Abstract
Background
Immunoglobulin A (IgA) nephropathy (IgAN) treatment consists of maximal supportive care and, for high-risk individuals, immunosuppressive treatment (IST). There are conflicting results regarding IST. Therefore, we aimed to investigate IST results among IgAN patients in Turkiye.
Method
The data of 1656 IgAN patients in the Primary Glomerular Diseases Study of the Turkish Society of Nephrology Glomerular Diseases Study Group were analyzed. A total of 408 primary IgAN patients treated with IST (65.4% male, mean age 38.4 ± 12.5 years, follow-up 30 (3–218) months) were included and divided into two groups according to treatment protocols (isolated corticosteroid [CS] 70.6% and combined IST 29.4%). Treatment responses, associated factors were analyzed.
Results
Remission (66.7% partial, 33.7% complete) was achieved in 74.7% of patients. Baseline systolic blood pressure, mean arterial pressure, and proteinuria levels were lower in responsives. Remission was achieved at significantly higher rates in the CS group (78% vs. 66.7%, p = 0.016). Partial remission was the prominent remission type. The remission rate was significantly higher among patients with segmental sclerosis compared to those without (60.4% vs. 49%, p = 0.047). In the multivariate analysis, MEST-C S1 (HR 1.43, 95% CI 1.08–1.89, p = 0.013), MEST-C T1 (HR 0.68, 95% CI 0.51–0.91, p = 0.008) and combined IST (HR 0.66, 95% CI 0.49–0.91, p = 0.009) were found to be significant regarding remission.
Conclusion
CS can significantly improve remission in high-risk Turkish IgAN patients, despite the reliance on non-quantitative endpoints for favorable renal outcomes. Key predictors of remission include baseline proteinuria and specific histological markers. It is crucial to carefully weigh the risks and benefits of immunosuppressive therapy for these patients.
Introduction
Immunoglobulin A (IgA) nephropathy (IgAN) is the leading cause of primary glomerulonephritis in Turkiye, as well as all over the world [Citation1,Citation2]. Regarding the renal outcome, studies revealed an end-stage renal disease risk of 10–50% within 10–20 years [Citation3]. Along with the frequent occurrence, deleterious renal outcome odds make treatment approaches necessary. According to the studies and the latest KDIGO guidelines, supportive care is an indispensable treatment component. Additionally, immunosuppressive treatment (IST) is recommended for high-risk individuals [Citation4,Citation5]. However, studies that have been done so far have not revealed satisfactory results regarding IgAN treatment.
Besides unanswered questions about treatment, the pathogenesis of IgAN is enlightened. The pathogenesis of IgAN is explained by a multi-hit hypothesis, which consecutively consists of elevated serum galactose-deficient IgA1 levels, circulating anti-glycan autoantibodies, formed pathogenic IgA1-containing circulating immune complexes, and eventually deposition of the immune complexes in the glomeruli [Citation6]. These depositions trigger several immunological pathways and lead to kidney injury [Citation7]. Consequently, inflammation and immunological mechanisms are the main players in pathogenesis. With this regard, IST still holds its place. Corticosteroids (CS) are the mainstay agents of IST. Although the efficiency of other immunosuppressive agents is controversial, additional immunosuppressive agents are an option for high-risk individuals in practice. The main issues about the IST are efficacy and adverse effects. Favorable renal outcome results with IST could be valid for high-risk patients [Citation8–12]. However, serious adverse events (AEs) associated with IST are the common caution of clinical trials and guidelines [Citation4,Citation8,Citation13–17]. STOP-IgAN trial showed that additional IST to supportive care did not improve renal outcome and was associated with more adverse effects in IgAN patients [Citation13]. Recently, favorable results with IST were observed in several studies [Citation9,Citation10,Citation12]. On the other hand, numerous researches are going on for novel insights into IgAN management by targeting therapies and new agents. Moreover, there may be regional differences in response to IST. For example, in some Asian studies, the effectiveness of MMF has been shown to have positive results, unlike in other regions. As IgAN is the leading glomerular disease in Turkiye, management practice and outcome results are a considerable issue in our country.
Although, there are more studies comparing supportive care and IST, knowledge about efficacy of other immunosuppressive regimens is limited and controversial. Therefore, we aimed to investigate the results of the initial IST and outcome determinants among high-risk IgAN patients.
Material and methods
Study population
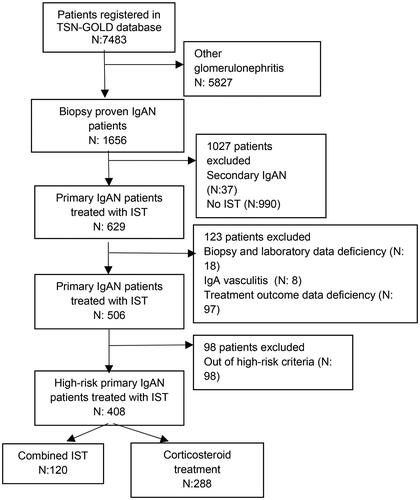
The data were obtained from a national database, the Primary Glomerulonephritis Registry of the Turkish Society of Nephrology Glomerular Diseases Study Group (TSN-GOLD) which was established on 04.04.2008 within the body of the Turkish Society of Nephrology. Biopsy-proven 1656 IgAN patients in the database, including 7483 patients, were analyzed. Patients with liver disease, inflammatory bowel disease, and rheumatologic disease (n:37) and patients not-administered IST (n:990) were excluded. A total of 629 primary IgAN patients who were treated with IST were evaluated. Patients with inaccurate biopsy diagnosis (n:8), IgA vasculitis (n:8), patients with laboratory data deficiency (n:9), and treatment response data deficiency (n:98) were excluded (). A total of 506 primary IgAN patients who received IST (63.4% male, mean age 38.9 ± 12.5 years) were reviewed. Ninety-eight patients who did not meet high-risk criteria were excluded. Eventually, 408 high-risk primary IgA patients who received IST were evaluated.
Demographics, body mass index (BMI), clinical presentation, blood pressure, laboratory and histopathological findings, treatment responses, and AEs were reviewed. Estimated glomerular filtration rate (eGFR) was calculated by CKD-EPI formula [Citation18]. The follow-up data were registered at 3, 6, and 12 months in the first year of the treatment, and annually after the first year.
Definitions
In this study, high-risk patients were defined as those with an eGFR greater than 30 mL/min and proteinuria exceeding 0.75 g/d under optimal supportive care [Citation4]. The biopsy indications were categorized as follows: asymptomatic urinary abnormalities (AUA), which included persistent non-nephrotic proteinuria and/or isolated microscopic hematuria; nephrotic syndrome, characterized by proteinuria exceeding 3.5 g/d along with hypoalbuminemia, edema, and hyperlipidemia; nephritic syndrome, indicated by hematuria, proteinuria less than 3.5 g/d, hypertension, and decreased GFR; rapidly progressive glomerulonephritis (RPGN), defined by a rapid decrease in GFR within days or weeks; mixed nephrotic syndrome, which combined features of nephrotic and nephritic syndromes; and others. RPGN patients were included in the nephritic syndrome subgroup.
Adverse events
Treatment-associated AEs declared by the centers in the database were evaluated. Severe infection was defined as an infection that required hospitalization, like pneumonia, severe pyelonephritis, sepsis, etc. Mild infection was defined as an infection that could be controlled with oral antibiotics. Steroid-associated AEs were Cushingoid appearance, glucose intolerance, osteoporosis, hirsutism, acne, and myopathy. Other AEs were defined as nonspecific complaints such as diarrhea, dyspepsia, headache, and itching.
Treatment protocols
The data were obtained from 26 centers in Turkiye. All centers recorded their data through a national web-based database. Treatment protocols were consistent with the KDIGO guidelines [Citation4] and the TSN Consent Report on Diagnosis and Treatment of Primary Glomerular Diseases [Citation19]. The individuals were divided into CS alone (70.6%) and combined IST (29.4%) groups according to the treatment protocols. Supportive care was administered to all individuals, including an ACEI or ARB at the maximum tolerated dose, blood pressure control, dietary counseling, smoking cessation, and weight control.
Systemic CS protocols were [Citation1] oral administration with a dose of 0.5–1 mg/kg/d prednisolone (maximum 60 mg/d) tapering within 4-8 months according to the response at least for 3–6 months, [Citation2] Pozzi protocol (intravenous methylprednisolone for three consecutive days in the 1st-3rd-5th months and 0.5 mg/kg/d oral prednisone every other day for 6 months). Combined therapy is a combination of CS with one of the other immunosuppressive agents, including azathioprine (AZA), cyclophosphamide, calcineurin inhibitors (CNIs), and mycophenolate mofetil (MMF) [Citation19].
Treatment outcomes
Treatment responses were recorded according to the declaration of the centers and obtained from the database. In this registration system, it is required from the participant centers to input the ‘‘treatment response to initial immunosuppressive treatment’’ information of patients treated with IST as below; 1. Remission achieved. 2. Remission not achieved. Afterward, the remission type was determined by the acknowledged definitions as partial or complete. Treatment responses were defined as complete remission (proteinuria <0.5 g/d with stable or improved serum creatinine level), partial remission (proteinuria 0.5–3.5 g/d, >%50 decrease, and <3.5 g/d with stable or improved serum creatinine level) and unresponsive. Relapse was defined as proteinuria >3.5 g/d after complete remission has been achieved [Citation4].
Histopathological evaluation
The authorized pathologist of each center performed the histopathological evaluation. Light and immunofluorescence microscopic (IF) evaluations were performed in all specimens. For light microscopic (LM) evaluation, paraffin-hidden tissue was examined under hematoxylin-eosin, periodic acid–Schiff, aldehyde fuchsin orange G, and periodic acid-silver methenamine stains. The fluorescence intensity of immunoglobulins (IgG, IgA, and IgM) and complement components (C3 and C1q) was assessed by means of a semi-quantitative scale ranging between 0 and +3. The number of total, global sclerotic, and segmental sclerotic glomeruli, the presence of thickening of the basal membrane, mesangial proliferation, interstitial inflammation, and tubular atrophy were evaluated for each biopsy sample. Kidney biopsy demonstrating dominant or codominant mesangial IgA staining is required for definitive diagnosis of IgAN. Oxford MEST-C classification was performed for every specimen with IgAN features according to LM and IF findings.
Ethical approval
Ethical approval was obtained from the institutional Ethics Committee of Istanbul University, Istanbul Faculty of Medicine.
Statistics
The suitability of continuous variables to normal distribution was examined with the Shapiro-Wilk test. Continuous variables were evaluated using mean ± standard deviation or median (minimum:maximum) values; Categorical variables are expressed as n(%). In comparisons between groups, independent paired sample t-test was used if there were two groups and normal distribution was observed, and Mann–Whitney U test was used if normal distribution was not observed. Categorical variables were analyzed using the Chi-square test, Fisher’s Exact Chi-Square test, and Fisher Freeman Halton tests.
The risk factors affecting the development of remission were examined using Cox regression analysis. To determine the risk factors thought to be effective on the development of remission, univariate Cox regression analysis was first performed, and after the analysis, variables that met the p < 0.25 criterion were included in the multivariate Cox regression model. The variables of age, serum creatinine level, proteinuria, immunosuppressive protocol, and Oxford MEST-C classification were examined with univariate Cox regression analysis. Variables found to be significant as a result of univariate analyses were included in the multivariate analysis and the results of the relevant analysis are reported in the table. The regression model created as a result of multivariate analysis was found to be significant (p < 0.001). In the Cox regression analysis, the results of the Wald test were given. The Wald test gives the ratio of the regression coefficient to the standard error. SPSS (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.) program was used for statistical analysis, and the type I error level was accepted as 5% in statistical analyses.
Results
The study cohort included 408 primary IgAN patients, of whom 65.4% were male, 37.7% were hypertensive, 7.8% were diabetic, 13.7% were active smokers, and the mean age was 38.4 ± 12.5 years. Other demographic features, baseline laboratory findings, biopsy indications, and biopsy findings are presented in . The median follow-up duration was 30 (3–218) months.
Table 1. Demographic and baseline laboratory data of patients according to initial immunosuppressive treatment protocol.
The initial IST protocol two treatment groups were well balanced in demographic features, comorbidities, baseline laboratory findings, biopsy indications, and Oxford MEST-C classification. The participants in the combined group had higher proteinuria (3365 (750–10,885) vs. 2565 (790–23,300) mg/d, p = 0.007), and a lower rate of segmental glomerulosclerosis (S1 lesion) (49.6% vs. 61.1%, p = 0.036) ().
Treatment outcomes
Remission was achieved in 74.7% of the patients in a median duration of 4 months (1–69 months), with partial remission in 65.5% and complete remission in 34.5% (). Remission was maintained for a median of 12 months (1–126 months).
Table 2. Treatment responses.
When the patients grouped according to the remission achievement, responsive patients had lower systolic blood pressure, mean arterial pressure, and proteinuria levels (). Regarding Oxford classification, the remission rate was significantly higher among patients with S1 lesions (60.4% vs. 49%, p = 0.047) (). Additionally, regarding remission types, partial remission rate was significantly higher than complete remission (73.1% vs. 26.9%, p:0.004) among responsive patients with S1 lesions.
Table 3. Laboratory and demographic data of patients according to treatment responses.
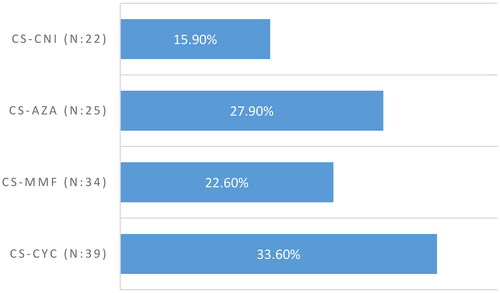
When the patients grouped according to the treatment, remission was achieved at significantly higher rates in the CS group (78% vs. 66.7%, p = 0.016). In the combined group, treatment response was compared between treatment subgroups. There was no significant difference in remission rates between treatment subgroups (). When we compared the remission types, partial remission rate and complete remission rate were similar between treatment groups. Partial remission was the prominent treatment achievement among responsive patients (). During the follow-ups, relapse was declared in 32.3% (n = 94/291) of responsive patients.
Figure 2. Remission rates of other immunosuppressive protocols. CS: corticosteroid, CYS: cyclophosphamide, MMF: mycophenolate mofetil, CNI: calcineurin inhibitor, AZA: azathioprine.
The outcome data of 173 individuals was declared. A total of 24 patients (13.9%) developed ESRD and 2 patients (1 in the CS group, 1 in the CS-cyclophosphamide group) died due to sepsis, which was considered related to IST.
Adverse events
Steroid-associated AEs (n = 74) were significantly higher in the CS group (93.4% vs. 56.7%, p < 0.001). Both mild infection (n = 1), severe infection (n = 6), and other treatment-associated AEs (n = 10) were prominent in the combined IST group (3.3% vs. 0, 13.3% vs. 3.3%, 26.7% vs. 3.3%, respectively, p < 0.001). In the sub-group analysis, severe infection rate was higher in the CS-cyclophosphamide group and mild in the MMF group (p < 0.001). The laboratory findings, age, gender, BMI, or presence of diabetes that could be associated with AEs were analyzed. And only proteinuria was higher in patients who were exposed to an AE (4038 ± 3147 g/d vs. 3285 ± 2321 g/d, p = 0.024).
Cox regression results
The results of multivariate cox regression analysis are reported in . In the multivariate analysis, MEST-C S1 (HR 1.43, 95% CI 1.08–1.89, p = 0.013), MEST-C T1 (HR 0.68, 95% CI 0.51–0.91, p = 0.008) and combined treatment (HR 0.66, 95% CI 0.49–0.91, p = 0.009) were found to be significant regarding remission (). Remission expectancy was determined to be 1.43 times higher in patients with S1 lesions than in patients with S0. Remission achievement was 32% less likely in patients with T1 than in patients with T0 features, and 34% less likely in patients treated with combined protocol than individuals treated with CS ().
Figure 3. Cumulative incidence of primary outcome, remission, according to remission-associated risk factors during follow-ups. CI: Confidence Interval.
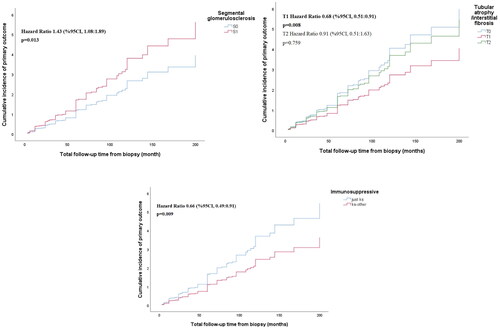
Table 4. Analysis of factors associated with remission by multivariate cox regression.
Discussion
In this study, we investigated the impact of initial IST on high-risk IgAN patients. We focused on treatment outcomes, AEs, and factors influencing remission. Our study utilized a national database, enabling us to analyze a substantial cohort of biopsy-proven primary IgAN patients. High-risk individuals were identified based on specific criteria involving eGFR and proteinuria levels after a period of supportive care. According to our findings, remission was achieved with a significant rate of 74.7% among high-risk primary IgAN patients with initial IST. However, our results were based on non-quantitative endpoints which were obtained from registered data. Consistent with recent studies, systemic CSs might have favorable beneficial effects in high risk IgAN patients.
The mainstay of IgAN management is to be beneficial and harmless. Optimal supportive care is the mainstay of IgAN treatment which is consisting of an ACEI or ARB at the maximum tolerated dose, blood pressure control, dietary counseling, smoking cessation, and weight control. Afterwards, determination of progression risk is essential. In the recent KDIGO guideline high risk of progression in primary IgAN was defined as proteinuria >0.75–1 g/d despite 90 d of optimized supportive care and for these high risk patients a 6-month course of glucocorticoid therapy is recommended. Other immunosuppressive agents of which the safety and efficacy are uncertain, were recommended only for patients who remained at high risk of progressive CKD, with patient decision-sharing [Citation4]. In our study, IST provided beneficial effect for high risk primary IgAN patients. However, remission rate were lower with other immunosuppressant agents combined with CS. Additionally, in our study AEs were considerable issue with IST. Because of considerable side effects, repeated or prolonged treatment with CSs in patients with smoldering or relapsing inflammatory activity should be avoided.
Systemic CS are former and well-studied drugs in IgAN. Favorable long-term renal outcome results with 6–8 months long CS treatment was reported in several historical studies [Citation20–22]. However, in these studies there are several methodological limitations such as small sample sizes, inadequate optimization of supportive treatment, and preserved baseline kidney function. Beneficial effects of CS were mentioned in the Cochrane review which were higher complete remission rates and lower risk of progression in patients with proteinuria >1 g/day compared to placebo or standard of care. In this review 58 randomized controlled trials (RCTs) and quasi-RCTs were evaluated and the main criticism was not optimizing supportive care and RAASi during a standardized run-in phase of the reviewed studies [Citation8]. Recent RCTs overcoming these shortages showed CS efficiency on renal function. However, significant treatment-associated AEs and undistinguished long-term outcomes compared with supportive care provoked conflicts [Citation9,Citation11–17,Citation23]. In the TESTING trial, the primary outcome, defined as a composite of 40% decline in eGFR, kidney failure (dialysis, transplant), or death due to kidney disease occurred in 74 participants (28.8%) in the CS group compared with 106 (43.1%) in the placebo group. Taking backwards the results, renal functions were preserved in 71.2% of the participants with CS [Citation9]. Consistent with these findings in our study CS had beneficial effects in high risk IgAN patients. However, our results were based on non-quantitative endpoints.
Along with CS, protocols with additive immunosuppressive agents are used in practice. This management approach is commonly preferred in patients with higher progression risk, features of active proliferative lesions (cellular and fibrocellular crescents, endocapillary hypercellularity, or necrosis) that predict worse outcomes. MMF is the leading agent of which the efficacy was shown in several studies particularly consisting of Asian patients [Citation10,Citation12,Citation24]. Clinical benefit with other conventional immunosuppressive agents is uncertain. In the recent guideline, because these agents have no proven efficacy, it is recommended to enroll high-risk patients in a clinical trial instead [Citation4]. In our study, remission rates were higher in the CS group compared with combined group. Whereas partial remission was the prominent outcome. According to the logistic regression analysis, patients treated with CS were more advantageous for remission compared with other immunosuppressive agents. No additional beneficial effect was provided with other traditional immunosuppressive drugs in our patients.
Besides positive results with CS, treatment-associated toxicity is the major obstacle. Infections requiring hospitalization, diabetes, and death due to sepsis are leading consequences associated with CS toxicity. The concern about systemic CS markedly came up after STOP-IgAN and TESTING studies [Citation9,Citation13,Citation17]. To overcome CS toxicity lower CS dose regimen was used in the continued TESTING study [Citation17]. Although the efficacy was maintained with higher tolerability, still treatment-associated AEs were reported. Treatment- associated AEs were significantly reported among our patients. Although most of the practitioners are mostly aware of treatment-associated AEs and take care of possible expected ones, there were remarkable reported AE in our cohort. Furthermore, death of two patients was considered associated with treatment and reported due to sepsis. AEs could be more common among patients with decreased GFR, obesity, diabetes, and frailty. In our study, AEs did not differ among patients regarding age, gender, BMI, or presence of diabetes. Only proteinuria was higher in patients who had treatment-associated AEs.
The diagnosis of IgAN relies on kidney biopsy, and several histopathological features have been associated with progression. Because of issues about treatment approach, determining the patients at risk of disease progression who could have beneficial effect with IST is essential. The determinants that can predict the patient and renal outcome are specified as the degree of proteinuria, the presence of persistent microscopic hematuria, and the rate of eGFR loss, combined with the MEST-C score. However, there are some controversial inferences about the prognostic value of histopathologic lesions of the Oxford MEST-C classification regarding renal outcomes. According to our findings, tubular atrophy is a negative predictor for remission. This finding is consistent with previous studies, and supports to avoid aggressive immunosuppression in patients with chronic changings in the biopsy [Citation25]. The significance of glomerulosclerosis in tailoring a treatment approach is controversial. Presence and intensity of sclerotic lesions are generally considered as unfavorable findings regarding renal outcome. Besides, impact of segmental sclerotic lesion types on renal outcome is a considerable issue. Patients with segmental glomerulosclerosis (S1 lesions) tended to have the benefit of IST in our study. The reason for this remarkable result may be related to the characteristics and intensity of sclerosis. In a recent study, it was stated that rather than presence, characteristics of segmental sclerotic lesion are a better predictor of outcome [Citation26]. In this study, sclerotic lesions were defined as perihilar, tip, collapsing, and not otherwise specified (NOS; segmental sclerosis without characteristics associated with other subtypes) variants. And the authors stated that sclerotic lesion subtypes such as S-NOS, global sclerotic glomeruli, segmental adherence, and perihilar glomerular sclerosis were associated with adverse outcomes. Additionally, they claimed that prognostic impact of S-NOS and perihilar glomerular sclerosis was more significant than other subtypes and sclerotic lesions. With another suggestion, because patients with <25% and >25% of segmental sclerotic lesions had different prognoses, segmental sclerotic lesions could be subclassified into S0, S1, and S2 [Citation27]. In this study, favorable outcomes in patients with S1 lesions under IST could be associated with sclerosis type or severity of glomerulosclerosis. We classified sclerosis according to Oxford classification and for that reason we could not evaluate the association of segmental glomerulosclerosis rate and variant. In Oxford classification presence of segmental glomerulosclerosis is defined as S1 lesion independent of rate. The low rate of S1 lesion (<25%) and low rate of aforementioned variants associated with adverse outcomes could be an explanation of the favorable outcome in our patients with S1 lesions.
The major limitations of this study were retrospective pattern, and heterogeneity. Our registry data is nationwide in Turkiye, and centers that register in this database are all experienced and competent in glomerular diseases. Regarding the heterogeneity, when we reviewed the IST protocols we noticed that the protocols were all consistent with guidelines, or studies. And all the treatment approaches were based on intention to treat. The RCT are mostly performed a while after the kidney biopsy, that duration could alter the outcome. As IgAN can be on progressive pattern, the initial IST is important. We evaluated initial IST results which could better reflect real-life outcome. Moreover, we could not obtain the histopathologic findings regarding segmental sclerosis rate and sclerosis type from our database. Because of that we only could speculate the reason of favorable outcome in patients with S1 lesions. Our registry’s binary classification of treatment response does not allow for nuanced analysis based on varying degrees of proteinuria reduction, a limitation that is compounded by incomplete data on follow-up laboratory parameters such as eGFR and proteinuria levels. While this approach facilitated a standardized analysis across multiple centers, it may not fully capture the complexity of treatment outcomes in IgAN. On the other hand, since treatment responses can vary between populations, especially between Asian patients and Europeans, given the substantial cohort of IgAN patients represented from Turkiye, our study contributes valuable insights into treatment responses in a geographically and genetically diverse population.
In conclusion, systemic CS treatment in IgAN patients with high risk of progression may lead to significant remission rates in the Turkish cohort. In contrast, conventional immunosuppressive drugs do not provide a clear benefit. However, it is important to exercise caution due to the potential for serious side effects associated with IST. Key determinants of remission include initial proteinuria, and specific histological findings. The treatment approach should be tailored to individual patient characteristics, as remission rates tend to be lower, especially in cases with chronic tubular changes. However, reasonable CS administration can be considered even in patients with segmental sclerotic lesions. And further studies are needed to support these findings. Although, favorable renal outcome with CS was based on non-quantitative endpoints, this study underscores the need for a personalized and well-informed strategy for managing high-risk IgAN patients.
Ethics Committee approval
The study was conducted according to the guidelines of the Declaration of Helsinki and approval by the Ethics Committee of Istanbul University Faculty of Medicine (Number: 09/28.06.2011).
Informed consent
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
We would like to express our endless thanks to the Turkish Society of Nephrology, who organized the background of the study, and to the pathologists in each centre for their contributions to patient care and their help in providing these data.
Disclosure statement
The authors report there are no competing interests to declare.
Additional information
Funding
References
- Turkmen A, Sumnu A, Cebeci E, et al. Epidemiological features of primary glomerular disease in turkiye: a multicenter study by the Turkish society of nephrology glomerular diseases working group. BMC Nephrol. 2020;21(1):1.
- Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med. 2013;368(25):2402–10.
- Coppo R, D’Amico G. Factors predicting progression of IgA nephropathies. J Nephrol. 2005;18(5):503–512.
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4S):S1–S276.
- Zhang YM, Lv JC, Wong MG, Zhang H, Perkovic V. Glucocorticoids for IgA nephropathy-pro. Kidney Int. 2023;103(4):666–669.
- Novak J, Rizk D, Takahashi K,. New insights into the pathogenesis of IgA nephropathy. Kidney Dis (Basel). 2015;1(1):8–18.
- Kant S, Kronbichler A, Sharma P, Geetha DAdvances in understanding of pathogenesis and treatment of ımmune-Mediated kidney disease: a review. Am J Kidney Dis. 2022;79(4):582–600.
- Natale P, Palmer SC, Ruospo M,. Immunosuppressive agents for treating IgA nephropathy. Cochrane Database Syst Rev. 2020;3(3):CD003965.
- Lv J, Wong MG, Hladunewich MA, et al. Effect of oral methylprednisolone on decline in kidney function or kidney failure in patients With IgA nephropathy: the TESTING randomized clinical trial. JAMA. 2022;327(19):1888–1898.
- Hou FF, Xie D, Wang J, et al. Effectiveness of mycophenolate mofetil Among patients With progressive IgA nephropathy: a randomized clinical trial. JAMA Netw Open. 2023;6(2):e2254054.
- Liang M, Xiong L, Li A, et al. The effectiveness and safety of corticosteroid therapy for IgA nephropathy with crescents: a prospective, randomized, controlled study. BMC Nephrol. 2022;23(1):40.
- Zhao H, Li Y, Sun J, et al. Immunosuppression versus supportive care on kidney outcomes in IgA nephropathy in the Real-World setting. Clin J Am Soc Nephrol. 2023;18(9):1186–1194.
- Rauen T, Eitner F, Fitzner C, et al. Intensive supportive care plus ımmunosuppression in IgA nephropathy. N Engl J Med. 2015;373(23):2225–2236.
- Rauen T, Fitzner C, Eitner F, et al. Effects of two ımmunosuppressive treatment protocols for IgA nephropathy. J Am Soc Nephrol. 2018;29(1):317–325.
- Rauen T, Wied S, Fitzner C, et al. After ten years of follow-up, no difference between supportive care plus immunosuppression and supportive care alone in IgA nephropathy. Kidney Int. 2020;98(4):1044–1052.
- Lv J, Xu D, Perkovic V, et al. Corticosteroid therapy in IgA nephropathy. J Am Soc Nephrol. 2012;23(6):1108–1116.
- Lv J, Zhang H, Wong MG, et al. Effect of oral methylprednisolone on clinical outcomes in patients With IgA nephropathy: the TESTING randomized clinical trial. JAMA. 2017;318(5):432–442.
- Levey AS, Stevens LA, Schmid CH. A new equation to estimate glomerular filtration rate Ann Intern Med. 2009;150(9):604–612.
- Turkish society of nephrology consent report on diagnosis and treatment of primary glomerular diseases. Ankara (Turkiye): Turkish Society of Nephrology; 2019.
- Pozzi C, Andrulli S, Del Vecchio L, et al. Corticosteroid effectiveness in IgA nephropathy: long-term results of a randomized, controlled trial. J Am Soc Nephrol. 2004;15(1):157–163.
- Manno C, Torres DD, Rossini M, Pesce F, Schena FPRandomized controlled clinical trial of corticosteroids plus ACE-inhibitors with long-term follow-up in proteinuric IgA nephropathy. Nephrol Dial Transplant. 2009;24(12):3694–3701.
- Lv J, Zhang H, Chen Y, et al. Combination therapy of prednisone and ACE inhibitor versus ACE-inhibitor therapy alone in patients with IgA nephropathy: a randomized controlled trial. Am J Kidney Dis. 2009;53(1):26–32.
- Gleeson PJ, O’Shaughnessy MM, Barratt J. IgA nephropathy in adults-treatment standard. Nephrol Dial Transplant. 2023;38(11):2464–2473.
- Hou JH, Le WB, Chen N, et al. Mycophenolate mofetil combined with prednisone versus full-dose prednisone in IgA nephropathy with active proliferative lesions: a randomized controlled trial. Am J Kidney Dis. 2017;69(6):788–795.
- Cambier A, Troyanov S, Tesar V, et al. Indication for corticosteroids in IgA nephropathy: validation in the European VALIGA cohort of a treatment score based on the oxford classification. Nephrol Dial Transplant. 2022;37(6):1195–1197.
- Haaskjold YL, Lura NG, Bjørneklett R, et al. Long-term follow-up of IgA nephropathy: clinicopathological features and predictors of outcomes. Clin Kidney J. 2023;16(12):2514–2522.
- Yu F, Zhu X, Yuan S, et al. Predictive value of Sub classification of focal segmental glomerular sclerosis in oxford classification of IgA nephropathy. Ann Med. 2021;53(1):587–595.