Abstract
Background
Acute kidney injury (AKI) is recognized as a common complication following cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy (CRS-HIPEC). Characterized by prolonged renal function impairment, acute kidney disease (AKD) is associated with a higher risk of chronic kidney disease (CKD) and mortality.
Methods
From January 2018 to December 2021, 158 patients undergoing CRS-HIPEC were retrospectively reviewed. Patients were separated into non-AKI, AKI, and AKD cohorts. Laboratory parameters and perioperative features were gathered to evaluate risk factors for both HIPEC-induced AKI and AKD, with the 90-day prognosis of AKD patients.
Results
AKI developed in 21.5% of patients undergoing CRS-HIPEC, while 13.3% progressed to AKD. The multivariate analysis identified that ascites, GRAN%, estimated glomerular filtration rate (eGFR), and intraoperative (IO) hypotension duration were associated with the development of HIPEC-induced AKI. Higher uric acid, lessened eGFR, and prolonged IO hypotension duration were more predominant in patients proceeding with AKD. The AKD cohort presented a higher risk of 30 days of in-hospital mortality (14.3%) and CKD progression (42.8%).
Conclusions
Our study reveals a high incidence of AKI and AKI-to-AKD transition. Early identification of risk factors for HIPEC-induced AKD would assist clinicians in taking measures to mitigate the incidence.
Keywords:
Introduction
Acute kidney injury (AKI) has been increasingly identified as an intractable postoperative complication [Citation1]. It has been reported that postoperative AKI accounts for one-third of all hospital-acquired cases of AKI [Citation2] and is associated with potentially serious consequences, including proceeding to chronic kidney disease (CKD) [Citation3], end-stage renal disease (ESRD) [Citation4], and increased mortality [Citation5]. Persistent AKI stage 1 status or greater beyond seven days but for less than 90 days is considered an occurrence of acute kidney disease (AKD) [Citation6]. Indeed, AKD showed lower chances of renal function recovery at 90 days and accelerated progression of further renal function deterioration, reflecting the association with short-term and long-term mortality outcomes [Citation7]. Therefore, this group of patients may require more exhaustive monitoring and follow-up care.
Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) have transformed from palliative therapy into curative therapy [Citation8]. Through this operation, the macroscopic lesions are treated by careful total resection, and the residual microscopic tumors are treated with HIPEC [Citation9]. The research shows that, compared with simple CRS, CRS combined with HIPEC can improve the overall survival and recurrence-free survival time of patients with gastric cancer peritoneal tumor without increasing complications and mortality, especially for patients with limited peritoneal metastasis and satisfactory tumor reduction [Citation10]. HIPEC has a unique therapeutic effect on PC and its complicated malignant ascites caused by peritoneal metastasis of gastric cancer [Citation11], colorectal cancer [Citation12], ovarian cancer [Citation13], pseudomyxoma peritoneum [Citation14], malignant peritoneal mesothelioma, pancreatic cancer [Citation15], cholangiocarcinoma [Citation16], and liver cancer. Nevertheless, the incidence of postoperative complications remains high, significantly influencing the survival of patients [Citation9,Citation17]. To date, only a handful of studies have revealed the morbidity of perioperative complications such as AKI, ranging from 1% to 48% [Citation18,Citation19]. Independent risk factors for HIPEC-associated AKI have been revealed, consisting of age, body mass index (BMI), baseline serum creatinine (SCr), estimated glomerular filtration rate (eGFR), preoperative albumin (ALB), intraoperative (IO) bleeding loss, and nephrotoxicity of intraperitoneal cisplatin therapy [Citation20–24]. Along with the highlighted concepts of the AKI-to-AKD-to-CKD transition, many researchers have paid attention to AKD prevention and management. However, there is a paucity of the prevalence of HIPEC-associated AKD, and it is not clear what risk parameters are associated with AKD.
The first aim of this study was to reveal the incidence and risk factors for AKI in patients undergoing CRS combined with hyperthermic intraperitoneal chemotherapy (CRS-HIPEC). The second purpose was to investigate the prevalence of AKI-to-AKD transition, identify possible factors contributing to its development, and evaluate its impact on short-term and long-term outcomes. Given that early recognition of patients at high risk of progression AKD may assist clinicians in providing timely implementation of interventions to facilitate recovery to mitigate poorer outcomes.
Patients and methods
Study population
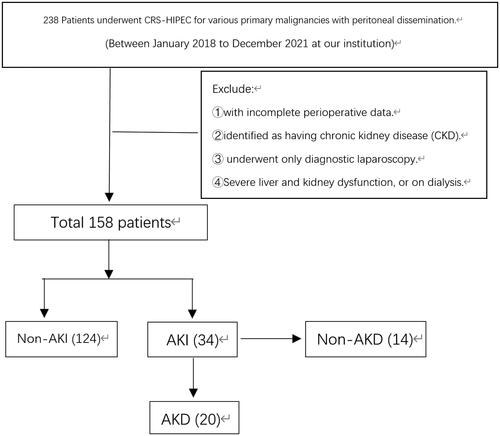
Between January 2018 and December 2021, we retrospectively studied a series of patients who underwent CRS-HIPEC for various primary malignancies with peritoneal dissemination at our institution. Patients were excluded from this study if they had incomplete perioperative data, had severe liver and kidney dysfunction, were identified as having CKD, or underwent only diagnostic laparoscopy. Patients on dialysis were also excluded. Patients were first distinguished into two cohorts according to the occurrence (AKI) or nonoccurrence of postoperative AKI (non-AKI). We further identified AKD group members according to the AKI duration. The study was approved by our Institutional Review Board (7222199).
Variables
Clinicopathologic variables, laboratory parameters, and perioperative and HIPEC details were collected from the electronic clinical records. Preoperative characteristics collected included age, gender, BMI, comorbidities (hypertension, diabetes mellitus, heart disease, and hepatitis), use of angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blockers (ARB), ascites, neoadjuvant therapy, Peritoneal Carcinomatosis Index (PCI), completeness of cytoreduction (CC), American Society of Anesthesiologists (ASA) score, primary tumor, hemoglobin (Hb), red blood cell (RBC), hematocrit (HCT%), white blood cell (WBC), platelet (PLT), neutrophil ratio (GRAN%), neutrophil (GRAN), lymphocyte ratio (LYM%), lymphocyte (LYM), SCr, urea, uric acid, eGFR, ALB, C-reactive protein (CRP), procalcitonin (PCT), and urine microalbumin.
Included in the IO variables were ureteral catheter implementation, operative duration, blood loss, fluid volume, urine output volume, hypotension duration (defined as episodes of systolic blood pressure (SBP) < 100 mmHg), RBC transfusion, plasma transfusion, use of vasopressors, and use of furosemide. HIPEC-related features included regimens (5-fluorouracil (5-FU), cisplatin, paclitaxel (TAX), and raltitrexed), cycles, and intervals between neoadjuvant chemotherapy and HIPEC.
Primary postoperative outcomes were assessed as the development of AKI, AKD, in-hospital mortality (at 30 days), length of intensive care unit (ICU) stay, and length of hospital stay. We followed up on all patients in the AKD group 90 days after the diagnosis of AKD to identify whether they developed CKD.
Definition of AKI and AKD
Baseline SCr was defined as the first record after patient admission. The AKI diagnosis of an abrupt decrease in renal function that occurs ≤7 days was based on the Kidney Disease Improving Global Outcomes (KDIGO) criteria. Accordingly, AKI stage I was defined as an increase in SCr >0.3 mg/dL within 48 h; stage II was described as an increase in creatinine 2–2.9 times baseline; stage III was described as an increase in SCr >4 mg/dL or three times baseline, or the need for renal replacement therapy [Citation25]. According to the ADQI 16 Workgroup, diagnosis of AKD was determined by the persistence of stage I or greater AKI (KDIGO criteria) beyond 7–90 days after the initial recognized AKI. CKD is renal structure or function abnormalities that persist for >90 days [Citation6]. Stage 1 AKD was described as an increase of SCr level to 1.5–1.9 times the baseline level, stage 2 AKD was defined as an increase of SCr level to 2.0–2.9 times the baseline level, and stage 3 AKD was described as an increase of SCr level ≥3.0 times the baseline level. Patients with a rise of SCr level to less than 1.5 times the baseline level and evidence of persistent renal damage, repair or regeneration, or decreased glomerular and tubular reserve function were defined as stage 0 AKD [Citation26].
Cytoreductive surgery and HIPEC
All patients underwent CRS, which included primary tumor resection, involved regional viscera dissection, lymphadenectomy, and peritoneal resection [Citation27]. Intraoperative or postoperative HIPEC followed the completion of CRS. Closed-abdomen HIPEC was performed, and the chemotherapy agent was delivered by two inflow catheters drained via two outflow probes. For perfusion, the temperature was administered at 43 °C for 60 min. The perfusate volume and chemotherapy dose varied according to the abdominal cavity volume and body surface area (BSA) (mg/m2) area-based dosing protocol [Citation28], respectively.
Statistical analyses
Continuous variables were expressed as mean ± standard deviation (SD), and categorical variables were reported as the frequency and percentage (n, %). Differences in categorical variables were compared using Pearson’s Chi-squared test or Fisher’s exact test, while the nonparametric Mann–Whitney test was used to assess continuous variables. Associations between risk factors and the occurrence of AKI and AKD were tested using univariate logistic regression. Variables with p < .05 on univariate analysis were included in multivariate logistic regression analysis. Adjusted odd ratios (ORs) with 95% confidence intervals (CIs) were calculated. We considered a p value <.05 (two-tailed) statistically significant. Statistical analyses were performed using IBM SPSS Statistics for Windows, version 26.0 (IBM Corp. Released 2019, Armonk, NY).
Results
A total of 158 patients who underwent CRS-HIPEC between January 2018 and December 2021 were identified (). The characteristics of all patients are presented in . The mean age was 60, and the majority were female (60.1%). Hypertension (21.5%) and diabetes mellitus (12.0%) were the most common comorbidities. Half of the patients presented ascites, and over 60% had an ASA score of 2. A minor percentage of patients (19.6%) underwent neoadjuvant therapy.
Table 1. Patient demographics and clinical characteristics.
One-third of patients were treated with CRS-HIPEC for colorectal cancer (32.3%). Other primary tumors included gastric (26.6%), appendiceal (20.9%), ovarian (12.7%), and other cancers (7.6%). The duration of CRS was 242.8 ± 111.0 min, and the majority of patients underwent three cycles (43.0%) of HIPEC. The mean ICU length of stay was 1.1 ± 2.7 days, and the hospital length of stay was 19.4 ± 11.1 days.
Comparisons of clinical features between AKI and non-AKI cohorts
Patients with ascites before surgery were more likely to develop AKI compared to those without (27 [79.4%] vs. 52 [41.9%], p < .001). Before CRS-HIPEC therapy, the functions of vital organs such as the blood, liver, and kidney were evaluated. As compared with the non-AKI cohort, the counts of WBC (5.7 ± 2.4 vs. 6.9 ± 3.3, p < .05), GRAN% (60.2 ± 13.3 vs. 70.2 ± 11.8, p < .001), CRP (18.6 ± 40.2 vs. 41.4 ± 44.6, p < .05), PCT (0.1 ± 0.2 vs. 0.5 ± 1.1, p < .01), and urea (4.8 ± 1.7 vs. 6.1 ± 3.6, p < .05) were significantly higher in the AKI cohort, while the LYM% was lower (26.4 ± 9.5 vs. 19.8 ± 10.0, p < .001). In contrast, the serum levels of eGFR in the non-AKI group were notably higher than those in the AKI group (96.4 ± 14.0 vs. 84.2 ± 25.9, p < .05). The percentage of patients who encountered IO hypotension duration was significantly longer in the AKI cohort than in the non-AKI cohort (31.7 ± 28.0 vs. 18.7 ± 16.5 min, p < .05). In addition, AKI patients (32.4%) received more IO plasma transfusions than non-AKI patients (16.9%) (p < .05). The mean ICU length of stay was significantly prolonged in AKI patients compared with non-AKI patients (2.4 ± 4.3 vs. 0.6 ± 1.7, p < .01) ().
Features of patients in the AKD cohort
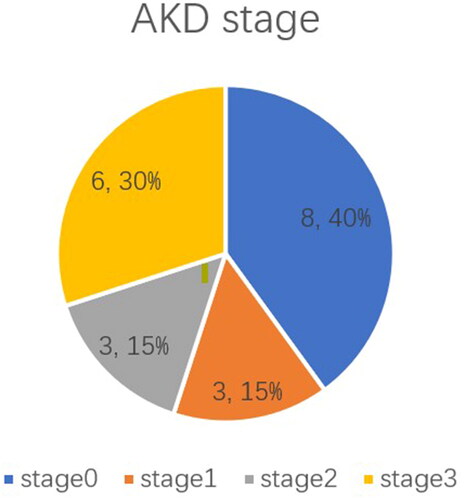
According to AKD grade, grade 0 AKD accounted for the majority of AKD patients (38%) (). Patients with AKD had the highest incidence of ascites manifestation (76.2%). Higher GRAN% (69.4 ± 11.6 vs. 60.2 ± 13.3, p < .01), urea (6.0 ± 2.8 vs. 4.8 ± 1.7, p < .05), and uric acid (381.9 ± 181.0 vs. 295.1 ± 79.2, p < .05), but lower LYM% (20.3 ± 9.9 vs. 26.4 ± 9.5, p < .05) and eGFR (80.3 ± 27.8 vs. 96.4 ± 14.0, p < .05) were present in patients diagnosed with postoperative AKD than those in the non-AKI group. Intraoperative hypotension duration was notably longer in the AKD cohort than in the non-AKI cohort (32.2 ± 27.3 vs. 18.7 ± 16.5 min, p < .05) ().
Postoperative outcomes and follow-up results
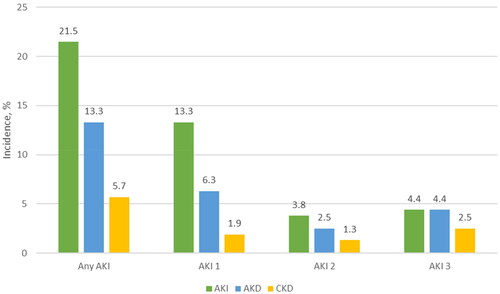
Overall, 34 (21.5%) patients developed postoperative AKI. According to the KDIGO classification, 20 (13.3%) patients suffered from stage I, 6 (3.8%) developed stage II, and 7 (4.4%) occurred from stage III (). Additionally, 20 (61.8%) of these AKI patients coincided with the AKD diagnosis. During the 90-day follow-up for the AKD group, 42.8% of patients were diagnosed with CKD ().
Table 2. Postoperative outcomes of patients undergoing CRS-HIPEC.
The recorded 30-day mortality was 3.2% (n = 5) of the total patients, while all these adverse events occurred in the AKI group (14.7%) (). In-hospital mortality was owing to suffering acute kidney failure, infectious shock, and multiple organ dysfunction syndromes (MODS) in all three patients who belonged to the AKD cohort. In addition, one patient developed cerebral infarction and MODS, while hemorrhagic shock was due to a lower gastrointestinal hemorrhage in another ().
Table 3. Characteristics and causes for patients occurred in-hospital death.
Factors associated with AKI
We performed univariate and multivariate logistic regression to identify the risk factors for AKI development in this patient population. By the former, the univariate model identified HCT, WBC, GRAN%, LYM%, baseline SCr, urea, uric acid, eGFR, ASA status, IO blood loss, duration of IO hypotension, and use of 5-FU as associated factors of the occurrence of postoperative AKI. After multivariable analysis, patients with AKI were more likely to have ascites (adjusted OR, 3.501; 95% CI, 1.149–10.663; p < .05), present with higher GRAN% (adjusted OR, 1.195; 95% CI, 1.022–1.399; p < .05), show lower eGFR (adjusted OR, 0.949; 95% CI, 0.905–0.995; p < .05), and experience the prolonged duration of IO hypotension (adjusted OR, 1.030; 95% CI, 1.005–1.054; p < .05) ().
Table 4. Univariate and multivariate analyses of risk factors for AKI after CRS-HIPEC procedure.
Risk factors for AKD
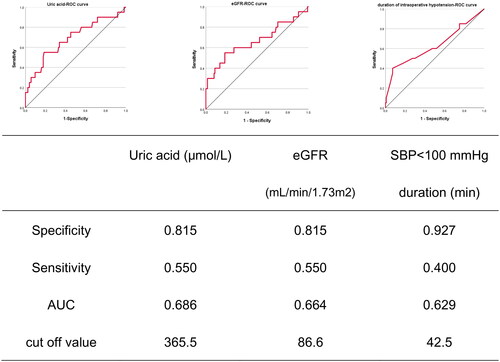
Univariate analysis for predictors associated with AKD is shown in . After multivariate regression analysis, uric acid (adjusted OR, 1.012; 95% CI, 1.001–1.023; p < .05), eGFR (adjusted OR, 0.942; 95% CI, 0.889–0.998; p < .05), and duration of IO hypotension (adjusted OR, 1.036; 95% CI, 1.003–1.070; p < .05) remained independent predictors of AKD. According to the receiver operating characteristic curve (ROC) () analysis, uric acid specificity was 0.815, sensitivity was 0.550, and the area under the curve was 0.686 (eGFR specificity of 0.815, the sensitivity 0.550, and the area under the curve 0.664). The hypotension duration specificity is 0.927, but the sensitivity is only 0.400, and the area under the curve is 0.629.
Table 5. Univariate and multivariate analyses for predictors of HIPEC-induced AKD.
Discussion
Our study found a significant increase in the incidence of AKI after CRS-HIPEC. The multivariate analysis identified that ascites, GRAN%, eGFR, and IO hypotension duration were associated with the development of HIPEC-induced AKI. AKI patients who progress to AKD, most of which are accompanied by high uric acid, low eGFR, and longer IO hypotension duration. The AKD cohort outcomes presented a higher risk of 30 days of in-hospital mortality and CKD progression.
The incidences of HIPEC-induced AKI varied widely between 1% and 48% [Citation18,Citation19]. This paper detected that 21.5% of patients experienced AKI after the CRS-HIPEC procedure. Hakeam et al. found that 3.7% of patients in their study developed AKI after CRS-HIPEC [Citation22]. In another study, Sin et al. reported that AKI occurred in 40.4% of ovarian cancer patients undergoing the CRS-HIPEC procedure [Citation23]. Additionally, with the larger sample size (n = 475) and the AKIN criteria, Cata et al. presented an AKI rate similar to ours. They found that 21.3% of patients had HIPEC-induced AKI [Citation21].
Prior studies have noted several risk factors for HIPEC-associated AKI. In our study, we demonstrated that ascites was significantly associated with the development of AKI. This may be explained by the fact that ascites can increase intra-abdominal pressure, which negatively affects renal function [Citation29]. For those patients who suffered from advanced carcinoma, peritoneal carcinomatosis increased the incidence of ascites, leading to higher intra-abdominal pressure. In addition, patients with higher preoperative GRAN% and LYM%, but not high GRAN and LYM, were confirmed at an incremental risk for HIPEC-induced AKI. Indeed, inflammatory processes mediated by the immune system are pivotal in mediating renal injury [Citation30]. Based on the individual differences of patients, there may be individual differences in the values of GRAN and LYM, while GRAN% and LYM% are relatively comparative. High GRAN% and LYM% presented the pre-disposing risk factor status, and the preoperative levels of inflammatory factors such as WBC and CRP in CKD patients are obviously higher, suggesting that the increase of inflammatory factors may be a sign of the aggravation of the secondary inflammatory process of kidney disease [Citation31]. MODS and septic shock have also appeared many times in the etiology of CKD patients, and the immune inflammatory system plays an essential role in the progress of the disease.
Our results proved that those with lower preoperative eGFR were more likely to experience HIPEC-induced renal impairment, and this finding was similar to what has been described in previous studies [Citation23]. Identifying patients at risk preoperatively, especially those with lower eGFR, would assist us in adopting suitable preventive measures. Furthermore, our study found that IO blood loss and duration of hypotension were significantly higher in patients suffering from HIPEC-induced AKI, suggesting that hypotension substantially contributes to causing ischemia–reperfusion injury, which may manifest as postoperative AKI [Citation32]. It is recommended that keeping blood pressure stable during operation and avoiding hypotension may reduce the risk of postoperative AKI [Citation33].
Unlike previous research results that cisplatin can increase the incidence of AKI in patients [Citation34], our statistical results show that the relationship between cisplatin and AKI is insignificant. It may be related to the administration of cisplatin. In previous studies, cisplatin was administered intravenously [Citation35], while ours was administered intraperitoneally. Different administration methods may result in different results. In the future, we plan to expand the sample size and continue to explore the differences between intraperitoneal administration and intravenous administration of cisplatin.
Among those who developed HIPEC-associated AKI, 61.8% of patients occurred AKI-to-AKD transition. Nonetheless, a previous study by Kellum et al. found that 36% of patients diagnosed with AKI have proceeded to AKD [Citation36]. Although this figure is lower than in our study, the authors only included critically ill patients with stage 2 or 3 AKI. Based on a retrospective study including 1341 patients, Peerapornratana et al. reported the association of AKD with deficient kidney recovery at hospital discharge among critically ill septic patients [Citation37]. Indeed, a recent meta-analysis demonstrated that prolonged AKI duration was independently associated with cardiovascular adverse events, development of incident CKD, and long-term mortality [Citation38]. Taken together, these findings punctuate the clinical influence of persistently reduced kidney function from AKI-to-AKD. Therefore, we drew attention to examining the prevalence and independently associated factors of AKD in patients with CRS-HIPEC procedures. In our population, 42.8% of patients with AKD propagated the transition to CKD, while 14.3% of in-hospital mortality occurred in the AKD cohort.
Our study also demonstrated that the development of AKD was significantly associated with preoperative eGFR, uric acid level, and IO hypotension duration. Patients with lower preoperative eGFR were more prone to beget AKI and proceed with AKD than those with normal parameters. Consistent with our present findings, previous studies have shown that lower eGFR was a risk factor for hospitalization, which was a risk factor for renal function impairment [Citation39–41]. The uric acid level was affirmed as a possible determinant of transient and persistent kidney dysfunction [Citation42]. Furthermore, baseline uric acid was presented to increase the incidence rate of both CKD and ESRD, with accelerated CKD progression. Our patient collective recognized preoperative uric acid as the leading risk factor for AKD after HIPEC. It is well known that hyperuricemia is closely associated with AKI/CKD and is a risk factor for renal insufficiency in the general population [Citation43]. However, there are few studies on AKD and high uric acid. This study found that preoperative hyperuricemia is one of the independent risk factors for AKD in patients undergoing CRS-HIPEC, which may be because long-term high uric acid level keeps the kidney in a damaged state, renal overload is likely to occur after CRS-HIPEC, resulting in AKD. ROC curve analysis showed that although uric acid and eGFR had low sensitivity (0.55) in predicting the occurrence of AKD, they had high specificity (0.815), suggesting that under the premise of maintaining the patient’s physical condition stable, reducing the preoperative uric acid as much as possible may reduce the incidence of postoperative AKD. It is generally believed that the average arterial pressure of the injury threshold for organ damage is about 60–70 mmHg, and the systolic pressure is about 90–100 mmHg [Citation44]. Patients with hypertension may be less able to tolerate hypotension than those with normal blood pressure and may require higher perioperative blood pressure [Citation45]. Prolonged episodes of hypotension during the IO period may decrease renal perfusion, resulting in renal function injury in patients with impaired autoregulation [Citation46]. However, the ROC curve indicates only high specificity (0.927) and a sensitivity of only 0.4. Overall, it is rational to recommend that the duration of the IO hypotensive episode should be kept as short as possible [Citation47].
CRS-HIPEC has become a standard treatment for peritoneal metastasis in selected patients, delivering more prolonged survival [Citation27,Citation48]. Nevertheless, several relevant morbidities were identified after this procedure [Citation49,Citation50]. Among these complications, AKI has been recognized as the most common complication following CRS-HIPEC [Citation51]. Compared to other studies investigating the incidence of AKD after AKI in hospitalized patients, this is the first study explicitly examining the epidemiology and risk factors of HIPEC-associated AKI and AKD. With the popular concepts of the AKI-to-AKD-to-CKD interplay and the association between AKD and incremental future mortality risk, many researchers have drawn attention to AKD prevention and management. However, little is known about the incidence of HIPEC-associated AKD, and it is unclear what parameters are associated with AKD. The study may provide a reference for reducing the incidence of postoperative AKI or AKI progression to AKD in patients undergoing CRS-HIPEC.
The shortcomings of the current study include our data from a single medical center, which limits generalizability. Additionally, this is a retrospective analysis of prospectively collected data, and as a result, there is an inherent selection bias. We attempted to select patients by two investigators using narrow inclusion criteria to mitigate this, and all records were reviewed. Finally, we only used SCr concentration to define AKI, while urine output was not included in diagnosing postoperative AKI due to incomplete data on urine output. And in the future, we intend to expand the sample size to continue our research. There is abundant room for further progress in designing prospective randomized trials regarding the sample size necessary to detect the long-term renal function difference in AKI and AKD cohorts.
Conclusions
Our present study implies that the incidence of AKI in patients undergoing CRS-HIPEC is high, and the transition to AKD is a common outcome following AKI, which confirms the association with more risk for both in-hospital mortality consulting from renal function failure and CKD progression. Patients at higher risk of AKI after the CRS-HIPEC procedure include ascites, incremental GRAN%, lower eGFR, and prolonged IO hypotension duration. Further independent risk factors for developing AKD are higher preoperative uric acid levels, lower eGFR, and longer IO hypotension duration. The indispensability of early recognition of HIPEC-induced AKD is beyond diagnostic purposes because it provides the opportunity for timely interventions to mitigate detrimental post-AKI complications.
Supplemental Material
Download PDF (125.9 KB)Acknowledgements
This work was preprinted at Research Square [https://doi.org/10.21203/rs.3.rs-1820140/v1]. The citation was Yunwei Lu, Xiujuan Zhao, Yingjiang Ye, et al. Incidence, risk factors, and outcomes of transition of HIPEC-induced acute kidney injury to acute kidney disease: a retrospective study, 15 July 2022, PREPRINT (Version 1).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Thakar CV. Perioperative acute kidney injury. Adv Chronic Kidney Dis. 2013;20(1):1–13. doi: 10.1053/j.ackd.2012.10.003.
- Thakar CV, Christianson A, Freyberg R, et al. Incidence and outcomes of acute kidney injury in intensive care units: a veterans administration study. Crit Care Med. 2009;37(9):2552–2558. doi: 10.1097/CCM.0b013e3181a5906f.
- Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442–448. doi: 10.1038/ki.2011.379.
- Coca SG, Yusuf B, Shlipak MG, et al. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53(6):961–973. doi: 10.1053/j.ajkd.2008.11.034.
- Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365–3370. doi: 10.1681/ASN.2004090740.
- Chawla LS, Bellomo R, Bihorac A, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13(4):241–257. doi: 10.1038/nrneph.2017.2.
- Matsuura R, Iwagami M, Moriya H, et al. The clinical course of acute kidney disease after cardiac surgery: a retrospective observational study. Sci Rep. 2020;10(1):6490. doi: 10.1038/s41598-020-62981-1.
- Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221(1):29–42. doi: 10.1097/00000658-199501000-00004.
- Bhatt A, Sheshadri D, Chandan G, et al. Outcomes of cytoreductive surgery and HIPEC for pseudomyxoma peritonei of appendiceal origin from two Indian centers: a preliminary five-year experience. J BUON. 2017;22(1):251–257.
- Bonnot P-E, Piessen G, Kepenekian V, et al. Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy for gastric cancer with peritoneal metastases (CYTO-CHIP study): a propensity score analysis. J Clin Oncol. 2019;37(23):2028–2040. doi: 10.1200/JCO.18.01688.
- Yang X-J, Huang C-Q, Suo T, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18(6):1575–1581. doi: 10.1245/s10434-011-1631-5.
- Esquivel J, Lowy AM, Markman M, et al. The American Society of Peritoneal Surface Malignancies (ASPSM) multiinstitution evaluation of the Peritoneal Surface Disease Severity Score (PSDSS) in 1,013 patients with colorectal cancer with peritoneal carcinomatosis. Ann Surg Oncol. 2014;21(13):4195–4201. doi: 10.1245/s10434-014-3798-z.
- van Driel WJ, Koole SN, Sikorska K, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378(3):230–240. doi: 10.1056/NEJMoa1708618.
- Baratti D, Kusamura S, Cabras AD, et al. Diffuse malignant peritoneal mesothelioma: long-term survival with complete cytoreductive surgery followed by hyperthermic intraperitoneal chemotherapy (HIPEC). Eur J Cancer. 2013;49(15):3140–3148. doi: 10.1016/j.ejca.2013.05.027.
- Schwarz L, Votanopoulos K, Morris D, et al. Is the combination of distal pancreatectomy and cytoreductive surgery with HIPEC reasonable? Results of an international multicenter study. Ann Surg. 2016;263(2):369–375. doi: 10.1097/SLA.0000000000001225.
- Mehta S, Schwarz L, Spiliotis J, et al. Is there an oncological interest in the combination of CRS/HIPEC for peritoneal carcinomatosis of HCC? Results of a multicenter international study. Eur J Surg Oncol. 2018;44(11):1786–1792. doi: 10.1016/j.ejso.2018.05.021.
- Lee L, Alie-Cusson F, Dubé P, et al. Postoperative complications affect long-term outcomes after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal peritoneal carcinomatosis. J Surg Oncol. 2017;116(2):236–243. doi: 10.1002/jso.24632.
- Angeles MA, Quenet F, Vieille P, et al. Predictive risk factors of acute kidney injury after cytoreductive surgery and cisplatin-based hyperthermic intra-peritoneal chemotherapy for ovarian peritoneal carcinomatosis. Int J Gynecol Cancer. 2019;29(2):382–391. doi: 10.1136/ijgc-2018-000099.
- Haslinger M, Francescutti V, Attwood K, et al. A contemporary analysis of morbidity and outcomes in cytoreduction/hyperthermic intraperitoneal chemoperfusion. Cancer Med. 2013;2(3):334–342. doi: 10.1002/cam4.80.
- Bouhadjari N, Gabato W, Calabrese D, et al. Hyperthermic intraperitoneal chemotherapy with cisplatin: amifostine prevents acute severe renal impairment. Eur J Surg Oncol. 2016;42(2):219–223. doi: 10.1016/j.ejso.2015.07.016.
- Cata JP, Zavala AM, Van Meter A, et al. Identification of risk factors associated with postoperative acute kidney injury after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy: a retrospective study. Int J Hyperthermia. 2018;34(5):538–544. doi: 10.1080/02656736.2017.1368096.
- Hakeam HA, Breakiet M, Azzam A, et al. The incidence of cisplatin nephrotoxicity post hyperthermic intraperitoneal chemotherapy (HIPEC) and cytoreductive surgery. Ren Fail. 2014;36(10):1486–1491. doi: 10.3109/0886022X.2014.949758.
- Sin EI-L, Chia CS, Tan GHC, et al. Acute kidney injury in ovarian cancer patients undergoing cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy. Int J Hyperthermia. 2017;33(6):690–695. doi: 10.1080/02656736.2017.1293304.
- Ye J, Ren Y, Wei Z, et al. Nephrotoxicity and long-term survival investigations for patients with peritoneal carcinomatosis using hyperthermic intraperitoneal chemotherapy with cisplatin: a retrospective cohort study. Surg Oncol. 2018;27(3):456–461. doi: 10.1016/j.suronc.2018.05.025.
- Fliser D, Laville M, Covic A, et al. A European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines on acute kidney injury: part 1: definitions, conservative management and contrast-induced nephropathy. Nephrol Dial Transplant. 2012;27(12):4263–4272. doi: 10.1093/ndt/gfs375.
- Hsu C-K, Wu I-W, Chen Y-T, et al. Acute kidney disease stage predicts outcome of patients on extracorporeal membrane oxygenation support. PLOS One. 2020;15(4):e0231505. doi: 10.1371/journal.pone.0231505.
- Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21(20):3737–3743. doi: 10.1200/JCO.2003.04.187.
- Albanese AM, Albanese EF, Miño JH, et al. Peritoneal surface area: measurements of 40 structures covered by peritoneum: correlation between total peritoneal surface area and the surface calculated by formulas. Surg Radiol Anat. 2009;31(5):369–377. doi: 10.1007/s00276-008-0456-9.
- Dalfino L, Tullo L, Donadio I, et al. Intra-abdominal hypertension and acute renal failure in critically ill patients. Intensive Care Med. 2008;34(4):707–713. doi: 10.1007/s00134-007-0969-4.
- Gonçalves GM, Zamboni DS, Câmara NO. The role of innate immunity in septic acute kidney injuries. Shock. 2010;34(7):22–26. doi: 10.1097/SHK.0b013e3181e7e69e.
- Shankar A, Sun L, Klein BEK, et al. Markers of inflammation predict the long-term risk of developing chronic kidney disease: a population-based cohort study. Kidney Int. 2011;80(11):1231–1238. doi: 10.1038/ki.2011.283.
- POISE Study Group, Devereaux PJ, Yang H, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371(9627):1839–1847.
- Walsh M, Devereaux PJ, Garg AX, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology. 2013;119(3):507–515. doi: 10.1097/ALN.0b013e3182a10e26.
- McSweeney KR, Gadanec LK, Qaradakhi T, et al. Mechanisms of cisplatin-induced acute kidney injury: pathological mechanisms, pharmacological interventions, and genetic mitigations. Cancers. 2021;13(7):1572. doi: 10.3390/cancers13071572.
- Manohar S, Leung N. Cisplatin nephrotoxicity: a review of the literature. J Nephrol. 2018;31(1):15–25. doi: 10.1007/s40620-017-0392-z.
- Kellum JA, Sileanu FE, Bihorac A, et al. Recovery after acute kidney injury. Am J Respir Crit Care Med. 2017;195(6):784–791. doi: 10.1164/rccm.201604-0799OC.
- Peerapornratana S, Priyanka P, Wang S, et al. Sepsis-associated acute kidney disease. Kidney Int Rep. 2020;5(6):839–850. doi: 10.1016/j.ekir.2020.03.005.
- Mehta S, Chauhan K, Patel A, et al. The prognostic importance of duration of AKI: a systematic review and meta-analysis. BMC Nephrol. 2018;19(1):91. doi: 10.1186/s12882-018-0876-7.
- Grams ME, Astor BC, Bash LD, et al. Albuminuria and estimated glomerular filtration rate independently associate with acute kidney injury. J Am Soc Nephrol. 2010;21(10):1757–1764. doi: 10.1681/ASN.2010010128.
- Grams ME, Sang Y, Ballew SH, et al. A meta-analysis of the association of estimated GFR, albuminuria, age, race, and sex with acute kidney injury. Am J Kidney Dis. 2015;66(4):591–601. doi: 10.1053/j.ajkd.2015.02.337.
- James MT, Grams ME, Woodward M, et al. A meta-analysis of the association of estimated GFR, albuminuria, diabetes mellitus, and hypertension with acute kidney injury. Am J Kidney Dis. 2015;66(4):602–612. doi: 10.1053/j.ajkd.2015.02.338.
- Ben-Dov IZ, Kark JD. Serum uric acid is a GFR-independent long-term predictor of acute and chronic renal insufficiency: the Jerusalem Lipid Research Clinic Cohort Study. Nephrol Dial Transplant. 2011;26(8):2558–2566. doi: 10.1093/ndt/gfq740.
- Jalal DI, Chonchol M, Chen W, et al. Uric acid as a target of therapy in CKD. Am J Kidney Dis. 2013;61(1):134–146. doi: 10.1053/j.ajkd.2012.07.021.
- Saugel B, Sessler DI. Perioperative blood pressure management. Anesthesiology. 2021;134(2):250–261. doi: 10.1097/ALN.0000000000003610.
- Futier E, Lefrant J-Y, Guinot P-G, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA. 2017;318(14):1346–1357. doi: 10.1001/jama.2017.14172.
- Dünser MW, Takala J, Ulmer H, et al. Arterial blood pressure during early sepsis and outcome. Intensive Care Med. 2009;35(7):1225–1233. doi: 10.1007/s00134-009-1427-2.
- Sun LY, Wijeysundera DN, Tait GA, et al. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology. 2015;123(3):515–523. doi: 10.1097/ALN.0000000000000765.
- Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004;22(16):3284–3292. doi: 10.1200/JCO.2004.10.012.
- Desantis M, Bernard J-L, Casanova V, et al. Morbidity, mortality, and oncological outcomes of 401 consecutive cytoreductive procedures with hyperthermic intraperitoneal chemotherapy (HIPEC). Langenbecks Arch Surg. 2015;400(1):37–48. doi: 10.1007/s00423-014-1253-z.
- Malfroy S, Wallet F, Maucort-Boulch D, et al. Complications after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis: risk factors for ICU admission and morbidity prognostic score. Surg Oncol. 2016;25(1):6–15. doi: 10.1016/j.suronc.2015.11.003.
- Naffouje SA, Tulla KA, Chorley R, et al. Acute kidney injury increases the rate of major morbidities in cytoreductive surgery and HIPEC. Ann Med Surg. 2018;35:163–168. doi: 10.1016/j.amsu.2018.09.036.