?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
To explore the relationship between lactate-to-albumin ratio (LAR) at ICU admission and prognosis in critically ill patients with acute kidney injury (AKI).
Methods
A retrospective analysis was conducted. Patients were divided into low (<0.659) LAR and high LAR (≥0.659) groups. Least absolute shrinkage and selection operator regression analysis was conducted to select variables associated with the 30-day prognosis. Cox regression analyses were performed to assess the association between LAR and mortality. Kaplan-Meier curves were plotted to compare cumulative survival rates between high and low LAR groups. Subgroup analysis was employed to assess the stability of the results. ROC curve was used to determine the diagnostic efficacy of LAR on prognosis.
Results
A nonlinear relationship was observed between LAR and the risk of 30-day and 360-day all-cause mortality in AKI patients (p < 0.001). Cox regulation showed that high LAR (≥ 0.659) was an independent risk factor for 30-day and 360-day all-cause mortality in patients with AKI (p < 0.001). The Kaplan-Meier survival curves demonstrated a noteworthy decrease in cumulative survival rates at both 30 and 360 days for the high LAR group in comparison to the low LAR group (p < 0.001). Subgroup analyses demonstrated the stability of the results. ROC curves showed that LAR had a diagnostic advantage when compared with lactate or albumin alone (p < 0.001).
Conclusion
High LAR (≥0.659) at ICU admission was an independent risk factor for both short-term (30-day) and long-term (360-day) all-cause mortality in patients with AKI.
Introduction
Acute Kidney Injury (AKI) is a clinically common and severe kidney disorder characterized by a rapid decline in kidney function over a relatively short period of time.
This results in insufficient removal of metabolic byproducts, waste, and electrolytes from the body, disrupting the internal equilibrium. AKI maintains a high morbidity and mortality currently [Citation1]. According to statistics, as high as 30% of hospitalized patients in Central Europe and the United States experience AKI [Citation2], with a short-term in-hospital mortality reaching up to 25% [Citation3]. However, in the Intensive Care Unit (ICU), the mortality rate may even exceed 50% [Citation2]. Surviving patients also face an increased risk of progressing to chronic kidney disease (CKD) [Citation4]. Thus, identifying indicators for predicting prognoses in patients with AKI is particularly crucial. Currently, indicators such as fibronectin/albumin ratio, liver-type fatty acid-binding protein (L-FABP), neutrophil gelatinase-associated lipocalin (NGAL), etc. have been demonstrated to be associated with the prognosis of patients with AKI [Citation5], yet their predictive efficacy remains limited.
Lactate is a product of anaerobic metabolism, reflecting tissue hypoperfusion. However, elevated lactate concentrations may be affected by various factors, including seizures, cardiac arrest, diabetic ketoacidosis, burns, liver dysfunction, and metformin overdose [Citation6]. Impaired liver function will affect lactate metabolism, leading to lactate accumulation. On the other hand, albumin, produced by the liver, can indicate liver function. Higher lactate levels or lower albumin levels are both indicators of lactate metabolic dysfunction. Thus, the lactate-to-albumin ratio (LAR) can provide a more accurate reflection of lactate metabolism. Moreover, research indicates that LAR holds predictive value for mortality in patients with heart failure [Citation7]. And LAR is better than lactate in predicting the outcomes of patients with severe sepsis [Citation8].
However, the research on LAR to predict the prognosis of patients with AKI is currently rarely seen. Therefore, the study aims to explore the correlation between LAR at ICU admission and the prognosis of patients with AKI, so as to provide help for clinicians to identify high-risk patients early.
Materials and methods
Population and data source
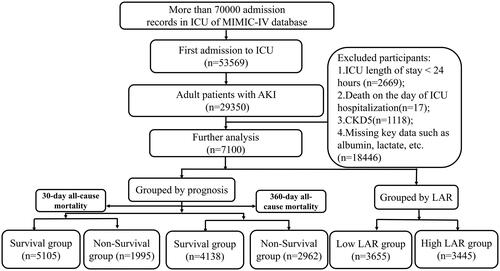
This study employed a retrospective research approach, with the study population consisting of eligible adult patients from the medical information mart for intensive Care (MIMIC-IV, v2.0) database from the years 2008 to 2019 [Citation9]. The database was approved by the Institutional Review Boards of Beth Israel Deaconess Medical Center and the Massachusetts Institute of Technology, and one author of the study was granted access to the database (ID number: 42303155).
Inclusion criteria for participant selection were as follows: (1) Patients diagnosed with AKI and aged 18 years or older, meeting the diagnostic criteria according to the Kidney Disease: Improving Global Outcomes (KDIGO) clinic practice guideline [Citation10]. (2) Patients who were admitted to ICU for the first time.
Exclusion criteria were as follows: (1) Patients with an ICU stay duration of less than 24 h; (2) Patients who experienced mortality on the same day of their ICU admission; (3) Patients diagnosed with stage 5 chronic kidney disease (CKD5); (4) Patients with incomplete data for variables such as lactate and albumin.
We collected the clinical data including age, sex, sequential organ failure assessment (SOFA) score, ICU length of stay (LOS), laboratory test, presence of oliguria on the first day (expressed as a percentage), presence of comorbidities (expressed as a percentage) such as hypertension, diabetes, chronic pulmonary disease, deep vein thrombosis (DVT), cerebrovascular disease, congestive heart failure, malignant tumors, acute myocardial infarction (AMI), acute pancreatitis, cardiac arrest (CA), and cardiogenic shock, treatments or examination (expressed as a percentage), including transthoracic echocardiography, blood transfusion (red blood cells, plasma, and platelets), norepinephrine usage, mechanical ventilation (MV), initiation of renal replacement therapy (RRT) on the first day, continuous renal replacement therapy (CRRT) during the period of ICU hospitalization.
Laboratory test included levels of white blood cell (WBC) count, hemoglobin, platelet count, red cell distribution width (RDW), anion gap, blood urea nitrogen (BUN), creatinine, prothrombin time (PT), glucose, serum magnesium, serum phosphate, lactate, albumin, LAR. All laboratory test results were recorded from the first-time assessments upon patient admission to the hospital as the period spanning the first 6 h before ICU admission through the initial 24 h after ICU admission.
Oliguria is defined as a urine output of less than 400 mL within a 24-h period.
Groups and endpoints
Based on the 30-day prognosis, all patients were divided into survival group (n = 5105) and non-survival group (n = 1995). Additionally, patients were divided into low LAR group (<0.659) and high LAR group (≥0.659) according to the LAR value (0.659) at 30-day death hazard ratio (HR) of 1 in restricted cubic spline (RCS) analysis.
The primary endpoint of the study was the 30-day all-cause mortality, while the secondary endpoint was the 360-day all-cause mortality.
Statistical analysis
We used the Kolmogorov-Smirnov test to assess whether the data followed a normal distribution. If the p-value was less than 0.05, we rejected the null hypothesis, indicating that the data did not adhere to a normal distribution. Conversely, if the p-value was greater than 0.05, it was considered to conform to a normal distribution. For continuous data that followed a normal distribution, it was presented as mean ± standard deviation , and the t-test was utilized for intergroup comparisons. If the data did not adhere to a normal distribution, it was reported as median (interquartile range) [M (QL, QU)], and intergroup comparisons were performed using the Mann-Whitney U test. Categorical data were represented as composition ratios (%) and analyzed using χ2 test for intergroup comparisons.
RCS method was used to analyze the correlation between LAR and the risk of 30-day and 360-day mortality, and a cutoff value was obtained. Variables with P-values less than 0.1 from the comparison of basic information were selected. Then we conducted a 10-fold cross-validation least absolute shrinkage and selection operator (LASSO) regression analysis on variables with P-values less than 0.1 from the comparison of basic information, in order to further select variables associated with the 30-day prognosis, and lambda (λ) value at the first standard error(se), that was λ.1se was calculated. In order to verify the stability of the results obtained by LASSO, we used the approach of elastic net to screen the variables. Then the Cox hazard proportional models of the association between LAR and 30-day and 360-day mortality were established, the results were presented as HR and 95% confidence intervals (CI). Kaplan-Meier curves were created and log-rank tests were used to compare the 30-day and 360-day cumulative survival rates between the high LAR group and the low LAR group. Subgroup analysis was used to demonstrate the stability of our results. ROC curve analysis was used to determine the diagnostic efficacy of LAR on prognosis.
Stata14.0 and R4.3.2 software was used for data analysis, and p < 0.05 was considered statistically significant.
Results
Participants and characteristic
Finally, 7100 eligible patients were enrolled, as shown in . Clinical data of the two groups of patients were compared in . The average age was 64.80 ± 16.72 years, with a mean SOFA score of 8. Age, SOFA score, WBC count, RDW, anion gap, BUN, creatinine, PT, magnesium, phosphate, lactate, LAR in non-survival group was higher (p < 0.05). Simultaneously, there were higher proportions of oliguria, receipt of treatments or examinations (transthoracic echocardiography, blood transfusion, norepinephrine, MV, RRT, CRRT), as well as complications (cerebrovascular diseases, malignancies, AMI, acute pancreatitis, CA, cardiogenic shock) in the non-survival group (p < 0.05), as shown in .
Table 1. Comparison of baseline characteristics between two groups segmented by 30-day mortality.
Non-linear association between LAR and all-cause mortality
RCS analyses showed that, there was a nonlinear trend relationship between LAR and the risk of 30-day all-cause mortality in patients with AKI (χ2 = 58.930, p < 0.001). Also, a similar trend was observed for the 360-day all-cause mortality rate (χ2 = 39.770, p < 0.001), as shown in . As the LAR level increased, the risk of 30-day all-cause mortality for patients at also increased, with the rate of increase gradually slowing down and approaching a plateau.
Figure 2. RCS analysis of the relationship between LAR and the risk of 30-day (left) and 360-day (right) all-cause mortality in patients with AKI.
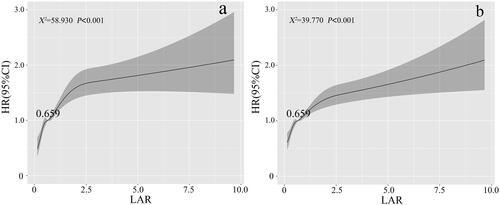
At the same time, we observed that the HR for both 30-day and 360-day mortality was 1 when the LAR value was 0.659, which was used as the cutoff value to categorize patients into high and low LAR groups.
Association between LAR and all-cause mortality
As shown in , the 30-day and 360-day all-cause mortality of the enrolled patients were 28.10% and 41.72% respectively. The 30-day and 360-day mortality in the high LAR group was significantly higher (37.94%, χ2 = 320.751, p < 0.001; 51.26%, χ2 = 250.725, p < 0.001).
Table 2. 30-Day and 360-day all-cause mortality in patients with AKI between the two groups.
With the 30-day prognosis as the dependent variable and the 28 variables with p < 0.1 in as the independent variables, a 10-fold cross-validation LASSO regression analysis was performed, and lambda.1se was calculated to be equal to 0.01. In the end, 20 variables were finally selected, including LAR, age, SOFA score, anion gap, BUN, creatinine, magnesium, WBC count, RDW, PT, cerebrovascular disease, malignant tumor, acute pancreatitis, CA, Cardiogenic shock, blood transfusion, MV, CRRT, norepinephrine and oliguria, as shown in . The aforementioned 28 variables and 20 selected variables were included in the Cox regression analysis respectively, as shown in and . In addition, variables selected by elastic net method was also included in the another Cox regression to verify the stability of the results.
Figure 3. a: Selection process of prognostic variables of AKI by LASSO regression. b: Selection process of the value of lambda by cross validation.
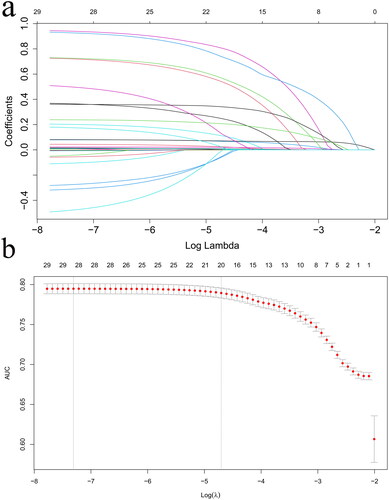
Table 3. Cox regression models of 30-day all-cause mortality in patients with AKI between the two groups.
Table 4. Cox regression models of 360-day all-cause mortality in patients with AKI between the two groups.
In the Cox regression analysis with variables of p < 0.1 in the basic data, it could be observed that without adjusting for confounding factors (Model I), high LAR was a risk factor for both 30-day and 360-day mortality in patients (p < 0.001). After adjusting for the 28 variables (Model III), the analyses showed that the OR (95%CI) of 30-day and 360 all-cause mortality was 1.474 (1.331–1.633) and 1.335 (1.230–1.447) respectively, indicating that high LAR (≥0.659) remained an independent risk factor for both 30-day and 360-day mortality in patients with AKI (p < 0.001).
The findings were also consistent in the Cox regression analysis with variables screened by LASSO. In Model I, without adjusting for multiple factors, high LAR was a risk factor for both 30-day and 360-day mortality in patients with AKI (p < 0.001). After adjusting for confounding factors in two instances, it was indicated that high LAR (≥0.659) was an independent risk factor for both 30-day and 360-day mortality in patients with AKI (HR 1.469, 95% CI 1.327–1.626; HR 1.320, 95% CI 1.218–1.431, both p < 0.001). Besides, another Cox regression with variables selected by elastic net method verified the stability of the results, showing that high LAR (≥0.659) remained an independent risk factor for both 30-day and 360-day mortality in patients with AKI (p < 0.001).
Kaplan-Meier survival curve
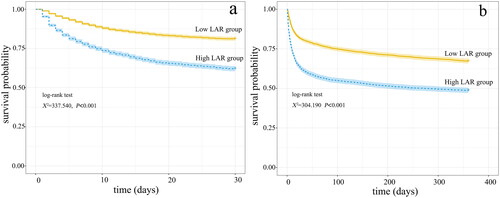
Kaplan-Meier survival curves in showed that compared with the low LAR group, the 30-day and 360-day cumulative survival rate of patients with AKI was significantly lower (log-rank test, χ2 = 337.540, p < 0.001; χ2 = 304.190, p < 0.001) in the high LAR group.
Subgroup analysis
Subgroup analyses which could further verify the stability of our results were performed to investigate the interaction between LAR and the variables which may play a role in the 30-day mortality of AKI patients, as shown in . The results indicated that there was an interaction between age, hypertension, cerebrovascular disease, MV and LAR on the 30-day all-cause mortality (interaction p < 0.05). No significant interactions were observed in other subgroups (interaction p > 0.05).
ROC curves analyses
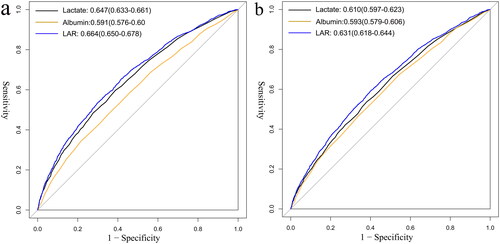
The ROC analyses showed that the optimal cutoff value for LAR to predict 30-day and 360-day mortality was 0.667 for both, with area under the curve (AUC) of 0.664 and 0.631, respectively. Also, LAR had a diagnostic advantage when compared with lactate or albumin alone (p < 0.001), as shown in and .
Table 5. Diagnostic value of lactate, albumin and LAR in the prediction of all-cause mortality in patients with AKI.
Discussion
The study found that there was a significant increase in 30-day and 360-day all-cause mortality in patients with AKI in high LAR group (p < 0.05). A nonlinear relationship was observed between LAR and the risk of 30-day and 360-day all-cause mortality in AKI patients (p < 0.001). After adjusting for confounding factors, Cox regulation showed that high LAR (≥0.659) was an independent risk factor for 30-day and 360-day all-cause mortality (p < 0.001) in patients with AKI. The Kaplan-Meier survival curves drawn the conclusion that compared with the low LAR group, the 30-day and 360-day cumulative survival rate of patients with AKI was significantly lower (p < 0.001). Subgroup analyses further confirmed the stability of the results of this study. Also, the ROC curves were demonstrated the diagnostic efficacy of LAR on prognosis in patients with AKI (p < 0.001). The conclusion offered clinicians a foundation for early identification of high-risk patients.
From , it was evident that the results were influenced by various confounding factors (p < 0.05). Therefore, we employed LASSO analysis to screen 20 confounding factors. In order to increase the confidence of the results, multiple methods of screening variables were employed and Cox regression analysis was performed for further adjustment. As a result, LAR emerged as an independent risk factor in patients with AKI. We used two methods to control the impact of confounding factors and obtained similar results, making the results more credible.
As a common disease with high mortality rate, AKI has attracted much attention and become one of the major medical challenges worldwide. The incidence of AKI has gradually increased over the years, and many factors, such as aging population, increased susceptibility to disease complications, increased use of nephrotoxic drugs, and invasive procedures may contribute to the increased incidence of AKI. At the same time, the occurrence of AKI is associated with increased mortality, prolonged hospital stay, and the risk of CKD [Citation11, Citation12]. Therefore, a large number of scholars have begun to explore the indicators for predicting renal function recovery or death in patients with AKI. Studies indicated that biomarkers such as Cystatin C (CysC), NGAL, and Kidney Injury Molecule-1 (KIM-1) may aid in detecting impaired kidney function during subclinical AKI stages, before serum creatinine elevation occurs [Citation13]. Researchers also studied the predictive value of different biomarkers for the prognosis of patients with AKI caused by various diseases. Reports suggested that hyperuricemia and lactate dehydrogenase/albumin ratio are independent risk factors for AKI patients with sepsis [Citation14, Citation15]. In critically ill patients with AKI receiving continuous renal replacement therapy (CRRT), higher albumin corrected anion gap (> 20 mmol/L) were significantly correlated with ICU all-cause mortality [Citation16]. Furthermore, other studies suggested that elevated levels of chloride in patients following ischemic stroke are associated with an increased occurrence of AKI and prolonged hospitalization [Citation17]. However, many of these studies have not undergone further validation, thus no definite conclusions have been drawn.
Lactate, as a serum biomarker, has been confirmed to be associated with the prognosis of many diseases. A study conducted by Seker et al. involving 1382 patients and found that lactate levels upon emergency department admission could predict outcomes of the patients [Citation18]. Meanwhile, abnormally elevated serum lactate levels might be a predictor of intra-abdominal infection after pancreatectomy [Citation19]. However, as a single indicator, lactate was affected by many factors, such as exercise, metabolic disorders, poor liver function, drugs and so on. Therefore, scholars recognized this limitation, integrated the two indicators of lactate and albumin so called LAR, as a breakthrough to explore its predictive role in different diseases. Cakir et al. compared the predictive value of lactate, albumin, and LAR for ICU patients with sepsis, concluding that LAR was better than lactate or albumin. LAR, being an easily accessible parameter, held potential value for critically ill patients [Citation20]. Kabra et al. even found that compared with lactate and albumin, LAR has outstanding predictive value in predicting the prognosis of patients with sepsis, with a sensitivity of 100% and a specificity of 88% [Citation21]. In 2020, some scholars proposed that LAR could be predictor for the mortality of ICU patients and could be an early prognostic marker for ICU patients [Citation6]. Meanwhile, LAR on admission was independently associated with 28-day mortality in severely burned patients [Citation22]. A recent study suggested that elevated LAR on admission was an independent predictor of high in-hospital mortality in patients with heart failure after myocardial infarction, and LAR was superior to lactate and SOFA Score [Citation23]. LAR was also associated with many other diseases, such as traumatic brain injury [Citation24] and urinary tract infection [Citation25]. In summary, LAR held significant clinical relevance in critically ill patients, including critically ill patients with heart failure, AKI and so on as mentioned before [Citation26, Citation27]. Given that patients in the ICU were more prone to experiencing AKI, the adoption of LAR to assess the prognosis of patients with AKI was advisable. However, there was little research on the association between LAR and prognosis in patients with AKI at present. A recent study similar to our reach got the conclusion that high LAR was an independent risk factor for in-hospital and ICU mortality in critically ill patients with AKI [Citation27]. This study only investigated the correlation between LAR and in-hospital mortality, which has its limitations. Our study made up for this regret, analyzed the relationship between LAR and the short-term and long-term prognosis of patients with AKI and obtained positive results. Moreover, the LAR values to predict prognosis in previous studies exhibited significant variability. In contrast, our study employed RCS analysis to establish a well-defined cutoff value (0.659) for LAR before conducting grouping. This methodological approach enhanced the scientific robustness of our study and offered valuable insights for clinical medical staff.
The potential mechanisms underlying the impact of LAR on the prognosis of critically ill patients with AKI are currently not yet understood. Research has confirmed that inflammation plays a crucial role in the occurrence and progression of AKI. The release of inflammatory mediators and infiltration of inflammatory cells can lead to renal tissue damage and functional impairment [Citation28]. Elevated lactate levels indicate the active degree of inflammation and have a promoting effect on the inflammatory response [Citation29]. Albumin also plays a role in regulating inflammation and immune response by increasing the production of anti-inflammatory substances such as lipoxins, solubilizers, and protectins, to promote wound/tissue healing [Citation30]. Therefore, the mechanism by which LAR affects the prognosis of critically ill patients with AKI may be mediated through two distinct inflammatory response mechanisms involving lactate and albumin. This dual mechanism reduces the influence of a single factor on regulatory mechanisms, thus providing a more accurate prediction of prognosis. Meanwhile, patients with AKI are in a state of high catabolism due to the release of endogenous inflammatory factors and catabolic hormones, accumulation of uremic toxins, metabolic acidosis, and insulin resistance. This catabolic state, along with RRT, leads to increased loss of nutrients and energy [Citation31]. Additionally, the kidneys not only maintain internal homeostasis by excreting water and metabolic waste but also participate in the metabolism of certain nutrients [Citation32]. LAR, as a biochemical marker, can be used to assess the metabolic status and nutritional condition of the body. In patients with AKI, impaired renal function may lead to metabolic abnormalities and malnutrition, thereby affecting changes in lactate and albumin levels. Through LAR, a better understanding of the metabolic disturbances and nutritional status of patients with AKI may be achieved, thereby exploring their relationship with disease progression and treatment outcomes. However, further basic research is needed to confirm the specific mechanisms by which LAR influences the prognosis of AKI patients.
This study had several strengths. Firstly, it explored not only the correlation between LAR and short-term prognosis but also the correlation with long-term prognosis. Additionally, the study benefited from a substantial sample size of 7100 cases, which enhanced the reliability of the findings. At the same time, the study employed three regression analyses and conducted subgroup analyses to verify the stability of the results. However, there were also limitations to consider. It was a retrospective study, which inherently introduced potential biases. Furthermore, the LAR indicator was not dynamically monitored, leaving room for future research in this direction.
Conclusion
High LAR (≥0.659) at ICU admission is an independent risk factor for both short-term (30-day) and long-term (360-day) all-cause mortality in patients with AKI. Clinical doctors should assess the prognosis of such patients as early as possible and raise vigilance accordingly.
Ethics approval and consent to participate
The database was approved by the Institutional Review Boards of Beth Israel Deaconess Medical Center and the Massachusetts Institute of Technology, and one author of the study was granted access to the database (ID number: 42303155).
Authors’ contributions
Penghui Gao contributed to design the study. Xiaoyun Shi wrote the manuscript. Jianhong Lu processed the data and did the analyses. Lei Zhong, Qikai Shen, Beiping Hu contributed to review and edit the manuscript. All the authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study were available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Mahowald M, Khayat M. Kidney disease: acute kidney injury. FP Essent. 2021;509:11–19.
- Hoste EAJ, Kellum JA, Selby NM, et al. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol. 2018;14(10):607–625. doi:10.1038/s41581-018-0052-0.
- Asmus K, Erfurt S, Ritter O, et al. AKI epidemiology and outcomes: a retrospective cohort study from the prenephrology era. Int J Nephrol. 2021;2021:5549316–5549318. doi:10.1155/2021/5549316.
- Kellum JA, Romagnani P, Ashuntantang G, et al. Acute kidney injury. Nat Rev Dis Primers. 2021;7(1):52. doi:10.1038/s41572-021-00284-z.
- Patschan D, Erfurt S, Oess S, et al. Biomarker-based prediction of survival and recovery of kidney function in acute kidney injury. Kidney Blood Press Res. 2023;48(1):124–134. doi:10.1159/000528633.
- Gharipour A, Razavi R, Gharipour M, et al. Lactate/albumin ratio: an early prognostic marker in critically ill patients. Am J Emerg Med. 2020;38(10):2088–2095. doi:10.1016/j.ajem.2020.06.067.
- Guo W, Zhao L, Zhao H, et al. The value of lactate/albumin ratio for predicting the clinical outcomes of critically ill patients with heart failure. Ann Transl Med. 2021;9(2):118–118. doi:10.21037/atm-20-4519.
- Shin J, Hwang SY, Jo IJ, et al. Prognostic value of the lactate/albumin ratio for predicting 28-day mortality in critically ILL sepsis patients. Shock. 2018;50(5):545–550. doi:10.1097/SHK.0000000000001128.
- Johnson A, Bulgarelli L, Pollard T, et al. 2022. MIMIC-IV (version 2.0). PhysioNet.
- Kellum JA, Lameire N, Group KAGW, KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (part 1). Crit Care. 2013;17(1):204. doi:10.1186/cc11454.
- Sohaney R, Yin H, Shahinian V, et al. In-hospital and 1-Year mortality trends in a national cohort of US veterans with acute kidney injury. Clin J Am Soc Nephrol. 2022;17(2):184–193. doi:10.2215/CJN.01730221.
- Su C-C, Chen J-Y, Chen S-Y, et al. Outcomes associated with acute kidney disease: a systematic review and meta-analysis. EClinicalMedicine. 2023;55:101760. doi:10.1016/j.eclinm.2022.101760.
- Turgut F, Awad AS, Abdel-Rahman EM. Acute kidney injury: medical causes and pathogenesis. J Clin Med. 2023;12(1):375. doi:10.3390/jcm12010375.
- Jiang Y-X, Gong C-L, Tang Y, et al. Association between hyperuricemia and acute kidney injury in critically ill patients with sepsis. BMC Nephrol. 2023;24(1):128. doi:10.1186/s12882-023-03129-x.
- Liang M, Ren X, Huang D, et al. The association between lactate dehydrogenase to serum albumin ratio and the 28-day mortality in patients with sepsis-associated acute kidney injury in intensive care: a retrospective cohort study. Ren Fail. 2023;45(1):2212080. doi:10.1080/0886022X.2023.2212080.
- Zhong L, Xie B, Ji XW, et al. The association between albumin corrected anion gap and ICU mortality in acute kidney injury patients requiring continuous renal replacement therapy. Intern Emerg Med. 2022;17(8):2315–2322. doi:10.1007/s11739-022-03093-8.
- Haller JT, Smetana K, Erdman MJ, et al. An association between hyperchloremia and acute kidney injury in patients with acute ischemic stroke. Neurohospitalist. 2020;10(4):250–256. doi:10.1177/1941874420913715.
- Seker YC, Bozan O, Sam E, et al. The role of the serum lactate level at the first admission to the emergency department in predicting mortality. Am J Emerg Med. 2021;45:495–500. doi:10.1016/j.ajem.2020.09.088.
- Li Y, Chen L, Xing C, et al. Changes in serum lactate level predict postoperative Intra-Abdominal infection after pancreatic resection. World J Surg. 2021;45(6):1877–1886. doi:10.1007/s00268-021-05987-8.
- Cakir E, Turan IO. Lactate/albumin ratio is more effective than lactate or albumin alone in predicting clinical outcomes in intensive care patients with sepsis. Scand J Clin Lab Invest. 2021;81(3):225–229. doi:10.1080/00365513.2021.1901306.
- Kabra R, Acharya S, Shukla S, et al. Serum lactate-albumin ratio: soothsayer for outcome in sepsis. Cureus. 2023;15(3):e36816. doi:10.7759/cureus.36816.
- Dudoignon E, Quennesson T, De Tymowski C, et al. Usefulness of lactate albumin ratio at admission to predict 28-day mortality in critically ill severely burned patients: a retrospective cohort study. Burns. 2022;48(8):1836–1844. doi:10.1016/j.burns.2022.01.003.
- Nishioka N, Kobayashi D, Izawa J, et al. Association between serum lactate level during cardiopulmonary resuscitation and survival in adult out-of-hospital cardiac arrest: a multicenter cohort study. Sci Rep. 2021;11(1):1639. doi:10.1038/s41598-020-80774-4.
- Wang R, He M, Qu F, et al. Lactate albumin ratio is associated with mortality in patients with moderate to severe traumatic brain injury. Front Neurol. 2022;13:662385. doi:10.3389/fneur.2022.662385.
- Madrazo M, López-Cruz I, Piles L, et al. Lactate/albumin ratio prognostic value for mortality in patients older than 65 years with complicated urinary tract infection. Rev Clin Esp (Barc). 2023;223(6):366–370. doi:10.1016/j.rceng.2023.04.006.
- Sai IN, Prasad R, T V. Assessing the prognostic value of crp/albumin ratio and lactate/albumin ratio in critically ill patients. J Assoc Physicians India. 2022;70(4):11–12.
- Zhu X, Xue J, Liu Z, et al. The lactate/albumin ratio predicts mortality in critically ill patients with acute kidney injury: an observational multicenter study on the eICU database. Int J Gen Med. 2021;14:10511–10525. doi:10.2147/IJGM.S339767.
- Gong L, Pan Q, Yang N. Autophagy and inflammation regulation in acute kidney injury. Front Physiol. 2020;11:639938. doi:10.3389/fphys.2020.639938.
- Li X, Yang Y, Zhang B, et al. Lactate metabolism in human health and disease. Signal Transduct Target Ther. 2022;7:305.
- Liu Q, Zheng H-L, Wu M-M, et al. Association between lactate-to-albumin ratio and 28-days all-cause mortality in patients with acute pancreatitis: a retrospective analysis of the MIMIC-IV database. Front Immunol. 2022;13:1076121. doi:10.3389/fimmu.2022.1076121.
- Soto GJ, Frank AJ, Christiani DC, et al. Body mass index and acute kidney injury in the acute respiratory distress syndrome. Crit Care Med. 2012;40(9):2601–2608. doi:10.1097/CCM.0b013e3182591ed9.
- Wooley JA, Btaiche IF, Good KL. Metabolic and nutritional aspects of acute renal failure in critically ill patients requiring continuous renal replacement therapy. Nutr Clin Pract. 2005;20(2):176–191. doi:10.1177/0115426505020002176.