Abstract
Background
Neutrophil-to-lymphocyte ratio (NLR) has been suggested to be a prognostic marker for various diseases, but whether NLR dynamics (ΔNLR) is related to mortality and disease severity in patients with septic acute kidney injury (AKI) has not been determined.
Methods
Between August 2013 and August 2021, septic AKI patients at our center were retrospectively enrolled. ΔNLR was defined as the difference between the NLR at septic AKI diagnosis and at hospital admission. The relationship between the ΔNLR and mortality was evaluated by Kaplan–Meier curves, Cox proportional hazards, and cubic spline analyses. The prediction values were compared by area under the receiver-operating characteristic curve (AUROC), net reclassification improvement (NRI), and integrated discrimination improvement (IDI) analyses.
Results
Of the 413 participants, the mean age was 63 ± 17 years, and 134 were female (32.4%). According to the median value, patients in the high-ΔNLR group had significantly greater 90-d mortality (74.4% vs. 46.6%, p < 0.001). After adjustment for potential confounders, high ΔNLR remained an independent predictor of 90-d mortality (HR = 2.80; 95% CI = 1.74–4.49, p < 0.001). Furthermore, ΔNLR had the highest AUROC for 90-d mortality (0.685) among the various biomarkers and exhibited an improved NRI (0.314) and IDI (0.027) when incorporated with PCT and CRP. For secondary outcomes, patients with high ΔNLR had increased risk of 30-d mortality (p = 0.004), need for renal replacement therapy (p = 0.011), and developing stage-3 AKI (p = 0.040) according to the adjusted models.
Conclusions
High ΔNLR is independently associated with increased risk of patient mortality and adverse outcomes. ΔNLR might be utilized to facilitate risk stratification and optimize septic AKI management.
1. Introduction
Acute kidney injury (AKI) is defined as a sudden loss of kidney function for various reasons [Citation1]. Sepsis, a potentially fatal condition linked to multiple organ dysfunction [Citation2], is the leading cause of AKI, especially in the intensive care unit (ICU) [Citation3]. In sepsis patients, the development of AKI, especially a high degree of AKI, has been shown to be a significant predictor of death [Citation4,Citation5], while for patients with established septic AKI, the identification of other reliable and cost-effective biomarkers to assess the risk of adverse outcomes is of great clinical value.
Inflammation plays a critical role in the pathophysiology of septic AKI [Citation6]. The neutrophil-to-lymphocyte ratio (NLR), a readily available biomarker of inflammation, can be easily calculated based on the complete blood count and thus has gained increasing attention in the management of septic AKI patients. A meta-analysis demonstrated that an elevated NLR was associated with mortality in AKI patients [Citation7]. As septic AKI is dynamic during disease progression, inflammatory indices, such as the NLR are subject to changes over time, with marked variations in the kinetic profile. The NLR dynamic (ΔNLR) has previously been shown to predict outcomes in patients with metastatic gastric cancer [Citation8], patients receiving thrombolysis during stroke [Citation9], and patients with AKI after coronary artery bypass grafting [Citation10]. However, few studies have investigated the application of ΔNLR among septic AKI patients. In this retrospective study, we aimed to evaluate the predictive value of ΔNLR for mortality and other clinical outcomes in septic AKI patients.
2. Method
2.1. Participants
This was a single-center retrospective study of septic AKI patients at West China Hospital of Sichuan University between August 2013 and August 2021. Our inclusion criteria were as follows: i) ≥ 18 years of age; ii) developed sepsis during hospitalization according to the diagnostic criteria of Sepsis version 3.0 (New York, USA) [Citation11]; iii) developed AKI during hospitalization according to Kidney Disease: Improving Global Outcomes (KDIGO) guideline [Citation12]; and iv) had AKI occurring within 7 d following the diagnosis of sepsis. Patients who met the following criteria were excluded: i) had AKI secondary to other causes; ii) AKI occurred before the onset of sepsis; or iii) presented with sepsis or septic AKI prior to current admission. Patients with a diagnosis of sepsis or septic AKI prior to present hospitalization were excluded so as to accurately define the timeframe from sepsis to septic AKI and guarantee that the NLR at admission reflected the baseline level.
The study was approved by the Institutional Review Board of West China Hospital of Sichuan University (Approval No. of the ethics committee: 2022–1948) and abided by the Declaration of Helsinki and the Declaration of Istanbul. The Institutional Review Board waived patient informed consent, as this was a retrospective study.
2.2. Demographic and laboratory variables
Demographic and laboratory variables on the day of hospital admission, as well as comorbidities, were recorded from electronic health records (EHRs) on flowsheets. Comorbidities, including hypertension (HTN), diabetes mellitus (DM), coronary artery disease (CAD), acute respiratory distress syndrome (ARDS), multiple organ failure syndrome (MODS), urinary tract infection (UTI), hypoproteinaemia, and tumor history, were determined by ICD-9/10-CM codes. The laboratory parameters included neutrophil (Neu), lymphocyte (Lym), platelet (PLT), hemoglobin (Hb), white blood cell (WBC), red blood cell (RBC), blood urea nitrogen (BUN), serum creatinine (sCr), uric acid, and cystatin-c (Cys-C) levels; estimated glomerular filtration rate (eGFR) according to the modified MDRD equation based on sCr adjusted for age and sex; total protein (TP), albumin (ALB), low-density lipoprotein (LDL), high-density lipoprotein (HDL), total bilirubin (TBIL), triglyceride (TG), cholesterol (Chol), procalcitonin (PCT), and C-reactive protein (CRP) levels. In addition, Neus and Lyms were collected at the time of hospital admission and the time of septic AKI diagnosis, respectively. The NLR was calculated by dividing the absolute Neu count by the absolute Lym count. The change in the NLR between the time of hospital admission and the time of septic AKI diagnosis was calculated as ΔNLR (the NLR at AKI diagnosis minus the baseline NLR). Patients were divided into two groups based on the median ΔNLR: a high ΔNLR (≥1.71) group and a low ΔNLR (<1.71, including negative values) group. The patient mortality rate was calculated from the time of hospital admission to the date of death by censoring survivors at the date of last follow-up; data were collected from the EHR database or by telephone calls. Information on rehospitalization for up to 365 d was also collected by the same methods. Specific identifiers of protected individual health information, such as names, geographic subdivisions, and medical record numbers, were removed from the dataset.
2.3. Diagnosis of septic AKI
According to the 28th ADQI consensus, septic AKI was considered when AKI occurred within 7 d of sepsis diagnosis [Citation13]. Sepsis was defined according to ICD-10-CM) codes or Sepsis version 3.0 criteria (life-threatening organ dysfunction caused by a dysregulated host response to infection, with organ dysfunction identified as an acute change in total SOFA score ≥ 2 points) [Citation11]. AKI was diagnosed by either ICD-10-CM codes or identified by adopting previous criteria based on the KDIGO guidelines [Citation14]. The SCr criteria were used to define and stage AKI, while the urine output criteria were not used due to the unavailability of accurate data. The stage of AKI was determined according to the maximum disease severity.
2.4. Clinical outcomes
Our primary outcome was 90-d mortality from the time of hospital admission to the date of the last follow-up. The secondary outcomes were 30-day mortality, mechanical ventilation, septic shock, renal replacement therapy, Stage-3 AKI, length of hospital stay and ICU stay, major adverse kidney events (MAKEs: death, dependence on renal replacement therapy, or persistent kidney dysfunction with eGFR < 30 mL/min/1.73 m2 for 3 months) and 1-year rehospitalization.
2.5. Statistical analysis
Continuous variables are expressed as the mean and standard deviation or median with interquartile ranges and were analyzed by t test or Wilcoxon test as appropriate. The categorical data are presented as frequency rates and percentages and were analyzed using the chi-square test. Kaplan–Meier curves, Cox proportional hazards regression, and multivariate logistic regression were used to evaluate the associations between the ΔNLR and mortality and secondary outcomes. The results are expressed as HRs/ORs and 95% confidence intervals (CIs) after adjusting for relevant clinical variables according to the different models. In addition, the associations between mortality and ΔNLR were also evaluated by restricted cubic spline analysis, and the relationship between ΔNLR and eGFR variation from admission to septic AKI diagnosis was evaluated by Spearman correlation analysis. Furthermore, the predictive value of the ΔNLR and other biomarkers for patient mortality was compared by the area under the receiver-operating characteristic curve (AUROC) and Youden index. The net reclassification index (NRI) and integrated discrimination improvement (IDI) were employed to assess the predictive value of ΔNLR for mortality when combined with other inflammatory parameters. All the statistical analyses were performed using IBM SPSS software version 26.0 (IBM Corp., Armonk, NY) and R version 4.2.3 (R Foundation for Statistical Computing, Vienna, Austria). A two-tailed p value < 0.05 was considered to indicate statistical significance.
3. Results
3.1. Baseline characteristics of septic AKI patients
Between August 2013 and August 2021, a total of 413 septic AKI patients meeting the eligibility criteria were included in this study (). The baseline characteristics of the septic AKI patients are summarized in . The mean age of the septic AKI patients was 63 ± 17 years, of which 134 (32.4%) were female, and the body mass index (BMI) was 23.44 (20.16–26.57) kg/m2. Regarding comorbidities, 148 patients (35.8%) had HTN, 83 (20.1%) had DM, 51 (12.3%) had CAD, 325 (78.7%) had hypoproteinemia, and 107 (25.9%) had a history of cancer. The patients had a mean duration of 5 d between hospital admission and sepsis diagnosis and a mean duration of 3 d between sepsis diagnosis and septic AKI diagnosis.
Figure 1. Flow diagram of the study. AKI: acute kidney injury; ΔNLR: neutrophil-to-lymphocyte ratio dynamic.
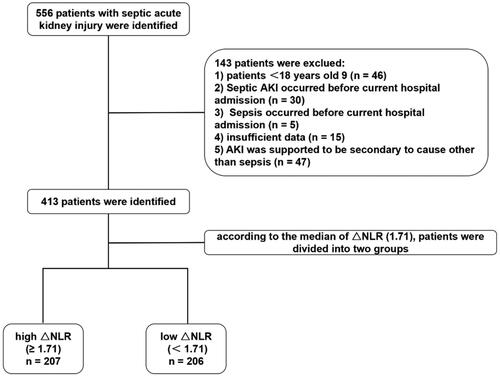
Table 1. Baseline characteristics of septic AKI patients.
Table 2. Patient outcomes basing on ΔNLR.
The median NLR at hospital admission, at the time of septic AKI diagnosis and the ΔNLR were 11.65 (6.24–18.4), 10.36 (4.38–23.59), and 1.71 (−5.55–11.42), respectively. According to the median ΔNLR (1.71), all patients were divided into a high ΔNLR (≥1.71) group and a low ΔNLR (<1.71) group. Compared with those in the low-ΔNLR group, patients in the high-ΔNLR group had lower Neu, TG, and CRP levels (p = 0.015, p = 0.041, and p = 0.006, respectively), and higher TP, LDL, HDL, and BUN levels (p = 0.013, p = 0.001, p = 0.026, and p = 0.011, respectively). The patients in the high-ΔNLR group had higher incidence of tumor (p = 0.006) and lower incidences of DM and UTI (p = 0.035 and p = 0.017) than those in the low-ΔNLR group. We explored the relationship between ΔNLR and eGFR variation from admission to septic AKI diagnosis, but no significant association was detected (r = −0.01, p = 0.430) (Figure S1). Other laboratory pretreatments and comorbidities were not significantly different between high and low ΔNLR groups (all p > 0.05).
3.2. Association between ΔNLR and mortality in septic AKI patients
The overall 90-d mortality was 60.5%. Univariate analysis revealed that patients in the high-ΔNLR group had significantly greater 90-d mortality than did those in the low-ΔNLR group (74.4% vs. 46.6%, p < 0.001) (). As shown by Kaplan–Meier survival analysis (), the cumulative 90-d survival probability was significantly lower for patients in the high ΔNLR group than for those in the low ΔNLR group (p < 0.001).
Figure 2. Kaplan–Meier survival curve for 90/30-d survival based on ΔNLR. A. Kaplan–Meier analysis for 90-d survival based on ΔNLR. B. Kaplan–Meier analysis for 30-dy survival based on ΔNLR. ΔNLR: neutrophil-to-lymphocyte ratio dynamic.
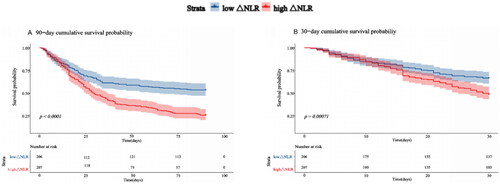
The relationship between ΔNLR and 90-d mortality remained statistically significant after adjusting for potential confounders according to Cox proportional regression (). According to the unadjusted model, a high ΔNLR was associated with a 1.95-fold increase in 90-d mortality (95% CI: 1.51–2.52, p < 0.001) compared with a low ΔNLR. In Model 2 adjusted for sex, age, BMI, HTN, DM, CAD status, UTI status, and tumor history, a high ΔNLR was associated with a 1.89-fold increase in 90-d mortality (95% CI: 1.41–2.55, p < 0.001). In Model 3, with additional adjustment for CRP, PCT, ALB, BUN, TG, TP, LDL, and HDL, a high ΔNLR remained an independent risk factor for 90-d mortality (HR = 2.80; 95% CI = 1.74–4.49, p < 0.001). The overall 30-d mortality was 42.6%. Analyses of 30-d mortality yielded similar results, and a high ΔNLR was also a prognosticator for 30-d mortality according to the fully adjusted Cox regression model (HR = 2.23; 95% CI = 1.29–3.83; p = 0.004). We also compared patient mortality based on the 25th and 75th percentiles of ΔNLR; however, the difference was not significant (Tables S3 and S4).
Table 3. Multivariate cox proportional hazard model for mortality based on ΔNLR.
Table 4. Multivariate logistic regression model showing relationship between ΔNLR and secondary outcomes.
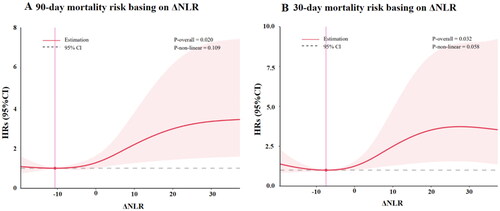
The association between ΔNLR and mortality was further evaluated by using restricted cubic spline analysis (). It was shown that 90/30-d mortality increased with the ΔNLR when the ΔNLR was ≥ 0. The HRs of 90/30-d mortality increased progressively with increasing ΔNLR, but the trend was no longer maintained when ΔNLR was greater than 30.
Figure 3. The relationship between ΔNLR and mortality risk by using cubic spline regression analyses. A. The 90-d mortality risk was analyzed by using cubic spline regression. B. The 30-d mortality risk was analyzed by using cubic spline regression. AKI: acute kidney injury; ΔNLR: neutrophil-to-lymphocyte ratio dynamic.
3.3. Association between ΔNLR and secondary outcomes in septic AKI patients
Among the 413 septic AKI patients in this study, 207 (50.1%) were transferred to the ICU, 172 (41.6%) received mechanical ventilation, 69 (16.7%) developed septic shock, 110 (26.6%) were diagnosed with stage-3 AKI, and 108 (26.2%) needed RRT. The median length of ICU stay was 11.0 (6.0–35.5) d, and the median length of hospital stay was 19.0 (13.0–37.0) d. A total of 288 (69.7%) patients developed MAKEs. Univariate analysis suggested that patients with a high ΔNLR had an increased incidence of developing Stage-3 AKI (30.9% vs. 22.3%, p = 0.048), need for RRT (30.4% vs. 21.8%, p = 0.047), MAKEs (p = 0.007), 1-year rehospitalization (p = 0.048), and longer length of ICU stay (p = 0.019). There was no significant difference in the incidences of mechanical intervention, transfer to the ICU, sepsis shock, or length of hospital stay (all p > 0.05) (). The association between the ΔNLR and secondary outcomes was further evaluated by multivariate logistic regression (). After adjusting for confounding factors, including sex, age, BMI, HTN, DM, CAD, ARDS, hypoproteinemia, MODS, LDL, HDL, Cys-C, Bun, CRP, and PCT, high ΔNLR was independently related with an increased risk of developing Stage-3 AKI (OR: 2.331, 95% CI: 1.054–5.343, p = 0.040) and need for RRT (OR: 3.390, 95% CI: 1.354–9.014, p = 0.011), while the risk of transfer to ICU (OR: 1.379, 95% CI: 0.554–3.522, p = 0.493), septic shock (OR: 2.328, 95% CI: 0.889–6.489, p = 0.093), mechanical ventilation (OR: 1.113, 95% CI: 0.527–2.361, p = 0.779), and MAKEs (OR: 1.86, 95% CI: 0.95–3.63, p = 0.070) were no longer significantly different between high and low ΔNLR groups.
3.4. Comparisons on ΔNLR with other biomarkers
The ability of the ΔNLR and other biomarkers to predict mortality was compared by ROC analysis (). For 90-d mortality, ΔNLR had a greater AUC (0.685, 95% CI: 0.633–0.737) than did the other inflammatory variables, such as NLR at hospital admission, NLR at AKI diagnosis, Neu, Lym, WBC, PLT, Bun, CRP, PCT, and cystatin C levels (). For 30-d mortality, ΔNLR similarly showed better predictive value than the other biomarkers (). The AUCs, sensitivities, specificities, and predictive accuracy values of these parameters for predicting 90/30-d mortalities are provided in Table S2. Furthermore, the NRI and IDI were analyzed to evaluate the performance of ΔNLR when integrated with other inflammatory biomarkers. The addition of ΔNLR to a PCT and CRP model increased the NRI (estimate, 0.314; 95% CI, 0.050–0.623; p = 0.047) and IDI (estimate, 0.027; 95% CI, 0.002–0.051; p = 0.031), suggesting that ΔNLR might help to improve the prediction accuracy of mortality in septic AKI patients (Table S5).
Figure 4. The predictive value of ΔNLR for 90/30-d mortality by using ROC analysis. A. The predictive value of ΔNLR for 90-d mortality by ROC analysis. B. The predictive value of ΔNLR for 30-d mortality by ROC analysis. AUC: area under the receiver.
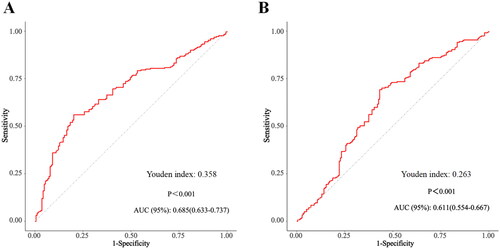
Table 5. The diagnostic accuracy of various prediction factors for 30/90-d mortality.
3.5. Subgroup analysis
The predictive value of ΔNLR for the primary outcome was assessed by subgroup analysis (Figure S2 and Table S1). Although there might be substantial interactions between ΔNLR and UTI, MODS, ALB, TP, and HDL (p interaction = 0.010, 0.006, 0.011, 0.036, and 0.003, respectively) in predicting 90-d mortality, most strata indeed displayed consistent trends showing an increased risk of death in the high-ΔNLR group. However, how the aforementioned parameters might confound the prognostic value of the ΔNLR needs further verification and explanation. Interactions between ΔNLR and age, HTN, DM, CAD, tumor, PCT, BUN, TG, and LDL were not detected (all p interactions > 0.05).
4. Discussion
This study suggested that the ΔNLR was independently associated with 90 and 30-d mortality in septic AKI patients. Moreover, patients in the high-ΔNLR group exhibited an increased risk of adverse outcomes, including need for renal replacement therapy and developing Stage-3 AKI. The ability of the ΔNLR to predict septic AKI prognosis outperformed that of other potential markers, such as Neu, Lym, WBC, PCT, PLT, and CRP, as well as NLR at hospital admission and NLR at the time of septic AKI diagnosis. ΔNLR might hence be used as a prognostic biomarker for septic AKI patients.
Septic AKI has been well recognized as a public health problem with high mortality and morbidity. Given that no effective pharmacological therapy is available for septic AKI, reliable prognostic biomarkers are warranted for the management and risk stratification of septic AKI patients. NLR, the fraction of Neu over Lym number in routine blood counts, is a readily available inflammation index that has attracted attention in recent years. A high NLR is an independent risk factor for mortality in patients with various diseases, such as sepsis, pneumonia, COVID-19, and cancer [Citation15]. We previously reported that a high NLR at hospital admission and at the time of AKI diagnosis was associated with 30/90-d mortality and disease severity in septic AKI patients [Citation16].
As sepsis is a dynamic disease process, inflammatory indices such as NLR are subject to changes over time, with marked variations in the kinetic profile. ΔNLR is used to demonstrate the dynamic change in NLR from its initial value. Several studies have shown that ΔNLR is associated with short-term mortality in a variety of diseases, such as sepsis, cancer, brain trauma, and acute ischemic stroke [Citation17–20]. In a single-institutional retrospective study enrolling 329 breast cancer patients, it was reported that there was a temporal relationship between dynamic NLR changes and oncologic outcomes, and the survival rate was significantly decreased in the high ΔNLR group divided by the maximal sensitivity and specificity point of AUC [Citation21]. Another retrospective study of 1322 coronary artery bypass surgery patients showed that a ΔNLR above the median value could be a predictor of higher long-term mortality [Citation22]. The correlation between the ΔNLR and the clinical outcomes of patients with acute ischemic stroke after reperfusion therapy was assessed in a meta-analysis pooling 17232 patients from 52 studies, which confirmed that ΔNLR was significantly associated with an increased risk of 90-d mortality [Citation23]. In a retrospective study of 396 patients undergoing cardiac surgery, ΔNLR on the first four postoperative days (p < 0.001) was significantly related to AKI onset in subsequent days (p < 0.001) and NLR values were significantly augmented before the development of AKI [10].
This study is among the first to apply ΔNLR as a potential prognosticator among septic AKI population, showing that high ΔNLR is suggestive for death and other secondary outcomes. Compared with other inflammatory markers, ΔNLR is obtained from the complete blood count and could be measured more frequently, which enables care givers to evaluate the severity of septic AKI dynamically. ROC analysis revealed that ΔNLR outperformed other inflammatory biomarkers and improved NRI/IDI when incorporated with CRP and PCT, justifying its value as a convenient and cost-effective prognostic marker in septic AKI patients. Therefore, the ΔNLR should be considered when evaluating the progression and prognosis of septic AKI. Notably, ΔNLR achieved predictive power on 90-day mortality with an AUC of approximately 0.7 in this study. Future studies are needed to explore the possibility of integrating ΔNLR into an established risk score system in sepsis patients rather than using ΔNLR alone.
This study has several limitations. First, our research was a single-center retrospective study with a comparatively small sample size and comparatively short follow-up duration, which might hamper the robustness of our study. Second, ΔNLR outperformed BUN and cystatin C in predicting patient mortality, while several other kidney-specific markers, such as KIM-1 and NGAL were not routinely tested in our center and were not included in our comparison. Third, the predictive value of ΔNLR in septic AKI patients was not verified in an external validation cohort. The applicability of our results to other centers requires additional investigation. Fourth, AKI was defined basing on the sCr criteria. Due to the inaccuracy and availability of urine output data, the urine output criteria were not used for the identification and staging of AKI patients, which might have missed a certain number of potential participants. Moreover, the lack of baseline sCr levels prior to hospital admission could have resulted in selection bias. The prognostic value of ΔNLR awaits further validation in multiple-center prospective studies with larger sample sizes.
5. Conclusion
In conclusion, this study revealed that there was a correlation between a high ΔNLR and mortality and other adverse outcomes in septic AKI patients. ΔNLR might be a convenient and cost-effective biomarker for predicting septic AKI prognosis. As a readily available inflammatory index, ΔNLR could be utilized to facilitate risk stratification and optimize management. Prospective studies with larger sample sizes are warranted to further validate our findings.
Informed consent statement
Patient informed consent was waived by the Institutional Review Board due to the retrospective design.
Author contributions
WW and YZ were responsible for literature research and manuscript drafting. LC, GS, and YL were responsible for literature research and data collection. JL, TY, BW, and YY were responsible for the statistical analysis. ML, LZ, FP, and ZY were responsible for the study design and data interpretation. All coauthors were involved in patient care and participated in manuscript revision.
Supplemental Material
Download MS Word (131.5 KB)Acknowledgments
None.
Disclosure statement
The authors declare that there are no conflicts of interest.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Kellum JA, Romagnani P, Ashuntantang G, et al. Acute kidney injury. Nat Rev Dis Primers. 2021;7(1):1. doi: 10.1038/s41572-021-00284-z.
- Gómez H, Kellum JA. Sepsis-induced acute kidney injury. Curr Opin Crit Care. 2016;22(6):546–10. doi: 10.1097/MCC.0000000000000356.
- Poston JT, Koyner JL. Sepsis associated acute kidney injury. BMJ. 2019;364:k4891. doi: 10.1136/bmj.k4891.
- Jiang W, Zhang C, Yu J, et al. Development and validation of a nomogram for predicting in-hospital mortality of elderly patients with persistent sepsis-associated acute kidney injury in intensive care units: a retrospective cohort study using the MIMIC-IV database. BMJ Open. 2023;13(3):e069824. doi: 10.1136/bmjopen-2022-069824.
- Shum HP, Kong HH, Chan KC, et al. Septic acute kidney injury in critically ill patients - a single-center study on its incidence, clinical characteristics, and outcome predictors. Ren Fail. 2016;38(5):706–716. doi: 10.3109/0886022X.2016.1157749.
- Rabb H, Griffin MD, McKay DB, et al. Inflammation in AKI: current understanding, key questions, and knowledge gaps. J Am Soc Nephrol. 2016;27(2):371–379. doi: 10.1681/ASN.2015030261.
- Chen D, Xiao D, Guo J, et al. Neutrophil-lymphocyte count ratio as a diagnostic marker for acute kidney injury: a systematic review and meta-analysis. Clin Exp Nephrol. 2020;24(2):126–135. doi: 10.1007/s10157-019-01800-y.
- Cousillas Castineiras A, Gallardo Martin E, Fernandez Montes A, et al. Dynamic perspective of the neutrophil-to-lymphocyte ratio in metastatic gastric cancer. J BUON. 2021;26:2131–2140.
- Guo Z, Yu S, Xiao L, et al. Dynamic change of neutrophil to lymphocyte ratio and hemorrhagic transformation after thrombolysis in stroke. J Neuroinflamm. 2016;13(1):199. doi: 10.1186/s12974-016-0680-x.
- Parlar H, Arıkan AA, Önmez A. Dynamic changes in perioperative cellular inflammation and acute kidney injury after coronary artery bypass grafting. Braz J Cardiovasc Surg. 2021;36(3):354–364. doi: 10.21470/1678-9741-2020-0163.
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287.
- Lameire NH, Levin A, Kellum JA, et al. Harmonizing acute and chronic kidney disease definition and classification: report of a kidney disease: improving global outcomes (KDIGO) consensus conference. Kidney Int. 2021;100(3):516–526. doi: 10.1016/j.kint.2021.06.028.
- Zarbock A, Nadim MK, Pickkers P, et al. Sepsis-associated acute kidney injury: consensus report of the 28th acute disease quality initiative workgroup. Nat Rev Nephrol. 2023;19(6):401–417. doi: 10.1038/s41581-023-00683-3.
- Kellum JA, Lameire N, KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (part 1). Crit Care. 2013;17(1):204. doi: 10.1186/cc11454.
- Buonacera A, Stancanelli B, Colaci M, et al. Neutrophil to lymphocyte ratio: an emerging marker of the relationships between the immune system and diseases. Int J Mol Sci. 2022;23(7):23. doi: 10.3390/ijms23073636.
- Wei W, Huang X, Yang L, et al. Neutrophil-to-lymphocyte ratio as a prognostic marker of mortality and disease severity in septic acute kidney injury patients: a retrospective study. Int Immunopharmacol. 2023;116:109778. doi: 10.1016/j.intimp.2023.109778.
- Chen S, Li R, Zhang Z, et al. Prognostic value of baseline and change in neutrophil-to-lymphocyte ratio for survival in advanced non-small cell lung cancer patients with poor performance status receiving PD-1 inhibitors. Trans Lung Cancer Res. 2021;10(3):1397–1407. doi: 10.21037/tlcr-21-43.
- Chen Y, Yan H, Wang Y, et al. Significance of baseline and change in neutrophil-to-lymphocyte ratio in predicting prognosis: a retrospective analysis in advanced pancreatic ductal adenocarcinoma. Sci Rep. 2017;7(1):753. doi: 10.1038/s41598-017-00859-5.
- Wang L, Guo W, Wang C, et al. Dynamic change of neutrophil to lymphocyte ratios and infection in patients with acute ischemic stroke. Curr Neurovasc Res. 2020;17(3):294–303. doi: 10.2174/1567202617666200408091131.
- Botoș ID, Pantiș C, Bodolea C, et al. The dynamics of the neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios predict progression to septic shock and death in patients with prolonged intensive care unit stay. Medicina (Kaunas). 2022;59(1):59. doi: 10.3390/medicina59010032.
- Moldoveanu D, Pravongviengkham V, Best G, et al. Dynamic neutrophil-to-lymphocyte ratio: a novel prognosis measure for Triple-Negative breast cancer. Ann Surg Oncol. 2020;27(10):4028–4034. doi: 10.1245/s10434-020-08302-2.
- Bae MI, Shim JK, Song JW, et al. Predictive value of the changes in neutrophil-lymphocyte ratio for outcomes after off-Pump coronary surgery. J Inflamm Res. 2023;16:2375–2385. doi: 10.2147/JIR.S411057.
- Wu B, Liu F, Sun G, et al. Prognostic role of dynamic neutrophil-to-lymphocyte ratio in acute ischemic stroke after reperfusion therapy: a meta-analysis. Front Neurol. 2023;14:1118563. doi: 10.3389/fneur.2023.1118563.

