Abstract
Objective
This single center retrospective study aimed to describe the variables associated with outpatient dialysis dependence in extracorporeal membrane oxygenation (ECMO) patients who needed continuous renal replacement therapy (CRRT) for acute kidney injury (AKI) during their hospitalization.
Methods
Retrospective study of patients who required ECMO-CRRT.
Results
Between the years of 2016 and 2022, 202 patients required ECMO-CRRT. One hundred and six patients (52.5%) survived their hospitalization and were followed up for a median of 391 [133, 1005] days. Eighty-one patients (76.5%) recovered kidney function and were dialysis-free before hospital discharge. Twenty-five patients (23.5%) were hemodialysis-dependent after hospitalization. On multivariate regression analysis, hyperlipidemia (odds ratio, OR 6.08 [1.67–22]) and CRRT duration (OR 1.09 [1.03–1.15]) were associated with the need for dialysis post-hospitalization. In this group, 16 patients eventually became dialysis-free, after a median of 49 [34.7, 78.5] days. These patients had a higher median baseline glomerular filtration rate (GFR) compared to those who never recovered renal function (93 mL/min/1.73 m2 [82.4, 104.3] vs. 63.8 mL/min/1.73 m2 [37.9, 83], p = .009). Their follow-up GFR was lower compared to those who recovered renal function before hospital discharge; (87 mL/min/1.73 m2 [68.2, 98.9] vs. 99 mL/min/1.73 m2 [79, 118], p = .07).
Conclusions
AKI requiring CRRT was associated with high mortality in patients receiving ECMO. Nonetheless, most ECMO survivors became dialysis-free before hospital discharge. Variables associated with the need for outpatient dialysis included hyperlipidemia and prolonged need for CRRT during hospitalization.
Introduction
Extracorporeal membrane oxygenation (ECMO) is being used to treat patients with severe respiratory failure, cardiac arrest, cardiogenic shock, post-cardiotomy shock, and combined cardiac and respiratory failure [Citation1].
The use of ECMO has grown significantly in recent years, with over 190,000 cases reported to the International Extracorporeal Life Support Organization Registry [Citation2].
Among critically ill patients, acute kidney injury (AKI) is associated with longer hospitalizations, prolonged mechanical ventilation, increased risk of chronic kidney disease (CKD), increased risk of infections and increased 90-day mortality among others [Citation3].
In the ECMO population, 40–50% of the patients develop severe AKI that requires renal replacement therapy, with a 3.7-fold higher hospital mortality risk [Citation4].
Risks factors for AKI in critically ill patients include multiorgan failure, hemodynamic instability, diffuse inflammation, ischemic events, microbial toxicity, nephrotoxins, and prior comorbid conditions (i.e., heart disease, hypertension, diabetes, and CKD). Specific risk factors for AKI in ECMO include hemodynamic changes during ECMO cannulation, veno-arterial ECMO (VA-ECMO), hemolysis, hyperinflammatory state associated with cannulation, microcirculatory dysfunction, and coagulopathy [Citation5–7].
In the non-ECMO population, it is estimated that 10–30% of hospitalized patients who required renal replacement therapy will need dialysis after hospital discharge. These patients have a higher risk of death, hospital readmission, and CKD [Citation8]. Furthermore, dialysis dependence for more than 90 days is associated with long term dialysis. There is a paucity of studies describing these outcomes in the ECMO population [Citation9].
Patients on ECMO-CRRT present a challenging scenario, due to issues with vascular access, anticoagulation, severe illness, fluid removal, and ECMO-CRRT interplay. The management of these complex patients requires an interdisciplinary approach with collaboration of multiple specialties.
Since most studies describing post-hospitalization dialysis are based on non-ECMO populations, we aimed to describe the variables associated with outpatient dialysis dependence in ECMO patients who required continuous renal replacement therapy (CRRT) during their hospitalization.
Materials and methods
Study design
Retrospective case control study (protocol number 1860629-1, 3 February 2022). The protocol was approved by the Institutional Review Board at WellStar Kennestone Hospital, Marietta, GA.
Population
ECMO patients who required CRRT.
Inclusion criteria: Consecutive patients on ECMO who developed AKI requiring CRRT at WellStar Kennestone Hospital (Marietta, GA) between June 2016 and February 2022.
Exclusion criteria: Patients on dialysis prior to hospital admission. Patients who were not on ECMO. Patients who did not require CRRT during their hospitalization.
ECMO protocols and indications
A multidisciplinary ECMO team consisting of interventional cardiologists, cardiothoracic surgeons, vascular surgeons, ICU nurses, ECMO nurses and intensivists assessed, cannulated, managed, and followed the patients throughout their hospitalizations.
Indications for VV ECMO: Severe ARDS and refractory hypoxemia (PaO2/FiO2 < 80 mm of mercury (mm Hg)), severe hypercapnic respiratory failure (pH < 7.25 with a PaCO2 ≥ 60 mm Hg) despite best standard of care (i.e., paralysis and proning).
Indications for VA ECMO: Cardiac arrest, cardiogenic/obstructive shock (i.e., myocardial infarction, post-cardiotomy, myocarditis, massive PE, cardiomyopathy, and toxic ingestions), combined cardiac-respiratory failure.
Hybrid ECMO: The combination of the above modalities.
CRRT protocols and indications
CRRT was delivered by either a temporary hemodialysis (HD) catheter or most commonly using a direct connection of the CRRT machine to the ECMO circuit. All patients on CRRT in our institution were treated with CVVHDF (continuous veno-venous hemodiafiltration), CVVH (continuous veno-venous hemofiltration), and CVVHD (continuous veno-venous HD) using the PrismaFlex® platform. The choice of modality was decided by the consulting nephrologist. Once liberated from ECMO and hemodynamically stable, patients were transitioned to intermittent HD using a HD catheter.
Indications for CRRT: Uremia, hyperkalemia, metabolic acidosis, and fluid overload. All ECMO patients with severe AKI were initially started on CRRT. The timing of CRRT initiation, prescription and fluid removal strategy was discussed in close collaboration between the Nephrology and the ECMO team, individualized to each patient.
Glomerular filtration rate (GFR) was calculated using the CKDP-EPI formula. AKI was defined using the AKIN classification [Citation10].
Statistics
Variables collected: Demographic, clinical, radiological, and laboratory values during hospitalization and after hospital discharge, from any visit to primary care, nephrology, cardiology, pulmonary medicine, subacute care facilities, and nursing homes. Most of the data are shared in a common electronic medical record system that allows longitudinal follow up.
Outcomes: Odds of dialysis dependence after hospitalization, renal function recovery, and time to dialysis independence.
Statistical analyses: The normality of the data was assessed using a frequency distribution histogram. Normally distributed data are described using mean and standard deviation. Differences in means were compared using Student’s t-test or analysis of variance. Differences in proportions were assessed using the Chi-square test.
Non-parametric data are presented with median [25% and 75% percentiles] or range. The Wilcoxon–Mann–Whitney/Kruskal–Wallis test was used to test differences. Differences in proportions were assessed with Fisher’s exact test.
Multivariate logistic regression analysis models with calculation of odds ratio (OR) were used to study the associations and to adjust for confounding factors. Variables with p ≤ .1 in univariate analyses were included in multivariate analyses. p Values p < .05 were considered statistically significant.
JMP software version 16 (SAS Campus Drive, Cary, NC) was used to perform the statistical analyses.
Results
The overall study flow is depicted in .
Figure 1. Flowchart of the study. *CRRT: continuous renal replacement therapy. Patients with ESRD on HD on admission were excluded.
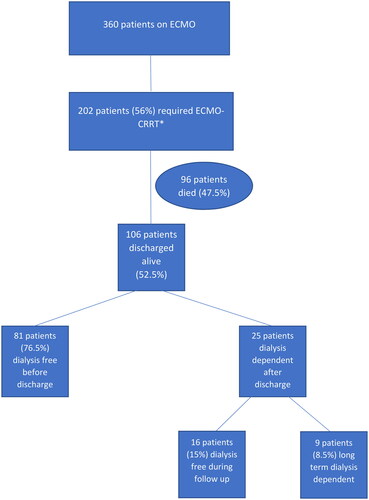
A total of 360 patients required ECMO support between 2016 and 2022. The main indications for ECMO support are described in Table S1.
Of these, 202 patients (56%) started CRRT due to AKI. CRRT was started at a median of 0 [−0.72, 1.55] days from ECMO initiation. Patients who required CRRT were older, had lower GFR on admission, required more pressor support, more blood products, were more commonly on venoarterial (VA), venovenous-venous (VVV), venovenous-arterial (VVA), and venoarterial-venous (VAV) ECMO, had lower GFR upon follow-up, and had increased mortality (Table S2).
shows the characteristics of ECMO-CRRT patients who survived their hospitalization. These patients were younger, with a higher BMI, and had a negative fluid balance. On the other hand, patients with a primary cardiac indication for ECMO and need for VA ECMO support had increased mortality.
Table 1. Characteristics of patients on ECMO-CRRT who survived their hospitalization.
Patients with sepsis accounted for 110 patients (54.4%). Among them, 10 patients had non-COVID viral pneumonias, 60 patients had COVID-19 pneumonia and 40 patients had bacterial infections (i.e., pneumonia, bacteremia). No difference in the need for CRRT was noted among the groups.
In the hybrid ECMO group, two patients transitioned from VAV to VV ECMO, four patients from VA to VV ECMO, eight patients from VA to VAV ECMO, three patients from VA to VAV to VV ECMO, one patient from VA to VV to VAV ECMO, four patients from VVA to VV ECMO, two patients from VV to VVV ECMO, one patient from VV to VAV ECMO, one patient from VV to VA to VAV to VV ECMO, and two patients from VV to VA to VV ECMO.
One hundred and six patients (52.5%) who required ECMO-CRRT survived their hospitalization and were followed up for a median of 391 [133, 1005] days. Of these, 81 patients (76.5%) recovered kidney function and were dialysis-free before hospital discharge. These patients had fewer days of CRRT and a more negative fluid balance than those who were dialysis-dependent at discharge ().
Table 2. Characteristics and outcomes of ECMO patients who became dialysis-free before hospital discharge.
Twenty-five patients (23.5%) were HD-dependent after hospitalization. On multivariate logistic regression analyses, each day on CRRT and hyperlipidemia remained associated with the need for dialysis after hospital discharge ().
Table 3. Logistic regression analyses of factors associated with the need for dialysis post-hospitalization.
Compared to those without hyperlipidemia, patients with hyperlipidemia had a lower baseline GFR: 60.2 mL/min/1.73 m2, [43, 87.6] vs. 77.8 mL/min/1.73 m2, [52.7, 99.4], p = .009. They also had more diabetes mellitus (42.7% vs. 24.3%), CHF (29% vs. 13.4%), CAD (50.6% vs. 14%), and HTN (93.3% vs. 52.2%), p < .001.
Sixteen patients who were discharged on dialysis became dialysis-free after a median of 49 [34.7, 78.5] days. These patients had a higher baseline GFR compared to those who remained long-term dialysis dependent: 93 mL/min/1.73 m2 [82.4, 104.3] vs. 63.8 mL/min/1.73 m2 [37.9, 83], p = .009. Nonetheless, their follow-up GFR was lower compared to those who recovered renal function before hospital discharge (87 mL/min/1.73 m2 [68.2, 98.9] vs. 99 mL/min/1.73 m2 [79, 118], p = .07).
Long-term survival was 93.4% in those who were dialysis-free before hospital discharge, 93.4% in those who were discharged on dialysis and eventually recovered kidney function, and 77.8% in those who remained dialysis dependent in the long term (p = .21).
Discussion
In this study, we described the variables associated with dialysis dependence after hospitalization in ECMO patients who required CRRT for AKI. There is a paucity of studies describing these outcomes in this population, as most previous studies involved patients who were not on ECMO. We also presented the time to renal recovery in patients who were discharged on dialysis but later became dialysis-free.
Acute kidney injury requiring renal replacement therapy is associated with increased short and long-term mortality. In the largest study of ECMO survivors using a national database, those who developed severe AKI requiring CRRT had higher long-term mortality rates [Citation11,Citation12].
We found that ECMO patients requiring CRRT were more likely to be on VA or hybrid ECMO modes. They also had more need for transfusions and had higher mortality. Among survivors, those who remained longer on CRRT with a more positive fluid balance were more likely to remain dialysis dependent. Nonetheless, we did not find a difference in the timing of CRRT initiation between the groups, suggesting that early initiation of CRRT was not associated with better renal outcomes.
In our study, ECMO-CRRT survivors were younger, had a higher BMI (similar to other reports in the literature) and had a negative fluid balance. On the other hand, patients with a primary cardiac indication for ECMO and VA ECMO support had increased mortality [Citation13].
De Corte et al. reported that in general ICU patients (non-ECMO) with AKI requiring renal replacement therapy, the proportions of complete renal recovery, incomplete renal recovery, and dialysis dependence were 48.4%, 32.6%, and 19.0%, respectively, after one year of follow-up [Citation14]. In a recent study of 57 patients treated with ECMO-CRRT who survived hospital discharge, 53 patients (93%) recovered renal function before hospital discharge, and four patients remained dialysis-dependent after discharge. Similarly, in our study, most survivors recovered renal function and became dialysis-free prior to hospital discharge [Citation15].
Unfortunately, we do not have the hourly urine output data or markers of inflammation/hemolysis in those on ECMO, but we found that patients who became dialysis-free before dismissal had fewer days on CRRT and a more negative fluid balance than those who required outpatient dialysis, probably indicating a faster recovery of renal function. This finding aligns with those of previous studies that showed that a positive fluid balance and longer CRRT duration increased the risk of end-stage renal disease [Citation16,Citation17]. Nonetheless, it is important to emphasize that even in patients who become dialysis-free, the risk of CKD is high. In a study of 51 survivors of VA ECMO-CRRT followed up for 1 year, 20 (39%) had a decline of GFR >30%. These patients had lower baseline GFR, higher AKI severity on ECMO cannulation, and an increased need for packed red blood cells transfusions on ECMO [Citation18].
In this study, patients who required long-term dialysis after hospitalization had a lower baseline GFR than those who recovered their renal function earlier. We also noted that earlier renal recovery was associated with higher GFR at follow-up. This finding should be interpreted with caution, as prolonged hospitalization, malnutrition, chronic disease, and inflammation might lead to a loss of muscle mass with subsequent lower creatinine levels and overestimation of the GFR, potentially missing the diagnosis of CKD in a significant number of patients. The association between hyperlipidemia and the need for outpatient dialysis could be related to the fact that patients with hyperlipidemia had more diabetes and cardiovascular comorbidities along with lower GFR at baseline that could have predisposed them to CKD later.
Although the numbers are small and need to be carefully interpreted, we found similar long-term survival rates in those who were discharged on dialysis but later became dialysis free compared to those who were dialysis free at the time of hospital discharge. On the other hand, we found a numerically lower survival rate in those who remained long-term dialysis dependent.
It is important to note this was a single center study, although patients requiring ECMO were referred from different hospitals. Given the relatively small number of patients in each specific subgroup (sepsis, pancreatitis, viral pneumonias, bacterial pneumonias, and burns), it was not feasible to include them in multivariate analyses. On the other hand, we included a significant number of COVID-19 patients, given the fact many of these patients required ECMO and CRRT [Citation11].
Because of this, the results might not be generalizable to other institutions. Despite the above, we believe that the present study adds to the body of literature by describing the long-term implications of severe acute kidney failure in the ECMO population, the natural history and timing of renal recovery, and the factors associated with outpatient dialysis dependence.
Ideally future studies should include a larger number of patients. Some areas that warrant further investigation include the association of renal replacement therapy modality, fluid management strategy and timing of renal replacement initiation with renal recovery in this population.
Conclusions
In conclusion, in this single center study, we found that AKI requiring CRRT was associated with high mortality in patients receiving ECMO. Of the survivors, a majority became dialysis-free before hospital discharge. Many patients who required dialysis after discharge eventually became dialysis-free within a couple of months. These findings will require confirmation with larger, multicenter studies.
Ethical approval
This study was approved by the Institutional Review Board of WellStar Kennestone Hospital, Marietta, GA. This study was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was waived, as it was determined that the proposed activity was exempt from further review by the Human Protection Administrator or the Institutional Review Board, based on 45 CFR 46.101(b) of the Code of Federal Regulations, Category 4: Research involving collection or study of existing identifiable private information or identifiable biospecimens, documents, records, or specimens if the sources were publicly available or if data were recorded in a way that subjects could not be identified.
Supplemental Material
Download MS Word (16.5 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data are available in a database from the corresponding author on reasonable request.
Additional information
Funding
References
- Ali J, Vuylsteke A. Extracorporeal membrane oxygenation: indications, technique and contemporary outcomes. Heart. 2019;105(18):1–8. doi: 10.1136/heartjnl-2017-311928.
- Wheeler CR, Bullock KJ. Extracorporeal membrane oxygenation. Respir Care. 2023;68(8):1158–1170. doi: 10.4187/respcare.10929.
- Delmas C, Zapetskaia T, Conil JM, et al. 3-month prognostic impact of severe acute renal failure under veno-venous ECMO support: importance of time of onset. J Crit Care. 2018;44:63–71. doi: 10.1016/j.jcrc.2017.10.022.
- Thongprayoon C, Cheungpasitporn W, Lertjitbanjong P, et al. Incidence and impact of acute kidney injury in patients receiving extracorporeal membrane oxygenation: a meta-analysis. J Clin Med. 2019;8:981.
- Al-Fares A, Pettenuzzo T, Del Sorbo L. Extracorporeal life support and systemic inflammation. Intensive Care Med Exp. 2019;7(Suppl. 1):46. doi: 10.1186/s40635-019-0249-y.
- Borasino S, Kalra Y, Elam AR, et al. Impact of hemolysis on acute kidney injury and mortality in children supported with cardiac extracorporeal membrane oxygenation. J Extra Corpor Technol. 2018;50(4):217–224. doi: 10.1051/ject/201850217.
- Kilburn DJ, Shekar K, Fraser JF. The complex relationship of extracorporeal membrane oxygenation and acute kidney injury: causation or association? Biomed Res Int. 2016;2016:1094296. doi: 10.1155/2016/1094296.
- Vijayan A, Abdel-Rahman EM, Liu KD, et al. Recovery after critical illness and acute kidney injury. Clin J Am Soc Nephrol. 2021;16(10):1601–1609. doi: 10.2215/CJN.19601220.
- Bhandari S, Turney JH. Survivors of acute renal failure who do not recover renal function. QJM. 1996;89(6):415–421. doi: 10.1093/qjmed/89.6.415.
- Mehta RL, Kellum JA, Shah SV, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713.
- Palacios CRF, Hoxhaj R, Thigpen C, et al. Outcomes of patients with COVID-19 requiring extracorporeal membrane oxygenation and continuous renal replacement therapy in the United States. Acute Crit Care. 2023;38(3):308–314. doi: 10.4266/acc.2023.00115.
- Chen S-W, Lu Y-A, Lee C-C, et al. Long-term outcomes after extracorporeal membrane oxygenation in patients with dialysis-requiring acute kidney injury: a cohort study. PLOS One. 2019;14(3):e0212352. doi: 10.1371/journal.pone.0212352.
- Peetermans M, Guler I, Meersseman P, et al. Impact of BMI on outcomes in respiratory ECMO: an ELSO registry study. Intensive Care Med. 2023;49(1):37–49. doi: 10.1007/s00134-022-06926-4.
- De Corte W, Dhondt A, Vanholder R, et al. Long-term outcome in ICU patients with acute kidney injury treated with renal replacement therapy: a prospective cohort study. Crit Care. 2016;20(1):256. doi: 10.1186/s13054-016-1409-z.
- Deatrick KB, Mazzeffi MA, Galvagno SM, et al. Breathing life back into the kidney-continuous renal replacement therapy and veno-venous extracorporeal membrane oxygenation. ASAIO J. 2021;67(2):208–212. doi: 10.1097/MAT.0000000000001210.
- Dado DN, Ainsworth CR, Thomas SB, et al. Outcomes among patients treated with renal replacement therapy during extracorporeal membrane oxygenation: a single-center retrospective study. Blood Purif. 2020;49(3):341–347. doi: 10.1159/000504287.
- Kuo G, Chen S-W, Fan P-C, et al. Analysis of survival after initiation of continuous renal replacement therapy in patients with extracorporeal membrane oxygenation. BMC Nephrol. 2019;20(1):318. doi: 10.1186/s12882-019-1516-6.
- Vinclair C, De Montmollin E, Sonneville R, et al. Factors associated with major adverse kidney events in patients who underwent veno-arterial extracorporeal membrane oxygenation. Ann Intensive Care. 2020;10(1):44. doi: 10.1186/s13613-020-00656-w.

