Abstract
Gastrointestinal and respiratory infections are common among children attending day-care, particularly among younger children. The aim of the present randomized, double-blind and placebo-controlled study was to investigate whether Biola, a commercial milk product with a combination of three different probiotic strains (Lactobacillus rhamnosus GG (LGG), L. acidophilus LA-5, and Bifidobacterium Bb-12) given daily to 240 children younger than 3 years, during 7 winter months of their first year in a day-care centre, could prevent such infections. Information about symptoms of respiratory and gastrointestinal infections was collected by use of a diary completed by the parents and the number of days with respiratory and gastrointestinal symptoms and absences from day-care because of illness were studied. There was no significant difference between the two groups when analysing the total number of days with gastrointestinal and/or respiratory symptoms (26.5 days for the Biola group versus 26.9 days for the placebo group, p=0.52). However, the results indicate that Biola may reduce the number of days with gastrointestinal symptoms only (1.7 days for the Biola group versus 3.0 days for placebo, p=0.02). No significant difference between treatments was seen with respect to respiratory symptoms alone.
Introduction
Respiratory tract infections and gastrointestinal infections like diarrhoea are more common in children attending day-care centres than in children at home or in family care Citation[1–3], with the relative risk assumed to be greatest for children below 2 years of age attending day-care. For example, in two Finnish studies, the relative risk for common cold in 1-year-old children attending day-care was 1.69, and 1.76, and 1.56 for diarrhoea in 1- and 2-year-old children, respectively Citation[3], Citation[4]. Illness, in particular infections, among children in day-care causes significant economic losses, i.a. due to the guardian's absence from work, deficient utilization of day-care centres and direct medical costs Citation[5], and prevention of infections in day-care children is of high interest for both society and individual families.
Functional foods, such as probiotic products, may be one way to prevent infections in children by simple means. Probiotics are defined as ‘living micro-organisms which upon ingestion in certain numbers exert health benefits beyond inherent general nutrition’ Citation[6]. It has been suggested that such bacteria may prevent infections caused by pathogenic intestinal bacteria by enhancing the production of specific immunoglobulins (sIgA) from the intestinal mucosa, stimulate re-establishment of normal intestinal microflora and lower the intestinal permeability Citation[7]. To obtain the best specific interaction with the host, the probiotic bacteria should be of human origin, and the consumption should exceed 109 probiotic bacteria per day Citation[8].
Several probiotic bacterial strains have been tested for prevention of various infectious diseases, primarily gastrointestinal and respiratory infections. Lactobacillus species, and in particular Lactobacillus rhamnosus GG (LGG), but also Bifidobacterium species like Bb-12, have been demonstrated to reduce the duration and to some extent the incidence of gastrointestinal infections in children Citation[9–12]. In particular, a significant preventive effect (in terms of lower frequency) on acute diarrhoea, both nosocomial and non-nosocomial, has been demonstrated with Bifidobacterium and Lactobacillus species in previous long-term studies in children (3–17 months duration) Citation[11], Citation[13], Citation[14].
A Finnish study by Hatakka et al. Citation[9] indicated that milk containing Lactobacillus GG may slightly reduce the incidence of respiratory infections and antibiotic use in children, resulting in fewer days of absence from day-care. However, the age-adjusted results were not significant.
Although studies in children may not have been consistent in demonstrating an effect on rate and duration of respiratory illnesses Citation[9], Citation[11], studies in adults have shown that regular intake of probiotic bacteria (Bifidobacterium, Lactobacillus species and Streptococcus thermophilus) can reduce the level of potentially pathogenic bacteria in the upper respiratory tract Citation[15].
Lactobacillus rhamnosus GG (LGG) is a bacterium isolated from human intestines, and survives all the way through the human gastrointestinal tract Citation[16]. When administered in fermented milk, it is recovered in the faeces to a larger extent than when administered as powder or frozen concentrate Citation[16], Citation[17]. A milk product is also beneficial for use in children as the administration is easier, which may give a higher compliance rate than tablets.
The aim of the present study was to investigate whether Biola, a commercial milk product with a combination of three different probiotic strains (Lactobacillus rhamnosus GG (LGG), L. acidophilus LA-5 and Bifidobacterium Bb-12) given daily to children during 7 winter months of their first year in day-care centre, can further support the Finnish results Citation[9] and also demonstrate an effect on prevention of gastrointestinal symptoms.
Subjects and methods
Study product
The active study product was a probiotic milk drink, Biola (manufactured by TINE BA), with three lactic acid bacteria, LGG, L. acidophilus LA-5 and Bifidobacterium Bb-12, to a minimum level of 108 colony forming units (cfu)/ml of LGG and Bb-12, and 107 cfu/ml of La-5 for the entire shelf life. The placebo product was an ordinary fermented milk drink (manufactured by TINE BA), heated to 75oC for 4 s to ensure absence of probiotic bacteria. Raspberry flavour was added to both investigational products to increase the compliance in children. The microbial count was regularly checked to ensure that the probiotic content was according to specifications during the study. To ease the administration of the study at the day-care centres, the products were delivered in 1 litre cartons coloured blue or yellow. All involved parties – children, parents, day-care staff and operational project staff – were blinded to which study group the colour codes represented. The children were asked to drink 1.5 dl investigational product every day, also on those days when they did not attend day-care.
Subjects
The study was conducted in 71 day-care centres in the Oslo area of Norway. The parents of 240 children aged 12–36 months gave their consent to participation. Eligible children had to have their first winter season in a day-care centre with at least 10 children and, in order to participate, the children had to be present 5 days per week (6 h per day). Children could not be breast-fed during the study, and children who had an allergy to milk proteins or strong lactose intolerance, or who would use other probiotic bacteria during the study, were excluded from participation.
Study design
The study was conducted over 7 months from October 2004 to April 2005. After obtaining written consent for participation from at least one of the parents, the children were randomly allocated to one of the two treatment groups. All participating subjects received the investigational milk in the day-care centre in addition to receiving the milk at home during weekends and vacations.
Information about the children's health was collected by use of a diary completed by the parents. Information about whether the child was healthy, information about absence and reason for absence from day-care, and information about disease symptoms was collected. In particular, questions regarding symptoms of respiratory and gastrointestinal infections were asked. Fever (37.5°C or above), cough, sore throat, running nose, ear pain and wheezing were listed as symptoms of respiratory infection, and diarrhoea (two or more loose or watery stools per 24 h), vomiting, nausea, abdominal pain and decreased appetite were listed as symptoms of gastrointestinal infection. The child was considered to have a respiratory or gastrointestinal infection if two of the listed possible symptoms for each disease group were present.
Compliance was assessed by the day-care staff according to six defined categories indicating how often the child did or did not drink the investigational milk product. The categories ranged from ‘the child always drinking the milk’ to ‘the child not drinking the milk several times every week on average’. In addition, the compliance for days away from day-care centre was calculated from information in the diaries.
Statistical analysis
The statistical analyses were undertaken on both the intention-to-treat and the per-protocol populations using the Statistical Analysis Systems software package version 9.1.3 (SAS Institute, Cary, NC, USA). The primary efficacy variable, ‘number of days with symptoms of gastrointestinal and/or respiratory infection’, was analysed using a Student's t test for independent groups, transforming the data taking the square root, as the assumption of normal distribution did not seem to hold for the untransformed data. Also the secondary variables were analysed using a Student's t test for independent groups, transforming the data taking the square root, or the Mann-Whitney test if the assumption of normal distribution could not be met for the transformed values. As transformed values were used for statistical tests, no confidence intervals have been given. The mean for non-transformed values has been stated, together with the p value and test used.
Ethics and administration
The study was conducted in accordance with the Edinburgh, Scotland (2000) amendment of the Declaration of Helsinki 1964, and the protocol and informed consent form were approved by the Regional Ethics Committee of Health Region East, Norway. TINE BA, Oslo, Norway was responsible for the monitoring and follow-up of the study and for overall quality assurance. An independent contract research organization (Smerud Medical Research International AS, Oslo, Norway) was responsible for the statistical analyses of the study.
Results
Six hundred subjects were planned for the study. Due to limitations in recruitment of day-care centres only 240 subjects were finally included in the study. Of these, 117 were randomized to the active Biola group and 123 to the placebo group. No diary data were available for 41 children, which excluded them from the intention-to-treat analysis. The remaining 199 children were defined as the intention-to-treat population of the study. The child was considered compliant if the intake of investigational milk was above 60% on days away from day-care, and the child had not more than three consecutive days without milk in addition to being assessed by day-care staff as drinking the milk at least ‘every day except once every two weeks on average’. As many as 180 children had a too low compliance rate to be included in the per-protocol population (almost all children drank milk on a daily basis in the day-care, but the compliance at home was low or not registered) or were excluded from the per-protocol population due to missing observations from more than 20 days, being breast-fed within the last 2 weeks prior to study start (indicating that they may have continued breast-feeding during the study) or being above the age of 36 months (1 child), leaving only 19 subjects in the per-protocol population ().
Figure 1. Disposition of subjects. Most children had several reasons for being excluded from the per-protocol population. PP, per-protocol; ITT, intention-to-treat.
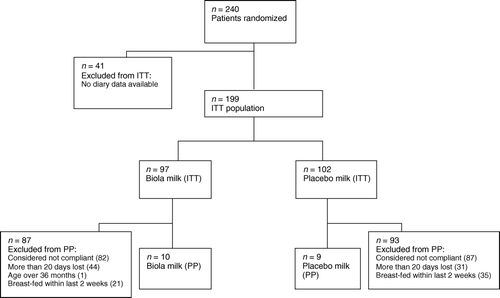
No significant differences were seen in characteristics at baseline for the two treatment groups with respect to gender, age, number of siblings, exposure to tobacco smoke, animals in household, asthma, allergies, atopic eczema, or mothers’ use of probiotics during breast-feeding. In all, 9% of the children in the Biola group and 4% of the children in the placebo group had been taking other probiotics prior to study inclusion. The mean age of the children at study start was 18 months.
Symptoms of gastrointestinal and respiratory infections
There was no significant difference between the two groups when analysing number of days with gastrointestinal and/or respiratory symptoms (26.5 days for the Biola group versus 26.9 days for the placebo group, p=0.52, t test). Looking at the gastrointestinal symptoms alone, subjects receiving Biola had a lower number of days with symptoms than the subjects receiving placebo (1.7 days for the Biola group versus 3.0 days for the placebo group, i.e. a 43% lower mean for the Biola group, p=0.02, Mann-Whitney test). When analysing the per-protocol population, the result was confirmed with a 74% lower mean for the Biola group (0.7 days for the Biola group versus 2.7 days for the placebo group, p=0.048, t test). No significant difference was seen with respect to number of days with respiratory symptoms alone (25.4 days for the Biola group versus 25.1 days for the placebo group, p=0.63, t test). See for details of symptoms and absence due to symptoms for the intention-to-treat population.
Table I. Details of symptoms and absence due to symptoms for the intention-to-treat population.
The number of days absent from day-care due to gastrointestinal or respiratory symptoms was not significantly different between the two groups (7.5 days for the Biola group versus 8.5 days for the placebo group, p=0.16, t test).
The length of periods (in days) with symptoms was significantly shorter for the Biola group as compared with the placebo group when analysing the per-protocol population (3.1 days for the Biola group versus 5.9 days for the placebo group, p=0.02). The same variable analysis of the intention-to-treat population did not support a conclusive interpretation of the data, as the mean value for number of days was slightly higher for the Biola group due to a higher maximum value (5.4 days for the Biola group versus 4.7 days for the placebo group, p=0.88, Mann-Whitney test). It should be noted that the number of periods with symptoms was slightly higher for the placebo group in the intention-to-treat population. However, the mean value and the difference between the two groups are not statistically significant for the intention-to-treat population.
Discussion
This placebo-controlled study indicated that Biola (a milk product with three probiotic bacteria: LGG, L. acidophilus LA-5 and Bifidobacterium Bb-12), may reduce the number of days with gastrointestinal symptoms in children having their first winter in day-care.
The mechanism of action for probiotics is considered to be related to both enhancement of the innate immune system and balance in the microbiota, in addition to more specific immune reactions. The local effects are considered important in the prevention of diarrhoea. By administration of probiotics the proportion of potential harmful bacteria is kept low, and mucosal permeability is decreased. The adhesion of pathogens on the intestinal mucosa further seems to be reduced in the presence of probiotics Citation[18]. Strengthening of local and systemic immune response is considered important in the probiotic effect of reducing risk of several kinds of infections.
In infants, where the intestinal microflora is not fully developed, the potential immune effects of probiotics are of particular importance, as the type of bacteria in the intestinal microflora and the timing of colonization might affect the immune development Citation[19], Citation[20]. A change in the microbiota is seen upon weaning and introduction of solid foods, and an intestinal microbiota similar to that of an adult is not seen until 2–3 years of age. In our study children were aged 12–36 months, and from the current knowledge, it is not expected that the three-strain probiotic administered should impact the normal development of the immune system by displacing naturally occurring bacteria and their immunomodulatory effects Citation[19]. Probiotics are generally considered safe Citation[21], but more studies on long-term effects on immune development in children are required. Probiotic bacteraemia or fungaemia have been described, but only in patients with underlying diseases Citation[19].
Several studies, including meta-analyses, have evaluated the effect of LGG on gastrointestinal symptoms, in particular diarrhoea in children Citation[22], Citation[23], and the indicative results of our study are in line with these previous studies, i.e. LGG is effective in the prevention and treatment of diarrhoea.
However, our study failed to demonstrate an effect on number of days with respiratory symptoms. It is important to notice that the previously referenced Finnish study Citation[9] could also not demonstrate a significant difference between the Lactobacillus GG group and the control group with respect to the number of days with respiratory symptoms, although a relative reduction in the number of children suffering from respiratory infections was seen (unadjusted for age). The results of an Israeli study in 201 children (4–10-month-old babies) in day-care centres, i.e. approximately the same size as our study, demonstrated a positive effect of probiotics on diarrhoea, but no effect on respiratory illnesses Citation[11]. Thus neither the Israeli group nor our group were able to confirm the findings of Hatakka et al. with respect to respiratory symptoms Citation[9]. On the other hand Hatakka et al. had a much larger sample size compared with our studies.
Hatakka et al. Citation[9] speculated that their finding of negative correlation between the number of days with gastrointestinal symptoms and the dose of Lactobacillus milk may indicate a dose-dependent response for this variable. The amount of Lactobacillus GG was 103 higher in our study compared with the Finnish study, in addition to including two other probiotics in the test formulation, and our findings might consequently support this theory; i.e. the difference with respect to gastrointestinal symptoms in the two studies might be due to the dose difference. This is also in line with the theories for mechanism of action of probiotics including local intestinal effects, such as competition for nutrients and attachment sites in the intestine.
It is commonly known that, after initial colonization with probiotics, a strictly daily intake is not required for probiotics to exert their effect. Upon initial colonization with > 109 bacteria, the bacterial strains will normally be present in high numbers for 1–2 weeks Citation[24]. A strict compliance requirement for milk intake was set for the per-protocol population in our study. In practice, a few days missed during a 7 months study should not impact the results of the study. The majority of the children had a very good compliance in the day-care environment, although they did not drink the milk at home. Still it is worth noting that a larger difference with respect to number of days with gastrointestinal symptoms was seen for the group following the study protocol completely as compared with the intention-to-treat population.
Consumption of probiotic products (with the exception of the investigational milk) at home despite the information about such use being prohibited during the study, could have introduced bias to the study. Hatakka et al. Citation[9] found Lactobacillus GG-like bacteria in 15% of the control subjects in their Finnish study in day-care centre children. Although not tested, it is likely that a similar result is applicable to the Norwegian study, as Biola and similar commercially available probiotic products are quite common in a Norwegian household.
As evident from , 56 children were breast-fed within the last 2 weeks prior to study start and potentially also during the study, although prohibited according to the protocol. A study in breast-fed infants versus formula-fed infants Citation[25] demonstrated protection against acute respiratory infection and diarrhoea by lower incidence and percentage of days ill, and episodes of shorter duration. Breast-feeding in the present study could have introduced further bias to the interpretation of the results.
Conclusions
In conclusion, the present study indicates that the probiotics LGG, Bb-12 and LA-5 may reduce number of days with gastrointestinal symptoms in children experiencing their first winter in day-care (1.7 days for the Biola group versus 3.0 days for placebo, i.e. a 43% lower mean for the Biola group, p=0.02). No significant difference between treatments was seen with respect to respiratory symptoms.
Declaration of potential conflicts of interest
Stein-Erik Birkeland and Anette Roll Mosland are employed by TINE BA, which manufactures the study product, Biola, and supported the study financially.
Charlotte Ramstad Kleiveland, Gisle Grave and Hilde Kloster Smerud are all employed by Smerud Medical Research, a clinical research organization receiving consultancy fee from TINE BA for its work related to the study.
Acknowledgements
We would like to acknowledge the dedicated assistance of the staff of the participating day-care centres. The study products were provided by TINE BA, Oslo, Norway, which also supported the study financially. We would also like to thank Marianne Hope, TINE BA, for valuable comments on the manuscript. Stein-Erik Birkeland and Anette Roll Mosland are employed by TINE BA, which manufactures the study product, Biola, and supported the study financially. Charlotte Ramstad Kleiveland, Gisle Grave and Hilde Kloster Smerud are all employed by Smerud Medical Research, a clinical research organization receiving consultancy fee from TINE BA for its work related to the study.
References
- Wald ER, Guerra N, Byers C. Frequency and severity of infections in day care: three-year follow-up. J Pediatr 1991; 118(4 Pt 1)509–14
- Nafstad P, Hagen JA, Oie L, Magnus P, Jaakkola JJ. Day care centers and respiratory health. Pediatrics 1999; 103(4 Pt 1)753–8
- Louhiala PJ, Jaakkola N, Ruotsalainen R, Jaakkola JJ. Day-care centers and diarrhea: a public health perspective. J Pediatr 1997; 131: 476–9
- Louhiala PJ, Jaakkola N, Ruotsalainen R, Jaakkola JJ. Form of day care and respiratory infections among Finnish children. Am J Public Health 1995; 85: 1109–12
- Nurmi T, Salminen E, Ponka A. Infections and other illnesses of children in day-care centers in Helsinki. II: The economic losses. Infection 1991; 19: 331–5
- Guarner F, Schaafsma GJ. Probiotics. Int J Food Microbiol 1998; 39: 237–8
- Isolauri E, Kirjavainen PV, Salminen S. Probiotics: a role in the treatment of intestinal infection and inflammation? Gut 2002;50(Suppl 3):III54–III59.
- Ouwehand AC, Salminen S, Isolauri E. Probiotics: an overview of beneficial effects. Antonie Van Leeuwenhoek 2002; 82: 279–89
- Hatakka K, Savilahti E, Ponka A, Meurman JH, Poussa T, Nase L, et al. Effect of long term consumption of probiotic milk on infections in children attending day care centres: double blind, randomised trial. BMJ 2001; 322: 1327
- Rosenfeldt V, Michaelsen KF, Jakobsen M, Larsen CN, Moller PL, Tvede M, et al. Effect of probiotic Lactobacillus strains on acute diarrhea in a cohort of nonhospitalized children attending day-care centers. Pediatr Infect Dis J 2002; 21: 417–19
- Weizman Z, Asli G, Alsheikh A. Effect of a probiotic infant formula on infections in child care centers: comparison of two probiotic agents. Pediatrics 2005; 115: 5–9
- Arvola T, Laiho K, Torkkeli S, Mykkanen H, Salminen S, Maunula L, et al. Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respiratory infections: a randomized study. Pediatrics 1999; 104: e64
- Pedone CA, Arnaud CC, Postaire ER, Bouley CF, Reinert P. Multicentric study of the effect of milk fermented by Lactobacillus casei on the incidence of diarrhoea. Int J Clin Pract 2000; 54: 568–71
- Saavedra JM, Bauman NA, Oung I, Perman JA, Yolken RH. Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus. Lancet 1994; 344: 1046–9
- Gluck U, Gebbers JO. Ingested probiotics reduce nasal colonization with pathogenic bacteria (Staphylococcus aureus, Streptococcus pneumoniae, and beta-hemolytic streptococci). Am J Clin Nutr 2003; 77: 517–20
- Goldin BR, Gorbach SL, Saxelin M, Barakat S, Gualtieri L, Salminen S. Survival of Lactobacillus species (strain GG) in human gastrointestinal tract. Dig Dis Sci 1992; 37: 121–8
- Saxelin M, Ahokas M, Salminen S. Dose response on the faecal colonisation of Lactobacillus strain GG administered in two different formulations. Microb Ecol Health Dis 1993; 29; 6: 119–22
- Servin AL. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev 2004; 28: 405–40
- Boyle RJ, Robins-Browne RM, Tang ML. Probiotic use in clinical practice: what are the risks?. Am J Clin Nutr 2006; 83: 1256–64
- Gronlund MM, Arvilommi H, Kero P, Lehtonen OP, Isolauri E. Importance of intestinal colonisation in the maturation of humoral immunity in early infancy: a prospective follow up study of healthy infants aged 0–6 months. Arch Dis Child Fetal Neonatal Ed 2000; 83: F186–F192
- Salminen S, Bouley C, Boutron-Ruault MC, Cummings JH, Franck A, Gibson GR, et al. Functional food science and gastrointestinal physiology and function. Br J Nutr 1998; 80(Suppl 1)S147–S171
- Huang JS, Bousvaros A, Lee JW, Diaz A, Davidson EJ. Efficacy of probiotic use in acute diarrhea in children: a meta-analysis. Dig Dis Sci 2002; 47: 2625–34
- Van Niel CW, Feudtner C, Garrison MM, Christakis DA. Lactobacillus therapy for acute infectious diarrhea in children: a meta-analysis. Pediatrics 2002; 109: 678–84
- Alander M, Satokari R, Korpela R, Saxelin M, Vilpponen-Salmela T, Mattila-Sandholm T, et al. Persistence of colonization of human colonic mucosa by a probiotic strain, Lactobacillus rhamnosus GG, after oral consumption. Appl Environ Microbiol 1999; 65: 351–4
- Lopez-Alarcon M, Villalpando S, Fajardo A. Breast-feeding lowers the frequency and duration of acute respiratory infection and diarrhea in infants under six months of age. J Nutr 1997; 127: 436–43
