Abstract
Background/Aims: Mechanisms by which synbiotic treatment improves liver function in patients with cirrhosis are unknown. This study was performed to address this important issue. Patients and methods: Thirty cirrhotic patients were randomized to receive synbiotic or placebo preparations for 7 days. Viable faecal counts of Lactobacillus species, Child-Pugh class, plasma retention rate of indocyanine green (ICGR15), whole blood tumour necrosis factor alpha (TNF-α) mRNA and interleukin-6 (IL-6) mRNA, serum TNF-α, soluble TNF receptor (sTNFR)I, sTNFRII and IL-6 and plasma endotoxin levels were measured pre- and post-treatment. Results: Synbiotic treatment was associated with significantly increased faecal lactobacilli counts and significant improvements in ICGR15 and Child-Pugh class. Significant increases in whole blood TNF-α mRNA and IL-6 mRNA, along with serum levels of sTNFRI and sTNFRII, also occurred. TNF-α and IL-6 levels correlated significantly, both at baseline and post-synbiotic treatment. Synbiotic-related improvement in ICGR15 was significantly associated with changes in IL-6, both at mRNA and protein levels, and unrelated to plasma endotoxin values. No significant changes in any study parameter followed placebo treatment. Conclusions: Short-term synbiotic treatment proven to modulate gut flora significantly improves liver function in patients with cirrhosis. Benefit is unrelated to reduction in endotoxaemia and may be mediated, at least in part, by treatment-related induction of IL-6 synthesis by TNF-α.
Introduction
We have reported previously that short-term treatment with a synbiotic regimen comprising four different Gram-positive lactic acid bacterial species and bioactive fibres that alters the intestinal flora is associated with significant improvement in liver function in patients with cirrhosis, mostly due to hepatitis B virus or alcohol Citation[1]. A beneficial effect on hepatic function was subsequently reported with use of a probiotic preparation, comprising gut bacteria without supplementary fibre, in a cohort of patients with alcoholic cirrhosis Citation[2]. Mechanisms by which interventions to manipulate the gut flora may lead to improvement in liver function in patients with cirrhosis are currently uncertain.
Synbiotic treatment has been shown in patients with cirrhosis to increase peripheral blood monocyte expression of Toll-like receptor 2 (TLR2) Citation[3], signaling via which likely contributes significantly to production of tumour necrosis factor-α (TNF-α) in this group Citation[3]. In addition, short-term exposure to the TNF-inducible cytokine, interleukin-6 (IL-6) Citation[4], has been shown to reduce liver injury in experimental animal models of liver damage Citation[5–14]. Consequently, this study aimed to investigate whether the TNF-α/IL-6 cytokine pathway may possibly contribute to the hepatoprotective effect of short-term synbiotic treatment seen clinically. To this end, we studied in our cirrhotic patients whole blood levels of TNF-α messenger RNA (mRNA) and IL-6 mRNA and serum levels of TNF-α, soluble TNF receptor I (sTNFRI; p55), soluble TNF receptor II (sTNFRII; p75) and IL-6, along with plasma levels of endotoxin, pre- and post-synbiotic or placebo treatment and correlated treatment-related changes in these parameters with changes in liver function, as reflected by the plasma clearance of indocyanine green Citation[15], Citation[16] and Child-Pugh class Citation[17].
Patients and methods
Patients
The study group included 30 outpatients attending a specialist liver clinic at a university teaching hospital with biopsy-proven cirrhosis due to a range of aetiologies and covering the spectrum of degrees of hepatic functional impairment as reflected by the Child-Pugh classification Citation[17] (). Patients were considered to have alcohol-related cirrhosis if alcohol intake had been in excess of 80 g/day in males and 30 g/day in females for more than 5 years and if testing for viral, metabolic and immune aetiologies was negative. Only patients who had been abstinent from alcohol for at least 3 months, as corroborated by family members and/or carers, were included, as alcohol influences the sensitivity of macrophages to endotoxin and the production of TNF-α Citation[18], Citation[19]. Patients with histological features of alcoholic hepatitis were excluded. Exclusion criteria also included a history within the previous 6 weeks of factors that may influence the intestinal flora (treatment with lactulose β-galactofructosidase or antibiotics), factors that may influence extra-intestinal translocation of gut flora and, hence, circulating endotoxin levels (primary intestinal disorders and gastrointestinal haemorrhage) and factors that may influence circulating cytokine levels (infection, immunomodulatory drug use and renal impairment (serum creatinine > 120 µmol/L) Citation[1], Citation[3]. In addition to intercurrent infection and gastrointestinal haemorrhage, patients with other possible causes of reversible hepatic functional decompensation, such as drug-related hepatotoxicity and choledocholithiasis, were excluded Citation[1], as were those with iodine allergy, in whom administration of ICG is contra-indicated. No change to any medical therapy was permitted during the period of study. No patient with hepatitis C virus-related cirrhosis received antiviral treatment prior to or during the study period.
Table I. Clinical and demographic data in cirrhotic patients randomized to group A (synbiotic treatment) and group B (placebo treatment) and healthy controls.
Thirty asymptomatic volunteers of similar age and gender distributions to the cirrhotic group, with no history of liver disease, alcohol intake < 20 g/day, normal liver function tests and no predisposition to altered gut flora, extra-intestinal translocation of gut flora or circulating cytokine levels, as above, served as controls Citation[1], Citation[3], to establish normal ranges for whole blood TNF-α mRNA and IL-6 mRNA levels, serum TNF-α, IL-6, sTNFRI, sTNFRII levels and plasma endotoxin levels. Informed consent in writing was obtained from each patient. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the South Eastern Area Health Service Research Ethics Committee, Department of Health, New South Wales, Australia.
Supplementation with the synbiotic regimen
Coded sachets containing the study and placebo preparations (A, n=15 and B, n=15, respectively) were pooled. One sachet was randomly drawn from this pool for each patient at study entry. Patients drawn to receive treatment with sachets A (group A) received oral supplementation with a Gram-positive synbiotic preparation consisting of four freeze-dried, non-urease-producing bacteria, namely Pediacoccus pentoseceus 5-33:3, Leuconostoc mesenteroides 32-77:1, Lactobacillus paracasei subspecies paracasei 19 and Lactobacillus plantarum 2592, each at a dose of 1010 colony forming units (cfu) per sachet, along with 10 g of bioactive, fermentable fibre (betaglucan, 2.5 g; inulin, 2.5 g; pectin, 2.5 g and resistant starch, 2.5 g) (Synbiotic 2000; Medipharm, Kagerod, Sweden). Patients drawn to receive treatment with sachets B (group B) received a non-fermentable placebo, namely crystalline cellulose (Medipharm) Citation[1], Citation[3].
All patients in groups A and B received one sachet of the respective synbiotic or placebo preparation daily, taken in 150–200 ml of water, for 7 days. Patients in groups A and B were well-matched for clinical and demographic variables including age, gender, aetiology of cirrhosis, Child-Pugh classification and baseline ICG retention rate, whole blood TNF-α mRNA and IL-6 mRNA levels, serum TNF-α, IL-6, sTNFRI and sTNFRII levels and plasma endotoxin levels ().
The identity of the contents of sachets A and B (synbiotic or placebo preparations) was unknown to the investigators until after the study had been completed and the results had been analysed, when the code was broken Citation[1].
Quantitative bacteriological analysis of faecal samples for viable counts of Lactobacillus species
Faecal samples for determination of viable counts of Lactobacillus species, as a marker of effective colonization by bacteria included in the synbiotic preparation, were collected in sterile containers pre- and post-supplementation with the synbiotic and placebo preparations and cultured aerobically and anaerobically for 72 h. Representative bacterial colonies were identified to the genus level on the basis of their Gram stain reaction and colonial and cellular morphologies, as described previously Citation[1]. Viable faecal counts of Lactobacillus species were expressed as the logarithm of cfu per gram of dry faeces Citation[1].
Indocyanine green clearance
Plasma disappearance of ICG was measured using an intravenous ICG dose of 0.5 mg/kg ideal body weight and a non-invasive transcutaneous system based on a finger-tip sensor and pulse densitometry (LiMon, Pulsion Medical Systems, Munich, Germany). The accuracy of this non-invasive method of determining ICG clearance has been validated in comparison to both serial blood sampling with consecutive spectrophotometric analyses and an intra-aortic fibreoptic technique Citation[20], Citation[21]. Pre- and post-treatment ICG clearance measurements were performed following a fast of at least 6 h and at the same time of day so as to eliminate any possible confounding effects of post-prandial and diurnal variations of portal vein flow, respectively. Results were expressed as percentage plasma retention of ICG at 15 min (ICGR15). The normal ICGR15 using this methodology in subjects without liver disease is < 10% in our laboratory. The median (range) inter-assay variability of ICGR15 using our methodology, as assessed in eight healthy volunteers with normal liver function who received two intravenous ICG doses of 0.5 mg/kg body weight 2 h apart, was found to be 2.5% (0–3.6%).
Blood sampling
Peripheral blood was drawn using pyrogen-free needles, syringes and containers (Becton-Dickinson, Singapore). Whole blood was stored at –70°C until analysis within 6 weeks of collection. Where applicable, plasma and serum were separated in a refrigerated centrifuge at 4°C and stored at –70°C in pyrogen-free polyethylene cryotubes (Nunc, Denmark) until analysis – also within 6 weeks of collection.
Measurement of cytokine mRNA in whole blood
Total RNA was isolated from 250 µl whole blood, thawed on ice, with the addition of 750 µl triReagent LS (Molecular Research Center Inc., Cincinnati, OH, USA), 20 µl 5N acetic acid and 2 µl polyacryl carrier (Molecular Research Center Inc.), according to the manufacturer's protocol. Total RNA was dissolved in 100 µl nuclease-free water (Promega Corp., Madison, WI, USA). A 25 µl aliquot of RNA was reverse transcribed using M-MuLV reverse transcriptase (USB Corp, Cleveland, OH, USA) and pd(N)6 random primers (Amersham Pharmacia Biotech, Piscataway, NJ, USA) in a final reaction volume of 50 µl.
Real time PCR was performed using 2 µl of cDNA for amplification. Oligonucleotide primers used are listed in Citation[22], Citation[23]. In all, 900 nM of each forward and reverse primer (Geneworks, Adelaide, Australia) were used per 10 µl reaction. PCR was performed using SYBR Green PCR Master Mix (Aplied Biosystems, Foster City, CA, USA) in 384-well clear optical reaction plate (Applied Biosystems). Reaction conditions were 2 min at 50°C, 10 min at 95°C, 40 cycles of 15 s at 95°C and 1 min at 60°C. Melt curve analysis was performed. No unspecific amplification products were observed. All PCR reactions were performed in triplicate. Threshold cycle numbers were determined with Sequence Detector Software (version 2.0, Applied Biosystems). Standard curves for quantification were constructed by establishing the maximum mRNA production by peripheral blood monocytes in response to stimulation with endotoxin (10 µg/mL for 20 h) in vitro and performing serial dilutions. TNF-α mRNA and IL-6 mRNA levels in study patients and control subjects were expressed as a ratio to maximum stimulation values.
Table II. Oligonucleotide primers used for real-time PCR analysis.
Serum TNF-α assay
Serum TNF-α was measured using the Quantikine HS Human TNF-α Immunoassay (R&D Systems Inc., Minneapolis, USA), according to the manufacturer's instructions. The sensitivity of the assay was 0.5 pg/ml.
Serum sTNFR assays
Serum sTNFRI (p55) and sTNFRII (p75) were measured using Quantikine HS Human sTNFR Immunoassays (R&D Systems Inc.), according to the manufacturer's instructions. The sensitivity of the sTNFRI and sTNFRII assays was 3.0 pg/ml and 1.0 pg/ml, respectively.
Serum IL-6 assay
Serum IL-6 was measured using the Quantikine HS Human IL-6 Immunoassay (R&D Systems Inc.), according to the manufacturer's instructions. The sensitivity of the assay was 0.5 pg/ml.
Plasma endotoxin assay
Plasma endotoxin was measured using the chromogenic limulus amoebocyte lysate assay (Associate of Cape Cod Inc., MA, USA), according to the manufacturer's instructions. The sensitivity of the assay was 3 pg/ml.
Statistical analyses
Statistical analyses were performed using the Mann–Whitney rank sum test, Spearman's rank correlation test, the Wilcoxon rank sum test and Fisher's exact test, as appropriate (Systat for Windows, version 5.02, Systat Inc., Evanston, IL, USA). The probability level of p ≤ 0.05 was set for statistical significance.
Results
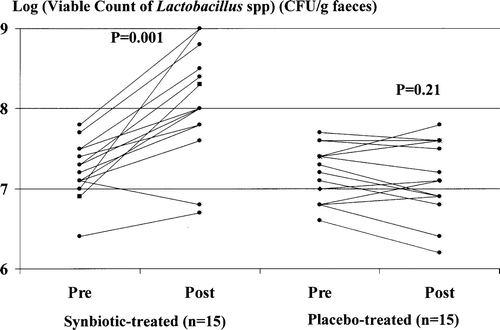
Viable faecal counts of Lactobacillus species pre- and post-synbiotic treatment
Synbiotic treatment was associated with a significant increase in viable faecal counts of Lactobacillus species (, left). No significant change in faecal counts of Lactobacillus species occurred in placebo-treated patients (, right).
ICG clearance and Child-Pugh class pre- and post-synbiotic treatment
Baseline ICGR15 was abnormal (≥10%) in 14/15 (93.3%) of cirrhotic patients randomized to treatment with the synbiotic preparation and in 14/15 (93.3%) of those randomized to treatment with the placebo preparation ().
Figure 2. Indocyanine green (ICG) clearance, expressed as the percentage plasma retention rate 15 min after a test dose of ICG of 0.5 mg/kg lean body weight (ICGR15), measured pre- and post-treatment with synbiotics (left) or placebo (right). Synbiotic, but not placebo, treatment was associated with a significant improvement in ICGR15.
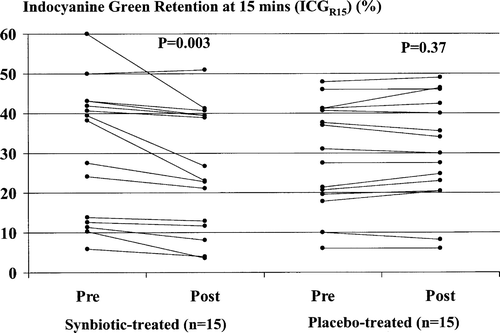
Synbiotic treatment was associated with a significant improvement in ICG clearance, with a median reduction in ICGR15 compared with baseline values of 15.2% (range 4.4–65.0%) occurring in 14/15 (93.3%) patients (, left). No significant change in ICGR15 followed treatment with the placebo (, right).
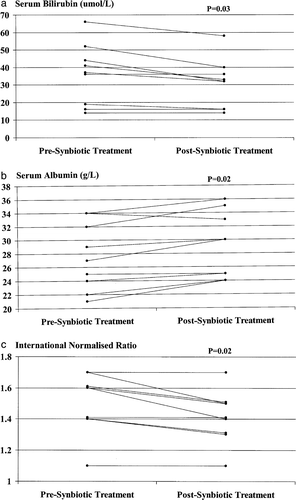
Improvement in Child-Pugh classification occurred in 4/9 (44.4%) patients who were initially Child-Pugh class B or C and who were treated with the synbiotic preparation (from class B to class A: n=2; from class C to class B: n=2) and in 0/10 (0%) such patients treated with the placebo (p=0.03). ICG clearance improved in all four synbiotic-treated patients in whom an improvement in Child-Pugh status was documented, as well as in 10/11 (90.9%) patients in whom the Child-Pugh grade remained stable during the period of the study. The improvement in Child-Pugh classification in initially decompensated synbiotic-treated patients resulted from significant improvements in serum bilirubin and albumin concentrations and in the prothrombin time, expressed as the international normalized ratio (INR) (a, b and c). No significant change in serum bilirubin or albumin levels or in the INR occurred in placebo-treated patients. No deterioration in Child-Pugh class occurred in any patient during the 7 day study period.
Figure 3. Serum concentrations of bilirubin (a) and albumin (b) and prothrombin time, as expressed by the international normalized ratio (INR) (c), in initially Child-Pugh class B and C patients (n=9) pre- and post-synbiotic treatment. Post-treatment values of each parameter were increased significantly compared with corresponding baseline values.
The synbiotic regimen was well tolerated with no change in general clinical condition. Two (13.3%) synbiotic-treated patients transiently complained of increased flatulence during the first few days of treatment.
Whole blood cytokine mRNA levels pre- and post-synbiotic treatment
Baseline whole blood TNF-α mRNA and IL-6 mRNA levels were each significantly higher in cirrhotic patients randomized to synbiotic or placebo treatment than in healthy controls, but did not differ significantly between the treatment groups ().
Synbiotic treatment was associated with further significant increases in both TNF-α mRNA () and IL-6 mRNA () levels, with post-treatment values increased in comparison to baseline levels by a median of 11.1% (range –33.3% to 600%) and a median of 70.0% (range –23.1% to 500%), respectively.
Figure 4. Whole blood TNF-α mRNA levels pre- and post-synbiotic treatment. Post-treatment values were significantly increased compared with corresponding baseline levels. *Ratio to maximum value resulting from in vitro stimulation of PBMCs by endotoxin (10 µg/ml for 20 h).
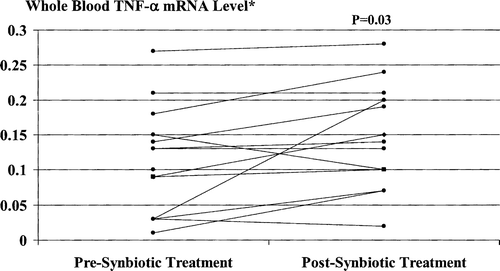
Figure 5. Whole blood IL-6 mRNA levels pre- and post-synbiotic treatment. Post-treatment values were significantly increased compared with corresponding baseline levels. *Ratio to maximum value resulting from in vitro stimulation of PBMCs by endotoxin (10 µg/ml for 20 h).
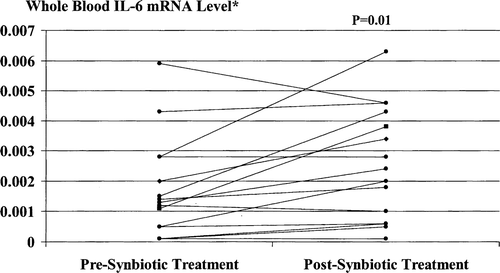
No significant change in either TNF-α mRNA or IL-6 mRNA levels occurred in placebo-treated patients (p>0.3).
Serum cytokine levels pre- and post-synbiotic treatment
Baseline serum TNF-α, sTNFRI, sTNFRII and IL-6 levels were each significantly higher in cirrhotic patients randomized to synbiotic or placebo treatment than in healthy controls, but did not differ significantly between the treatment groups ().
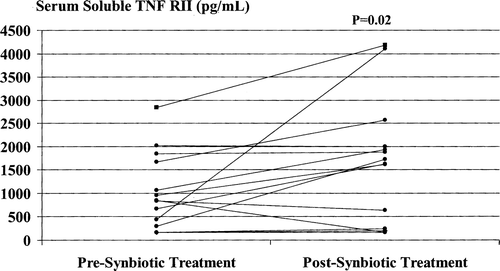
Synbiotic treatment was associated with further significant increases in serum sTNFRI and sTNFRII levels ( and ), with post-treatment values increased in comparison with baseline values by a median of 56.6% (range –52.7% to 177.6%) and a median of 42.2% (range –79.8% to 836.0%), respectively. Post-treatment serum IL-6 values were increased by a median of 100% (range 8.3–1500%) in 10/15 (66.7%) patients and reduced by a median of 87% (range 43–93%) in the remaining 5 (33.3%) patients (p=0.25). Serum TNF-α levels were increased by a median of 77% (range 10–133%) in 5/15 (33.3%) patients and reduced by a median of 35% (range 4–80%) in the other 10 (66.7%) patients (p=0.19).
Figure 6. Serum sTNFRI levels pre- and post-synbiotic treatment. Post-treatment values were significantly increased compared with corresponding baseline levels.
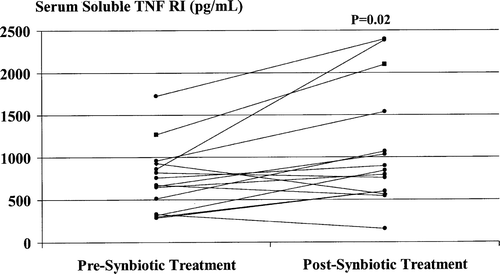
Figure 7. Serum sTNFRII levels pre- and post-synbiotic treatment. Post-treatment values were significantly increased compared with corresponding baseline levels.
No significant change in serum TNF-α, sTNFRI, sTNFRII or IL-6 levels occurred in placebo-treated patients (p>0.30).
Plasma endotoxin levels pre- and post-synbiotic treatment
Baseline plasma endotoxin values were detectable (> 3 pg/ml) in a significantly increased proportion of cirrhotic patients than healthy controls, yet still in a minority of cirrhotic patients randomized to synbiotic and placebo groups (3/15, 20.0% and 4/15, 26.7%, respectively) ().
Plasma endotoxin became undetectable in 1/3 (33.3%) synbiotic-treated patients with initially detectable plasma concentrations and 0/4 (0%) such placebo-treated patients. No patient with initially undetectable plasma endotoxin developed detectable plasma values during the study period.
Correlations between ICG clearance and other study parameters in synbiotic-treated patients
Following synbiotic treatment, significant positive correlations were evident between the degree of improvement in ICGR15 and degree of increase in circulating IL-6, both at mRNA and protein levels, when expressed as percentage change compared with baseline values ().
Table III. Correlations between degrees of improvement in ICGR15 and other study parameters, expressed as percentage increase or decrease compared to baseline values, in synbiotic-treated patients.
The degree of change in ICG clearance following synbiotic treatment did not correlate significantly with changes in whole blood TNF-α mRNA levels, serum TNF-α, sTNFRI or sTNFRII levels or plasma endotoxin values (). With regard to the latter, ICGR15 improved in all 12 of the synbiotic-treated patients with initially undetectable endotoxin in plasma in whom endotoxin remained undetectable following synbiotic treatment, along with 2/3 (66.7%) patients with initially detectable plasma endotoxin in whom endotoxin remained detectable despite synbiotic treatment. Conversely, ICGR15 failed to improve in the only synbiotic-treated patient with initially detectable plasma endotoxin whose plasma endotoxin concentration fell below the level of detection post-treatment.
Correlations between circulating cytokine and endotoxin levels in synbiotic-treated patients
Baseline circulating IL-6 values were significantly correlated with TNF-α values, both at mRNA and protein levels, in keeping with the known role of TNF-α in inducing production of IL-6 Citation[4]. The degree of change in IL-6 following symbiotic treatment, expressed as the percentage change compared with baseline values, also correlated significantly with the degree of change in TNF-α, both at mRNA and protein levels ().
Table IV. Correlations between circulating cytokine levels in patients randomized to synbiotic treatment.
Baseline plasma endotoxin values did not correlate significantly with levels of whole blood TNF-α mRNA or IL-6 mRNA or serum levels of TNF-α, sTNFRI, sTNFRII or IL-6. Similarly, the degree of change in plasma endotoxin concentrations following symbiotic treatment, expressed as the percentage change compared with baseline values, did not correlate significantly with the degree of change in any of these parameters (r<0.20, p>0.30).
Discussion
This report documents significant improvement in liver function in patients with cirrhosis, mostly due to chronic hepatitis C viral infection or alcohol, who underwent short-term treatment with a synbiotic preparation shown to significantly modulate the gut flora. In particular, enhanced clearance of ICG occurred in > 90% of treated patients, accompanied by improvement in the Child-Pugh class in nearly 50% of those initially categorized as Child-Pugh class B or C. Instances of improvement in Child-Pugh class resulted from modest yet statistically significant improvements in laboratory indices including the serum bilirubin and albumin concentrations and the INR. No deterioration in Child-Pugh class occurred in any synbiotic-treated patient. It is unlikely that the improvement in liver function seen in our synbiotic-treated patients was simply the consequence of improved clinical monitoring during the study period, as no significant change in ICG clearance or improvement in Child-Pugh class occurred in placebo-treated patients in our study, despite comparable baseline clinical and demographic profiles and clinical follow-up schedules. The degree of improvement in ICG clearance in synbiotic-treated patients in our study, although also only modest on occasion, was nonetheless in excess of inter-assay variability in all cases. Although the duration of synbiotic therapy was only short, it was sufficient to promote significant changes in the faecal flora of our patients.
We sought to identify possible mechanisms by which synbiotic treatment may lead to improved liver function in cirrhotic patients, focusing in particular on the possible importance of the TNF-α/IL-6 cytokine cascade. Notably, highly significant correlations were apparent between baseline IL-6 and TNF-α levels, both at the protein and mRNA levels, in the cirrhotic patients enrolled in our study, in keeping with the known effect of TNF-α in inducing IL-6 synthesis Citation[4]. We found evidence of up-regulation of the TNF-α/IL-6 pathway following synbiotic treatment, with significantly increased whole blood TNF-α mRNA and IL-6 mRNA levels and serum sTNFRI and sTNFRII concentrations compared with baseline occurring in synbiotic-treated but not in placebo-treated patients. As at baseline, the extent of changes in circulating IL-6 and TNF-α values following synbiotic treatment were significantly correlated at both mRNA and protein levels, suggesting that the increased IL-6 expression following synbiotic treatment is also TNF-α-induced.
The degree of improvement in ICG clearance that followed synbiotic treatment in our cirrhotic patients was significantly correlated with the magnitude of treatment-associated increases in circulating IL-6, both at protein and mRNA levels, raising the possibility that this pleiotropic cytokine may at least contribute to the hepatoprotective effect of synbiotic therapy in this setting. Recent experimental data support this concept. In particular, IL-6 has been shown in experimental animals to protect against liver injury induced by carbon tetrachloride Citation[5], alcohol Citation[6], ischaemia/reperfusion Citation[7], Fas Citation[8], concanavalin A Citation[9], haemorrhagic shock Citation[10] and following liver transplantation, including that involving steatotic donor organs Citation[11], Citation[12]. Recent experimental studies indicate that mechanisms of benefit of IL-6 include both pro-regenerative and anti-apoptotic effects on hepatocytes, evident within a few days of IL-6 exposure Citation[13], Citation[14], with this rapid onset of benefit of particular interest in view of our finding in this report of clinical benefit within 7 days of commencement of synbiotic treatment and associated increased whole blood IL-6 mRNA expression. Protection against sinusoidal cell necrapoptosis resulting in augmentation of the hepatic microcirculation has also been documented Citation[12]. Further studies in suitable experimental animal models, including IL-6 knockout mice in comparison to wild-type animals, will clarify to what extent and by what exact mechanisms IL-6 may promote the hepatoprotective effect of synbiotic treatment in cirrhosis.
TNF-α and IL-6 are cleared by the liver Citation[24], Citation[25] and cross-sectional analyses have shown that circulating levels are highest in those cirrhotic patients with more advanced degrees of hepatic dysfunction, implying that hepatic metabolism substantially influences the peripheral blood levels found clinically Citation[26]. We speculate that enhanced hepatic clearance of TNF-α and IL-6 consequent to improved liver function following synbiotic treatment, counter-balancing treatment-related increases in cytokine production, might account for our finding that the significant increases in TNF-α and IL-6 at the mRNA level associated with synbiotic supplementation were not replicated at the protein level. It is notable that significant increases in serum levels of sTNFRI and sTNFRII were documented following symbiotic treatment, providing further evidence of up-regulation of the TNF-α pathway in this circumstance.
Endotoxin, an essential cell wall component of Gram-negative bacteria, is an important cause of liver damage in experimental animals Citation[27–29] and we have previously documented that elevated circulating endotoxin levels in patients with cirrhosis are significantly reduced following synbiotic treatment Citation[1]. Our findings in this study, however, suggest that reduction in endotoxaemia is not necessary for synbiotic treatment-associated improvement in hepatic function to occur. Plasma endotoxin levels were elevated at baseline in only a minority of our study cohort and ICG clearance improved both in patients with initially undetectable plasma endotoxin levels and in those in whom the plasma endotoxin concentration remained elevated despite synbiotic treatment. Conversely, ICG clearance failed to improve in the only synbiotic-treated patient with initially elevated plasma endotoxin whose plasma endotoxin concentration fell below the level of detection post-treatment. We could not postulate a role for endotoxaemia in promoting TNF-α or IL-6 production in our patients, as no significant correlation was apparent between circulating endotoxin and either TNF-α or IL-6, either at mRNA or protein levels, at baseline or following synbiotic supplementation. These findings are in keeping with our earlier reported observation that signaling via TLR4, the endotoxin receptor, is unlikely to contribute significantly to circulating TNF-α and sTNFR levels in cirrhotic patients Citation[3].
We conclude that short-term use of a synbiotic regimen proven to modulate the gut flora significantly improves liver function in patients with cirrhosis. This beneficial effect is not dependent upon reduction in endotoxaemia and may be mediated, at least in part, by treatment-related induction of IL-6 synthesis by TNF-α.
References
- Liu Q, Duan ZP, Ha DK, Bengmark S, Kurtovic J, Riordan SM. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with liver cirrhosis. Hepatology 2004; 39: 1441–9
- Loguercio C, Federico A, Tuccillo C, Terracciano F, D'Auria MV, De Simone C, et al. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol 2005; 39: 540–3
- Riordan SM, Skinner N, Nagree A, McCallum H, McIver CJ, Kurtovic J, et al. Peripheral blood mononuclear cell expression of Toll-like receptors and relation to cytokine levels in cirrhosis. Hepatology 2003; 37: 1154–64
- Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, et al. Induction of cachexia in mice by systemically administered myostatin. Science 2002; 296: 1486–8
- Kovalovich K, DeAngelis RA, Li W, Furth EE, Ciliberto G, Taub R. Increased toxin-induced liver injury and fibrosis in interleukin-6-deficient mice. Hepatology 2000; 31: 149–59
- Hong F, Kim WH, Tian Z, Jaruga B, Ishac E, Shen X, et al. Elevated interleukin-6 during ethanol consumption acts as a potential endogenous protective cytokine against ethanol-induced apoptosis in the liver: involvement of induction of Bcl-2 and Bcl-x(L) proteins. Oncogene 2002; 21: 32–43
- Camargo CA, Jr, Madden JF, Gao W, Selvan RS, Clavien PA. Interleukin-6 protects liver against warm ischaemia/reperfusion injury and promotes hepatocyte proliferation in the rodent. Hepatology 1997; 26: 1513–20
- Kovalovich K, Li W, DeAngelis R, Greenbaum LE, Ciliberto G, Taub R. Interleukin-6 protects against Fas-mediated death by establishing a critical level of anti-apoptotic hepatic proteins FLIP, Bcl-2 and Bcl-xL. J Biol Chem 2001; 276: 26605–13
- Mizuhara H, Uno M, Seki N, Yamashita M, Yamaoka M, Ogawa T, et al. Critical involvement of interferon gamma in the pathogenesis of T-cell activation-associated hepatitis and regulatory mechanisms of interleukin-6 for the manifestations of hepatitis. Hepatology 1996; 23: 1608–15
- Meng ZH, Dyer K, Billiar TR, Tweardy DJ. Distinct effects of systemic infusion of G-CSF vs. IL-6 on lung and liver inflammation and injury in hemorrhagic shock. Shock 2000; 14: 41–8
- Selzner N, Selzner M, Tian Y, Kadry Z, Clavien PA. Cold ischemia decreases liver regeneration after partial liver transplantation in the rat: a TNF-alpha/IL-6-dependent mechanism. Hepatology 2002; 36: 812–18
- Sun Z, Klein AS, Radaeva S, Hong F, El-Assal O, Pan H-N, et al. In vitro interleukin-6 treatment prevents mortality associated with fatty liver transplants in rats. Gastroenterology 2003; 125: 202–15
- Jin X, Zimmers TA, Perez EA, Pierce RH, Zhang Z, Koniaris LG. Paradoxical effects of short- and long-term interleukin-6 exposure on liver injury and repair. Hepatology 2006; 43: 474–84
- Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, et al. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science 1996; 274: 1379–83
- Ganslmayer M, Ocker M, Zopf S, Schuppan D, Hahn EG, Herold C. Hepatocellular carcinomas do not compromise quantitative tests of liver function. Hepatogastroenterology 2005; 52: 881–4
- Imamura H, Sano K, Sugawara Y, Kokudo N, Makuuchi M. Assessment of hepatic reserve for indication of hepatic resection: decision tree incorporating indocyanine green test. J Hepatobiliary Pancreat Surg 2005; 12: 16–22
- Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973; 60: 646–9
- Manrekar P, Catalano D, Szabo G. Alcohol-induced regulation of nuclear regulatory factor-kappa beta in human monocytes. Alcohol Clin Exp Res 1997; 21: 1226–31
- Enomoto N, Ikejima K, Bradford B, Rivera C, Kono H, Brenner DA, et al. Alcohol causes both tolerance and sensitisation of rat Kupffer cells via mechanisms dependent on endotoxin. Gastroenterology 1998; 115: 443–51
- Sakka SG, Reinhart K, Meier-Hellmann A. Comparison of invasive and noninvasive measurements of indocyanine green plasma disappearance rate in critically ill patients with mechanical ventilation and stable hemodynamics. Intensive Care Med 2000; 26: 1553–6
- Faybik P, Krenn C-G, Baker A, Lahner D, Berlakovich G, Steltzer H, et al. Comparison of invasive and noninvasive measurement of plasma disappearance rate of indocyanine green in patients undergoing liver transplantation: a prospective investigator-blinded study. Liver Transpl 2004; 10: 1060–4
- Kruse N, Pette M, Toyka K, Rieckmann P. Quantification of cytokine mRNA expression by RT PCR in samples of previously frozen blood. J Immunol Methods 1997; 210: 195–203
- Starkie RL, Arkinstall MJ, Koukoulas I, Hawley JA, Febbraio MA. Carbohydrate ingestion attenuates the increase in plasma interleukin-6, but not skeletal muscle interleukin-6 mRNA, during exercise in humans. J Physiol 2001; 553: 585–91
- Tilg H, Vogel W, Wiedermann CJ, Shapiro L, Herold M, Judmaier G, et al. Circulating interleukin 1 and tumor necrosis factor antagonists in liver disease. Hepatology 1993; 18: 1132–8
- Diez-Ruiz A, Tilz GP, Gutierrez-Gea F, Gil-Extremera B, Murr C, Wachter H, et al. Neopterin and soluble tumor necrosis factor receptor type 1 in alcoholic cirrhosis. Hepatology 1995; 21: 976–8
- Lee FY, Lu RH, Tsai YT, Lin HC, Hou MC, Li CP, et al. Plasma interleukin-6 levels in patients with cirrhosis. Scand J Gastroenterol 1996; 31: 500–15
- Enomoto N, Ikejima K, Yamashina S, Hirose M, Shimizu H, Kitamura T, et al. Kupffer cell sensitization by alcohol involves increased permeability to gut-derived endotoxin. Alcohol Clin Exp Res 2001; 25(6 Suppl)51S–54
- Wheeler MD. Endotoxin and Kupffer cell activation in alcoholic liver disease. Alcohol Res 2003; 27: 300–6
- Morita T, Tanabe H, Takahashi K, Sugiyama K. Ingestion of resistant starch protects endotoxin influx from the intestinal tract and reduces D-galactosamine-induced liver injury in rats. J Gastroenterol Hepatol 2004; 19: 303–13