant medical data. Then, in order to verify our results, we conducted telephone interviews to inquire and check the reasons for patients withdrawing from PD. The whole follow-up process was completed by systematically trained nephrologists.
2.2. Data sources and data collection
The clinical data of these selected participants were collected from the electronic medical record system (EMRS) and paper medical records of our PD center. Demographics included age at catheterization for PD, gender, body mass index (BMI, weight(kg)/height(m)2), primary disease, blood pressure, history of diabetes mellitus (DM) and coronary artery disease (CAD), marital status, education level, and annual household income. Serum biochemical indexes included albumin, prealbumin, urea nitrogen, creatinine, uric acid, calcium, phosphorus, hemoglobin, fasting blood glucose, triglyceride, total cholesterol, HDL-C, LDL-C, and intact parathyroid hormone (iPTH). PD-related variables included types of PD (CAPD or APD), 24-h urine output, total Kt/V, total Ccr, and types of peritoneal solute transport based on peritoneal equilibration test (PET). All the above baseline data were obtained at the time of 1–3 months after the start of PD. Others included the duration of PD and whether peritoneal dialysis-associated peritonitis (PD peritonitis) occurred during PD treatment.
2.3. Follow-up and outcomes
We set the date of catheter insertion as the start date of PD [Citation3], and the date of extubation (or the date of initiation of regular HD, excluding heterozygous dialysis) or KTx as the end date of PD. Follow-up began at study enrollment and ended with the first of death, the end of the study period (February 2023), or a recent date of available data. Finally, the primary endpoint of our study was cardiovascular disease (CVD) events, and the secondary endpoint was all-cause mortality. CVD events was defined as the first appearance of severe coronary events (myocardial infarction, for example), severe heart failure, severe arrhythmia, cardiac arrest, cerebrovascular accident, or peripheral vascular disease after KRT conversion in PD patients [Citation22–24]. Death from any cause was defined as all-cause mortality. The above was based on the assessment or diagnosis of the clinician by telephone follow-up or patient reports from medical records.
Statistical analysis
Continuous variables with a normal or approximately normal distribution were expressed as mean ± standard deviation. The differences between the groups were determined using a T-test. Continuous variables with a skewed distribution were expressed as median (25th percentile, 75th percentile), and the differences between the groups were determined using the Mann-Whitney U test. Categorical variables were expressed as frequencies and percentages, and the differences between the groups were determined using the Chi-square test. Binary Logistic regression analysis was used to evaluate the correlation between physician-oriented or patient-oriented KRT conversion and other parameters. Variables with significant associations in the univariable analysis were included as adjustment variables in the multivariable analyses. The results were presented as odds ratio (OR) and 95% confidence interval (CI). Kaplan–Meier survival curves were used to analyze the cumulative survival rate. The differences in survival rates between the two groups were analyzed using the Log-Rank test. The Cox proportional hazards model was used to analyze the association between physician-oriented and patient-oriented KRT conversion and CVD events and all-cause mortality. Baseline variables significantly associated with the clinical outcomes were selected for the multivariate analysis. We used the Schoenfeld residual or compared Kaplan-Meier survival curves to ensure that the proportional hazards assumption was met for variables included in the Cox regression analysis. The results were expressed as hazard ratio (HR) and 95% CI. Due to the small sample size, in order to address sparse effects on the results of the study and reduce the inflation of OR and HR, we used Firth regression to optimize the main results and reconstruct the interval estimation based on profile penalized log likelihood [Citation25,Citation26]. For all tests, a P-value less than 0.05 was considered statistically significant. The above statistical analyses were performed by using SPSS software version 26.0 and R software version 4.3.2.
3. Results
3.1. Grouping and patient characteristics
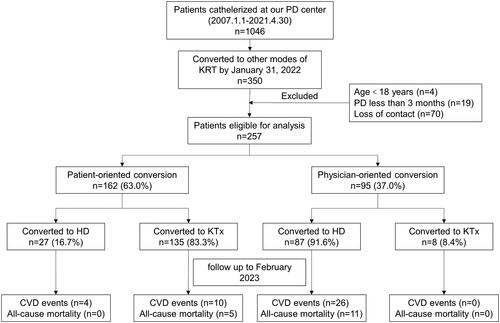
There were a total of 1046 patients who underwent PD catheter insertion at our PD center. Out of these, 350 patients switched to another form of KRT. We excluded 93 cases based on the specified exclusion criteria. Ultimately, this study included a total of 257 patients. The median age during catheterization was 35 (29, 47) years old, with 69.6% being male. Among the participants, 5.4% had DM, and the median duration of PD was 20.0 (11.00, 34.50) months. The primary cause of ESKD was primary glomerular disease (81.7%), followed by hypertensive nephropathy (8.6%) and diabetic nephropathy (3.5%). presents the differences in baseline demographic and clinical characteristics between the two groups. Significantly different factors included age at catheterization, BMI, history of DM, marital status, education level, annual household income, albumin, fasting blood glucose, LDL-C, PET results, duration of PD, and the incidence of PD peritonitis.
Table 1. Baseline characteristics of the included patients.
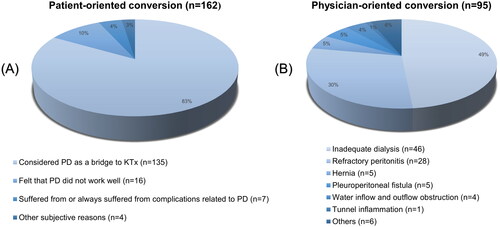
Approximately 162 patients (about 63.0%) underwent patient-oriented KRT conversion, while approximately 95 patients (about 37.0%) underwent physician-oriented KRT conversion. In the patient-oriented conversion group, 27 patients (about 16.7%) switched to HD and 135 patients (about 83.3%) switched to KTx. In the physician-oriented conversion group, 87 patients (about 91.6%) switched to HD and 8 patients (about 8.4%) switched to KTx (). Next, we examined the reasons behind the discontinuation of PD. In the patient-oriented conversion group, approximately 83% (135 patients) viewed PD as a temporary KRT while awaiting KTx. Around 10% (16 patients) stopped PD due to inadequate dialysis, while approximately 4% (7 patients) discontinued PD because of complications related to the treatment (). In the physician-oriented conversion group, the primary cause for ceasing PD was inadequate dialysis (n = 46, 49%), followed by PD-related complications ().
3.2. Influencing factors of physician-oriented and patient-oriented KRT conversion
As shown in , the results of the univariate logistic regression analysis indicated that certain factors had an impact on the occurrence of physician-oriented conversion compared to the patient-oriented conversion group. These factors included age, higher BMI, longer duration of PD, history of DM, higher fasting blood glucose and LDL-C levels, and peritonitis during PD. On the other hand, higher education levels, higher annual household income, and higher albumin levels had a hindering effect on the occurrence of physician-oriented conversion. Being married was associated with the occurrence of physician-oriented conversion compared to unmarried patients. Additionally, low average transport was associated with the occurrence of patient-oriented conversion compared to high transport patients.
Table 2. Odds ratios for the occurrence of physician-oriented conversion in PD patients based on logistic regression analysis.
Next, the following factors were included for multivariate logistic regression analysis in : age at catheterization, BMI, history of DM, education level, annual household income, albumin levels, and LDL-C levels. The analysis showed that age at catheterization (OR = 1.031, p = 0.036), history of DM (OR = 5.969, p = 0.009), education level (OR = 0.723, p = 0.011), and annual household income (OR = 0.749, p = 0.041) were independent factors associated with the occurrence of physician-oriented and patient-oriented KRT conversion.
3.3 Association of physician-oriented and patient-oriented KRT conversion with outcomes
The median follow-up time in this study was 39.00 (23.50, 62.00)months. During the follow-up period, there were 14 patients experienced CVD events, and 5 patients died in the patient-oriented conversion group. In the physician-oriented conversion group, there were 26 CVD events, and there were 11 deaths in this group. The detailed reasons are listed in Supplementary Figure 1.
The Kaplan-Meier survival curve indicated that, when compared to the patient-oriented conversion group, the physician-oriented conversion group had significantly higher rates of CVD events and all-cause mortality (). Furthermore, the univariate Cox regression analysis demonstrated that physician-oriented conversion increased the risk of CVD events when compared to patient-oriented conversion (HR = 3.818, 95%CI = 2.034-7.463, p < 0.001) (Supplementary Table 1). Even after adjusting for variables such as age at catheterization, history of DM, and albumin level, physician-oriented conversion remained significantly associated with the occurrence of CVD events (HR = 2.916, 95%CI = 1.501-5.846, p = 0.002) (). Furthermore, the results of the univariate Cox regression analysis indicated that compared to patient-oriented conversion, physician-oriented conversion was associated with a higher risk of all-cause mortality (HR = 3.956, 95%CI = 1.487-11.915, p = 0.006) (Supplementary Table 1). However, after adjusting for multiple variables, physician-oriented conversion was not found to be an independent risk factor for all-cause mortality ().
Figure 3. Kaplan–Meier survival curves for (A) cardiovascular events and (B) all-cause mortality of patients in the patient-oriented and physician-oriented KRT conversion groups. Kaplan–Meier survival curves for (C) cardiovascular events and (D) all-cause mortality of patients converted to HD or KTx in the patient-oriented conversion group. Kaplan–Meier survival curves for (E) cardiovascular events and (F) all-cause mortality of patients converted to HD or KTx in the physician-oriented conversion group.
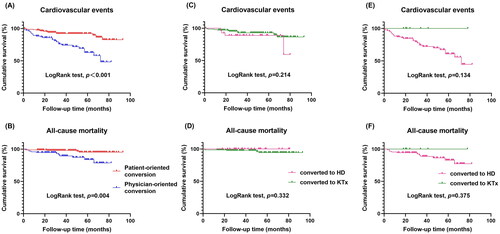
Table 3. The hazard ratios associated with physician-oriented conversion for cardiovascular events and all-cause mortality according to Cox regression analysis.
3.4 Subgroup analysis
Subsequently, we analyzed the factors that affect the conversion to different kidney replacement therapies based on subgroup analysis. In the patient-oriented conversion group, there were significant differences in age, education level, income, and peritonitis between patients converted to HD and patients converted to KTx. The univariate logistic regression analysis showed that higher education level and income promoted conversion to KTx, while older age and peritonitis hindered conversion. The multivariate logistic regression analysis revealed that age, education level, and income were independent factors for conversion to HD or KTx (Supplementary Table 2-3). The LogRank test showed no significant differences in CVD events and all-cause mortality between the two types of KRT ().
In the physician-oriented conversion group, significant differences were found in education level, types of PD, albumin, prealbumin, and PET results between patients converted to HD and patients converted to KTx. Univariate logistic regression analysis revealed that higher education levels and higher levels of albumin and prealbumin promoted conversion to KTx compared to conversion to HD. However, CAPD had a negative effect on conversion to KTx compared to APD (OR = 0.160, p = 0.018). This correlation remained significant when educational level and prealbumin level were analyzed together (Supplementary Table 4-5). In addition, there were no significant differences in CVD events and all-cause mortality between the two groups, as indicated by the LogRank test ().
4. Discussion
For all we know, this is the first study to explore the clinical outcomes of patients with ESKD who underwent either physician-oriented or patient-oriented conversion from PD to other modes of KRT. The results showed that higher rates of CVD events and all-cause mortality were observed in the physician-oriented conversion group, and it suggested that more care should be given to patients with physician-oriented conversion.
KRT for patients with ESKD is a continuous process until the patient dies, and there is the possibility of switching from one mode to another at any time. We found that, except for patients who withdrew from PD due to death or transferred to other centers so that they could not be followed, other patients who withdrew from PD had some decision-making power in the choice of KRT, which could not be ignored and had evidence to follow. An early attempt in the Netherlands to perform a randomized controlled trial comparing survival differences between HD and PD, however, of the 773 patients who met the inclusion criteria, the vast majority had a preference for either HD or PD, only 38 agreed to randomization, and the trial was prematurely stopped because of low enrollment, underscoring the important role of patients in decision-making about dialysis mode [Citation27]. In addition, a multinational, observational Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS) from 2014 to 2017 showed that HD transfer for psychosocial/medical reasons was at 8%-20% in other countries, except for Thailand [Citation28]. In our study, patient-oriented conversion from PD to HD accounted for approximately 10.5% of all enrolled patients, and these patients chose to HD transfer of their own volition, which did not mean that their PD could not be continued objectively. Whether it was the mode of PD itself or the related complications of PD, these patients subjectively felt that PD was a burden, which made it impossible for them to continue receiving it from the bottom of their hearts. In fact, patients were more concerned about how to improve their lives as well as meet their personal needs [Citation29]. Therefore, from this perspective, our study found that in our center, patient-oriented conversion accounted for a relatively high proportion of PD patients who were converted to other KRT modes, the most important of which was conversion to KTx. These patients indicated that they were simply waiting for a kidney source, which also explained the short median duration of PD in this group of patients. This was consistent with the conclusion that countries in the PDOPPS with higher rates of KTx tended to have a shorter median duration of PD [Citation28]. Meanwhile, we observed that the primary reason for physician-oriented conversion from PD to other KRT modes was inadequate dialysis, followed by refractory peritonitis, however about half of the patients with physician-oriented conversion had previously experienced PD peritonitis. These patients still emotionally wanted to be treated with PD but could not objectively continue. A substantial proportion of patients in this group (about 91.6%) were converted to HD. A multicenter study from Australia and New Zealand In 2015 reported that the most common reason for HD transfer in PD patients was peritonitis (42%), followed by inadequate dialysis (15%) [Citation30]. Another single-center study from China in 2019 reported that the main reason for HD transfer in PD patients was inadequate dialysis (about 45.8%) while peritonitis accounted for about 33.1% [Citation31], and this was generally consistent with our study. If realistic factors are not considered, KTx is recognized as the treatment first choice for patients with ESKD [Citation9]. KTx provides a better quality of life than dialysis [Citation32]. However, an international cross-sectional survey showed that the capacity to provide maintenance dialysis and KTx services varied around the globe, and that access to KRT was associated with national income [Citation33]. For people with higher incomes, KRT is more widely available and treatment choice is more often a reality [Citation34]. In reality, mode choice and conversion between modes are driven by a series of factors, such as patient characteristics, lifestyle constraints, clinical urgency, economics, mode availability, policy, and physician preferences [Citation29]. We found that PD patients with younger age, higher education, and higher household income reported higher levels of autonomy and flexibility, relatively easy ability to receive KRT-related knowledge, higher pursuit of quality of life, and were more likely to switch from PD to other KRT modes voluntarily. In addition, patients in the patient-oriented conversion group also had a better health status at baseline than the physician-oriented conversion group, such as higher albumin, lower BMI, fasting blood glucose and LDL-C levels, better results of PET, and lower incidence of DM and PD peritonitis. But notably, median or mean values for these blood biochemical indexes were within normal ranges in both groups.
In our study, a significantly higher rate of CVD events and all-cause mortality after KRT conversion was observed in the physician-oriented conversion group. Moreover, after adjusting for various confounding factors, physician-oriented conversion was still independently associated with the risk of CVD events. Patients with ESKD have a significantly increased cardiovascular (CV) risk compared with the general population. In addition to traditional CV risk factors such as advanced age, DM, hypertension, and dyslipidemia, which are all highly prevalent in this population, complications specific to HD or KTx may ensue when patients switch from PD to another mode of KRT. Previous studies have shown that HD patients also have nontraditional risk factors such as anemia, oxidative stress, chronic inflammatory state, malnutrition, calcium and phosphorus metabolism disorders, uremic toxin accumulation, volume overload, and vascular changes [Citation35]. KTx recipients have reported possible complications including reduced graft function, side effects of immunosuppression, and post-transplant DM [Citation36]. In addition, studies have shown that CVD is the leading cause of death in patients with normal kidney transplant function, but successful transplantation can reduce CV risk in ESKD patients, while CV risk is increased in dialysis patients [Citation36, Citation37]. To some extent, this might explain the lower CV risk among patients in the patient-oriented conversion group with a greater proportion of KTx. Furthermore, we analyzed that patients in the physician-oriented conversion group did not have smooth PD before conversion, as compared with those in the patient-oriented conversion group, and such an effect might persist.
Univariate Cox regression analysis showed that the underlying mechanisms of the patient’s death after KRT conversion were complex and multifaceted. Education and income were observed to be protective factors for survival in our study. And a multicenter study from China in 2012 generally supported our results, which found that PD patients with low income, especially in underdeveloped regions, with low education, bore a heavier burden of medical costs, significantly affecting their survival [Citation38]. However, in the classical multivariate Cox regression analysis, we noted that the history of DM and the duration of PD played a more important role in predicting the risk of all-cause mortality, and the effect of physician-oriented conversion was attenuated. It has been confirmed that DM is significantly and positively associated with an increased risk of all-cause mortality in PD patients [Citation39]. It was shown that rates of the combined outcome of HD transfer or death in PD patients were higher with higher PD vintage [Citation28]. Dextrose-containing dialysate used in PD patients can destroy peritoneal permeability, lead to decreased ultrafiltration due to easy absorption, and aggravate poor blood glucose control. As these patients are converted to HD or KTx, other special problems may arise. For example, HD patients are prone to hypoglycemia, and glycemic control may deteriorate after KTx because of the use of corticosteroids and calcineurin inhibitors [Citation40]. Therefore, good glycemic control is still a challenge in PD patients receiving KRT conversion and requires more attention from clinicians. Furthermore, it should be noted that the sample size of this study was too small, especially that only 5.4% of patients were diabetic, which means that our results were subject to sparse effects and might increase the probability of the occurrence of monotone likelihood. To deal with this issue, we adjusted for the inflated outcome by providing an implementation of the Firth regression. The results showed that the duration of PD still significantly affected the survival rate of patients with a narrowed 95% CI. But the above results need to be further verified by a larger sample size.
We reached similar conclusions by analyzing the factors related to conversion to HD or KTx in each group. In the patient-oriented conversion group, the patients who were converted to KTx were younger, had higher education and annual household income, and had less incidence of PD peritonitis. In the physician-oriented conversion group, patients with higher education and APD mode were more likely to choose KTx than CAPD, and their baseline albumin and prealbumin levels were higher than patients converted to HD. However, we found that no matter in the patient-oriented conversion group or the physician-oriented conversion group, there were no significant differences in CVD events and all-cause mortality between the patients converted to HD and KTx. We considered that this was largely due to the young age of the patients included in this study, who were only 35 (29, 47) years at the time of the catheterization, thus there was no difference in survival in the short term. By contrast, the mean age of PD patients ranged from 56 (±14) years in Thailand to 64 (±13) years in Japan [Citation28]. In our study, patients chose PD as the initial treatment mode mainly because they were informed through pre-dialysis education that PD provided a better quality of life than HD and preserved opportunities for learning and employment, and preemptive KTx was extremely difficult to achieve because of the shortage of kidney sources. It was also reported that PD might be a better dialysis option for young ESKD patients [Citation41]. However, with the progress of PD, each patient had their own cognition of PD. Some patients felt that in-center HD three times a week was more convenient, while the daily operation of PD brought them more trouble and the risk of infection caused by improper operation, and their interest gradually waned. Based on our findings, we suggest that clinicians should prioritize patients’ preferences and needs when faced with decision-making, especially for younger patients, and shift their focus to improving patients’ quality of life. In addition, for patients who prefer PD, reducing PD-related complications and lengthening the time on PD therapy are challenges that we need to face.
There were a few limitations in this study. Firstly, it is important to note that this study was conducted retrospectively and relied on patient-provided information, which may have introduced recall bias. Secondly, it is worth considering that the number of patients enrolled in this single-center study was relatively small, which may limit the generalizability of the findings due to regional differences. In addition, it is important to acknowledge that the small sample size and limited endpoint events in this study have restricted the scope of our multivariate analysis. The variables included in our analysis were selected based on differences observed in the univariate regression of baseline data. Moving forward, it is imperative to conduct further screening of additional variables for inclusion in the multivariate analysis with larger sample sizes in order to strengthen the support for our results. Additionally, the follow-up period in our study was relatively short, so it would be beneficial to conduct further research comparing the clinical outcomes of patients in multiple centers over a longer period of time. Lastly, it is important to acknowledge that we only collected clinical data from patients at the time of enrollment and did not investigate the long-term impact of changes in these measures on prognosis.
4. Conclusion
In conclusion, patients with PD in the physician-oriented conversion group might experience worse CVD events and overall death than those in the patient-oriented conversion group. PD patients who are younger, have higher education levels, higher annual household income, and have no DM at the time of catheterization are more likely to choose patient-oriented KRT conversion. These findings suggest that healthcare providers should receive adequate training to effectively guide patients in making decisions aligned with their preferences.
Disclosures
This work was supported by Joint Funds of the National Natural Science Foundation of China(No. U22A20279), Key projects Chongqing City technology development and application (cstc2021jcyj-msxmX0672), Key project of Chongqing technology development and application program (No. CSTB2023TIAD-KPX0060), Frontier-specific projects of Xinqiao Hospital (No. 2018YQYLY004), and Personal training Program for Clinical Medicine Research of Army Medical University (No. 2018XLC1007).
Author contributions
J.C.X. and J.H.Z. conceived and designed the study. X.G., L.L., and Y.H.H. collected the clinical data of patients and performed statistical analyses. L.Z. drafted the manuscript. J.C.X. checked and revised the article. All authors approved the final manuscript.
Supplemental Material
Download PDF (297 KB)Acknowledgments
We thank the nurses in our PD center and all subjects for participating in this study.
Conflict of interests
The authors have no financial conflicts of interest to declare.
References
- Cisneros-García DL, Sandoval-Pinto E, Cremades R, et al. Non-traditional risk factors of progression of chronic kidney disease in adult population: a scoping review. Front Med (Lausanne). 2023;10:1. doi: 10.3389/fmed.2023.1193984.
- Wang L, Xu X, Zhang M, et al. Prevalence of chronic kidney disease in China: results from the sixth China chronic disease and risk factor surveillance. JAMA Intern Med. 2023;183(4):298–12. doi: 10.1001/jamainternmed.2022.6817.
- Bello AK, Okpechi IG, Osman MA, et al. Epidemiology of peritoneal dialysis outcomes. Nat Rev Nephrol. 2022;18(12):779–793. doi: 10.1038/s41581-022-00623-7.
- François K, Bargman JM. Evaluating the benefits of home-based peritoneal dialysis. Int J Nephrol Renovasc Dis. 2014;7:447–455. doi: 10.2147/IJNRD.S50527.
- Imbeault B, Nadeau-Fredette AC. Optimization of dialysis modality transitions for improved patient care. Can J Kidney Health Dis. 2019;6:2054358119882664. doi: 10.1177/2054358119882664.
- Yi C, Guo Q, Lin J, et al. Clinical outcomes of remote peritoneal dialysis patients: a retrospective cohort study from a single center in China. Blood Purif. 2016;41(1-3):100–107. doi: 10.1159/000442516.
- Ngamvichchukorn T, Ruengorn C, Noppakun K, et al. Association between pretransplant dialysis modality and kidney transplant outcomes: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(10):e2237580. doi: 10.1001/jamanetworkopen.2022.37580.
- Hou YF, Wang XX, Yang HJ, et al. Impact of pre-transplant dialysis modality on kidney transplant outcomes: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. 2022;26(7):2292–2304. doi: 10.26355/eurrev_202204_28459.
- Torreggiani M, Piccoli GB, Moio MR, et al. Choice of the dialysis modality: practical considerations. J Clin Med. 2023;12(9):12.
- Liu FX, Gao X, Inglese G, et al. A global overview of the impact of peritoneal dialysis first or favored policies: an opinion. Perit Dial Int. 2015;35(4):406–420. doi: 10.3747/pdi.2013.00204.
- Wang J, Zeng J, Liu B, et al. Outcomes after transfer from hemodialysis to peritoneal dialysis vs peritoneal dialysis as initial therapy: a systematic review and meta-analysis. Semin Dial. 2020;33(4):299–308. doi: 10.1111/sdi.12896.
- Mehrotra R, Devuyst O, Davies SJ, et al. The current state of peritoneal dialysis. J Am Soc Nephrol. 2016;27(11):3238–3252. doi: 10.1681/ASN.2016010112.
- Wasse H. Factors related to patient selection and initiation of peritoneal dialysis. J Vasc Access. 2017;18(Suppl. 1):39–40. doi: 10.5301/jva.5000687.
- Chanouzas D, Ng KP, Fallouh B, et al. What influences patient choice of treatment modality at the pre-dialysis stage? Nephrol Dial Transplant. 2012;27(4):1542–1547. doi: 10.1093/ndt/gfr452.
- Dahlerus C, Quinn M, Messersmith E, et al. Patient perspectives on the choice of dialysis modality: results from the empowering patients on choices for renal replacement therapy (epoch-rrt) study. Am J Kidney Dis. 2016;68(6):901–910. doi: 10.1053/j.ajkd.2016.05.010.
- Devuyst O, Margetts PJ, Topley N. The pathophysiology of the peritoneal membrane. J Am Soc Nephrol. 2010;21(7):1077–1085. doi: 10.1681/ASN.2009070694.
- Tsai MH, Chen YY, Jang TN, et al. Outcome analysis of transition from peritoneal dialysis to hemodialysis: a population-based study. Front Med (Lausanne). 2022;9:876229. doi: 10.3389/fmed.2022.876229.
- Elphick E, Holmes M, Tabinor M, et al. Outcome measures for technique survival reported in peritoneal dialysis: a systematic review. Perit Dial Int. 2022;42(3):279–287. doi: 10.1177/0896860821989874.
- Ambruso SL, Teitelbaum I. Prevention of peritoneal dialysis drop-out. Adv Perit Dial. 2018;34(2018):19–23.
- Zhang L, Lee W-C, Wu C-H, et al. Importance of non-medical reasons for dropout in patients on peritoneal dialysis. Clin Exp Nephrol. 2020;24(11):1050–1057. doi: 10.1007/s10157-020-01948-y.
- Kolesnyk I, Dekker FW, Boeschoten EW, et al. Time-dependent reasons for peritoneal dialysis technique failure and mortality. Perit Dial Int. 2010;30(2):170–177. doi: 10.3747/pdi.2008.00277.
- Pugliese G, Solini A, Bonora E, et al. Distribution of cardiovascular disease and retinopathy in patients with type 2 diabetes according to different classification systems for chronic kidney disease: a cross-sectional analysis of the renal insufficiency and cardiovascular events (riace) italian multicenter study. Cardiovasc Diabetol. 2014;13(1):59. doi: 10.1186/1475-2840-13-59.
- Konerman MA, Fritze D, Weinberg RL, et al. Incidence of and risk assessment for adverse cardiovascular outcomes after liver transplantation: a systematic review. Transplantation. 2017;101(7):1645–1657. doi: 10.1097/TP.0000000000001710.
- Chen J, Zhong Z, Shi D, et al. Association between monocyte count to high-density lipoprotein cholesterol ratio and mortality in patients undergoing peritoneal dialysis. Nutr Metab Cardiovasc Dis. 2021;31(7):2081–2088. doi: 10.1016/j.numecd.2021.03.014.
- Tzeng IS. Examination of matching methods, sparse effects, and limitations in a nationwide database study on alzheimer’s disease. J Alzheimers Dis. 2023;96(1):73–75. doi: 10.3233/JAD-230701.
- Tzeng IS. To handle the inflation of odds ratios in a retrospective study with a profile penalized log-likelihood approach. J Clin Lab Anal. 2021;35:e23849.
- Korevaar JC, Feith GW, Dekker FW, et al. Effect of starting with hemodialysis compared with peritoneal dialysis in patients new on dialysis treatment: a randomized controlled trial. Kidney Int. 2003;64(6):2222–2228. doi: 10.1046/j.1523-1755.2003.00321.x.
- Lambie M, Zhao J, McCullough K, et al. Variation in peritoneal dialysis time on therapy by country: results from the peritoneal dialysis outcomes and practice patterns study. Clin J Am Soc Nephrol. 2022;17(6):861–871. doi: 10.2215/CJN.16341221.
- Lee MB, Bargman JM. Survival by dialysis modality-who cares? Clin J Am Soc Nephrol. 2016;11(6):1083–1087. doi: 10.2215/CJN.13261215.
- Lan PG, Clayton PA, Saunders J, et al. Predictors and outcomes of transfers from peritoneal dialysis to hemodialysis. Perit Dial Int. 2015;35(3):306–315. doi: 10.3747/pdi.2013.00030.
- Yifan W, Xiaojiang Z, Yanbing C, et al. Reasons for the dropout of peritoneal dialysis patients. Chin J Nephrol. 2019;35:275–280.
- Wang Y, Hemmelder MH, Bos WJW, et al. Mapping health-related quality of life after kidney transplantation by group comparisons: a systematic review. Nephrol Dial Transplant. 2021;36(12):2327–2339. doi: 10.1093/ndt/gfab232.
- Bello AK, Levin A, Lunney M, et al. Status of care for end stage kidney disease in countries and regions worldwide: international cross sectional survey. BMJ. 2019;367:l5873. doi: 10.1136/bmj.l5873.
- Davison SN, Levin A, Moss AH, et al. Executive summary of the kdigo controversies conference on supportive care in chronic kidney disease: developing a roadmap to improving quality care. Kidney Int. 2015;88(3):447–459. doi: 10.1038/ki.2015.110.
- Ahmadmehrabi S, Tang W. Hemodialysis-induced cardiovascular disease. Semin Dial. 2018;31(3):258–267. doi: 10.1111/sdi.12694.
- Stoumpos S, Jardine AG, Mark PB. Cardiovascular morbidity and mortality after kidney transplantation. Transpl Int. 2015;28(1):10–21. doi: 10.1111/tri.12413.
- Ali A, Macphee I, Kaski JC, et al. Cardiac and vascular changes with kidney transplantation. Indian J Nephrol. 2016;26(1):1–9. doi: 10.4103/0971-4065.165003.
- Xu R, Han Q-F, Zhu T-Y, et al. Impact of individual and environmental socioeconomic status on peritoneal dialysis outcomes: a retrospective multicenter cohort study. PLoS One. 2012;7(11):e50766. doi: 10.1371/journal.pone.0050766.
- Zhang J, Lu X, Li H, et al. Risk factors for mortality in patients undergoing peritoneal dialysis: a systematic review and meta-analysis. Ren Fail. 2021;43(1):743–753. doi: 10.1080/0886022X.2021.1918558.
- Ahmed Z, Simon B, Choudhury D. Management of diabetes in patients with chronic kidney disease. Postgrad Med. 2009;121(3):52–60. doi: 10.3810/pgm.2009.05.2002.
- He Z, Hou H, Zhang D, et al. Effects of dialysis modality choice on the survival of end-stage renal disease patients in Southern China: a retrospective cohort study. BMC Nephrol. 2020;21(1):412. doi: 10.1186/s12882-020-02070-7.