Abstract
The paper lays out the short scientific history and characteristics of the new probiotic Lactobacillus fermentum strain ME-3 DSM-14241, elaborated according to the regulations of WHO/FAO (2002). L. fermentum ME-3 is a unique strain of Lactobacillus species, having at the same time the antimicrobial and physiologically effective antioxidative properties and expressing health-promoting characteristics if consumed. Tartu University has patented this strain in Estonia (priority June 2001, patent in 2006), Russia (patent in 2006) and the USA (patent in 2007). The paper describes the process of the identification and molecular typing of this probiotic strain of human origin, its deposition in an international culture collection, and its safety assessment by laboratory tests and testing on experimental animals and volunteers. It has been established that L. fermentum strain ME-3 has double functional properties: antimicrobial activity against intestinal pathogens and high total antioxidative activity (TAA) and total antioxidative status (TAS) of intact cells and lysates, and it is characterized by a complete glutathione system: synthesis, uptake and redox turnover. The functional efficacy of the antimicrobial and antioxidative probiotic has been proven by the eradication of salmonellas and the reduction of liver and spleen granulomas in Salmonella Typhimurium-infected mice treated with the combination of ofloxacin and L. fermentum strain ME-3. Using capsules or foodstuffs enriched with L. fermentum ME-3, different clinical study designs (including double-blind, placebo-controlled, crossover studies) and different subjects (healthy volunteers, allergic patients and those recovering from a stroke), it has been shown that this probiotic increased the antioxidative activity of sera and improved the composition of the low-density lipid particles (LDL) and post-prandial lipids as well as oxidative stress status, thus demonstrating a remarkable anti-atherogenic effect. The elaboration of the probiotic L. fermentum strain ME-3 has drawn on wide international cooperative research and has taken more than 12 years altogether. The new ME-3 probiotic-containing products have been successfully marketed and sold in Baltic countries and Finland.
- animal experiments
- antimicrobial activity
- antioxidative activity
- atherosclerosis
- atopic dermatitis
- blood lipids
- cerebral stroke
- enteric infections
- food supplements
- functional food
- glutathione
- human clinical trials
- intestinal microbiota
- inflammation
- isoprostanes
- lactobacilli
- Lactobacillus fermentum ME-3
- oxidative stress
- oxidized LDL
- post-prandial lipidaemia
- probiotics
- probiotic patents
- typhoid nodules
Introduction
The host and its microbiota together form a complex ecological system. Despite temporary or sometimes even long-lasting imbalance due to various exogenous and endogenous influences, its individual stability is still achieved by different mechanisms. However, the impaired composition of the microbiota has been associated with several health consequences, which could be avoided by regulation of the microbe and host interrelations. Probiotics aimed at stabilizing the microbial communities and the health-promoting effects have an important position in the medical health care of different age groups and diseased persons.
Diversity and functions of intestinal microbiota
The gastrointestinal (GI) microbiota of human beings is a dynamic and complex mixture of microbes consisting of bacteria, archaea, protists (ciliates), anaerobic fungi and different bacteriophages and viruses Citation[1]. It has several diverse functions including the decomposition of different nutrients, the maturation of intestinal cells, morphology and gut physiology, stimulation of the immune system, systemic effects on blood lipids and inhibition of harmful bacteria Citation[2]. The number of microbes harboured in the human body is nearly 10 times more than the number of somatic cells: on the skin there are 1012, in the mouth 1010 and in the intestinal tract 1014 microbial cells. The interrelation between the host and its microbiota – both commensals and pathogens – is the subject of the microbial ecology of the human organism in health and disease.
The microbial biotope produces an environment for microorganisms with similar conditions and volume. New microorganisms are constantly arriving into the open system, yet they do not stay. During the years 1960–1970, research by R. Dubos, R.W. Schaedler and D. Savage introduced an understanding of indigenous microbial flora, which was composed from autochthonous (endogenous, specific for the host species) and allochthonous (exogenous, occasional) microbes Citation[3–5]. In addition, they recognized that two types of microbiota can be differentiated in the cavities of particular organs: the luminal and mucosal microbiota. In different microbial biotopes the mucosal microbiota is composed of the autochthonous microbes that have become resident due to their adhesion to eukaryotic cells via receptors or by harbouring in the mucin layer of mucosal membranes. The species composition of mucosal microbiota is individually specific and stable. In contrast, the luminal microbiota consists of some resident unattached microbes, autochthonous microbes released from different vertical biotopes of the GI tract and the transient allochthonous microbes from diet, water and the environment. The latter stay in the given biotope for a short time, then are killed or out-competed by the resident flora. The interrelations between luminal and mucosal microflora and its stability in experimental animals and humans, detected by earlier studies Citation[6–9], have been confirmed by modern molecular methods. By using denaturing gradient gel electrophoretic analysis (DGGE), the mucosa-associated bacterial community was found to be uniformly distributed along the complete colon Citation[10–12]. However, there are also quite different understandings available: applying the fluorescent situ hybridization method (FISH) using 16S rRNA-targeted oligonucleotide probes, some authors suggest that colonic microbes are not in close contact with the mucosa and no significant differences exist between the colonic biopsies and faecal samples Citation[13]. The individual specificity Citation[14], Citation[15] on one hand and the temporal modulation of the intestinal microbiota by different types of diet (vegetarian, western type, etc.) and stress on the other hand complicate the evaluation of different research results at the GI ecosystem level Citation[16–18]. The application of novel experimental sets Citation[19] and sequence-based methods for the determination of microbial diversity and their functions, aptly called ‘the second Human Genome Project’, will clearly advance our knowledge of human microbial ecology Citation[20–22].
Particular environmental microbes serve as an assumption for the individually specific composition of the indigenous microbiota of a newborn. It clearly depends on the spectrum of the mother's microbes, with which the newborn gets in close contact, on the one hand, and the individual receptor specificity/activity of its own cells, on the other hand Citation[14], Citation[22], Citation[23]. There is the possibility that the special genes of the host are involved in the determination of the particularly different characteristics of microbiocenosis. Some 20 years ago Dutch scientists showed that in the case of Crohn's disease in monozygotic twins, the quantitative composition of normal faecal microflora was genetically determined Citation[24]. Importantly, the data showed that the pattern of antibodies directed to the faecal bacteria of different morphotypes was unique for each individual, thus confirming the genetic influences of the host on their indigenous microbiota Citation[25], Citation[26].
Using bacteriological methods, we have postulated that the quantitative composition of the faecal microflora of adult monozygotic twins has the same degree of similarity as the paired samples of a single young healthy person Citation[8], Citation[14]. The comparison of DGGE profiles in the faecal samples of monozygotic twins and unrelated persons confirmed our earlier findings on the higher similarity of the twins Citation[22]. Monozygotic twins reveal the identity of many genetic markers that are important for the selective colonization of the indigenous microflora. It is well known that both the antigenic structure of somatic cells and their secretions, as well as the immune reactivity, are determined by the genotype.
The composition of the intestinal microbiota (IMB) has been determined by several endogenous mucosal and luminal factors in the GI tract () Citation[1], Citation[2], Citation[27–30].
Table I. Main endogenous factors determining the composition of the intestinal microbiota (IMB) Citation[1], Citation[2], Citation[27–30].
The colonization resistance (CR), defined in the early 1970s Citation[31], is the most important first-line defence against invasive pathogenic organisms such as Clostridium difficile, Salmonella, Shigella, Candida albicans, etc. and indigenous opportunistic microbes such as the urinary tract infection-causing Escherichia coli, Proteus sp., Pseudomonas sp. CR is a dynamic phenomenon influenced by the host, diet and medical interventions. Antibiotic therapy can decrease the CR some 1000-fold Citation[2]. There are several limitations in the protection by lactobacilli against the development of antibiotic-associated diarrhoea, although some association between the counts of intestinal lactobacilli and CR against Clostridium difficile has been found. Mainly, these depend on the species-specific antibiotic susceptibility of the individually different Lactobacillus spp. composition and whether or not the gut environment supports the survival of the administered probiotic lactic acid bacteria Citation[32].
The predominant microbial species of different GI tract niches (oral cavity, stomach, jejunum, ileum and colon) are not similar, depending on the individual conditions of the biotope. However, over the GI tract the same groups of bacteria predominate, e.g. firmicutes, bacteroidetes, actinobacteria, proteobacteria, fusobacteria and the uncultured groups Citation[33]. In the gastric cavity at physiological acidity, the number of bacteria reaches 103 cfu/g, with gram-positive microbes predominating (lactobacilli and streptococci). It is well known that the number of microbes (cfu/g) increases down the intestinal tract, with anaerobic bacteria outnumbering the aerobic ones by nearly a thousand times. In the colon at different ages (1010–11 cfu/g), anaerobes such as Bacteroides, eubacteria, bifidobacteria, peptostreptococci and fusobacteria predominate over the Lactobacillus/Enterococcus spp., coliforms and staphylococci assessed by cultivation on selective and non-selective media Citation[2]. The cultivable anaerobic clostridia are exceptions, including Clostridium perfringens and Clostridium difficile, whose counts do not exceed 105–6 cfu/g in adults Citation[2], Citation[8]. New possibilities for discovering the entity of the microbiota were achieved by the development of experimental animal studies, as well as studies on germ-free animals Citation[34].
A 100–1000-fold difference has been found when using bacteriological and molecular methods for IMB studies; however, the crude understanding of the predominant bacteria (bacteroids, anaerobic cocci, eubacteria, bifidobacteria) according to the bacteriological studies of our laboratory remains quite similar to those obtained by molecular methods Citation[35–40]. The main difference is in some unculturable genera like Atopobium,Clostridium coccoides and Clostridium leptum groups, whose relative abundance in total count is 3.1–11.9%, 23–28% and 21–25%, respectively, estimated by the application of 16S rDNA techiques and molecular genetic probes Citation[41–43].
As regards the other groups of bacteria, the main differences can be found in the susceptibility to environmental factors. For example, Bifidobacterium adolescentis is mainly detected by molecular methods due to its high susceptibility to oxygen Citation[44] and thus can be frequently omitted in bacteriological studies.
Impact of IMB on host metabolism
It is well known that the microbial community of the GI tract helps the host with the processing of nutrients, also producing vitamins and butyrate. The specific host–microbe interactions have been successfully studied by the estimation of different metabolites of the host, derived by microbiota. The concept of expression of the microflora-associated characteristics (MACs) in a macroorganism Citation[45] has been further elaborated for a microbial–host crosstalk Citation[46] having considerable value, especially when comparing the MACs with GACs, i.e. germ-free animal characteristics Citation[34]. Nowadays it has been shown that the Toll-like receptors on the different cells of the host recognize the microbiota and form the basis for the crosstalk, which can shape even the maturation and immunity of the GI cells Citation[29], Citation[47].
Nearly 20 years ago, we found a close correlation between the counts of different groups in the IBM and some secreted metabolic compounds (volatile phenols of urine) in monocygotic twins Citation[8], Citation[48]. To date, the microbial activity can be measured using culture, labelled biomarkers, RNAs, proteins and different metabolites. The new possibilities for linking the microbial and host metabolic activities evolved with the development of new molecular/biochemical technologies Citation[49]. The genomes of several bacteria have been sequenced, elucidating the ecological success of different bacterial groups in the different parts of the GI tract. For example, Lactobacillus plantarum has been characterized by a very large number of phosphotransferase systems granting their success in energy accumulation, but both bacteroidetes and some bifidobacteria had an enormous number of genes involved in the utilization of complex carbohydrates Citation[50]. The human metagenome is a composite of the individual host genes and the genes present in the genomes of the trillions of microbes colonizing human bodies. Our microbial genomes, i.e. microbiome, encode metabolic capacities that we have not had to evolve on our own Citation[51]. Several new methodical approaches are in development. Transcriptional profiling (transcriptomics) can be used in experimental models to find groups of bacteria that have switched to the expression genes involved in the utilization of different exposed substances Citation[52]. Specific activity can also be analysed using a metabolomic approach to study the influence of drugs and diet on the interactions between humans and the GI tract microbiota.
Members of the 70 well-known divisions of BacteriaCitation[53], such as the Bacteroidetes and Firmicutes, consist of more than 90% of all phylogenetic types in humans. Their role has remained largely unexplored; however, their balance was recently associated with the pathophysiology of obesity in animals and humans Citation[54]. It was shown that if fasting-induced adipocyte factor (FiaF) expression was repressed by microbes, the adipocytes increased triglyceride production. Thus, the GI microbiota also affects the energy harvest and storage Citation[55].
Recently, we have found in elderly persons over 65 years that the intestinal Lactobacillus sp. is closely bound to human inflammatory and metabolic markers of blood. The count of live lactobacilli showed a close negative correlation with an important lipid metabolism marker – the oxidized LDL (oxLDL) of blood. Moreover, colonization with L. fermentum was negatively associated with blood glucose level, seemingly showing its high potential for carbohydrate metabolism (both hexoses and pentoses) in the gut, thus limiting its absorbance into the blood (M. Mikelsaar et al., unpublished observations). The application of particular species of probiotic lactobacilli seemingly could be a challenge for health care to control the metabolic and systemic defence reactions of the elderly, including oxidative stress-related ones. This all suggests the possibility to influence the host metabolism by modulating the intestinal microbiota by administering useful beneficial bacteria.
Why do we need functional food and probiotics?
The disruption of the CR-granting indigenous microbiota, thus allowing potentially pathogenic microorganisms to multiply, can be attributed to a variety of illnesses. The high load of disinfectants
For health promotion: to compete withthe depletion of microbial variety, granting defence against infection and allergies; to compete with oxidative stress prone changes.
In wealthy societies the increased stress of life, the increasing number of elderly people and reduced physical activity are considered the reasons for the large spread of civilization-associated chronic diseases such as atherosclerosis, hypertonia, tumours, diabetes, peptic ulcers, neurodegenerative diseases and different syndromes such as adipositas, fatigue and depression. The crucial role of the impaired host functions is attributed to a wide consumption of processed foods that are very rich in sucrose, saturated fat and sodium. At the same time, a lot of foods are characterized by a deficiency of a number of human nutrients such as omega-3 fatty acids, arginine, glutamine, taurine, vitamins and antioxidants Citation[56].
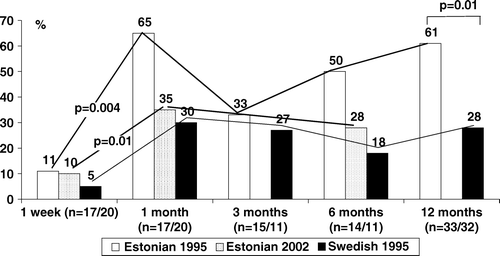
Artificially created environments free of bacteria contribute to the development of allergies. The important role of the IMB, particularly lactic acid bacteria, has been demonstrated in communities with different degrees of industrialization. At the beginning of the 1990s Strachan et al. Citation[57] presented the ‘hygiene hypothesis’ to explain the differences in the industrialized and non-industrialized world. In families with more siblings and living in rural areas, there were fewer allergies than in families with just one child and living in highly hygienic conditions; this was explained in terms of the increased priming of the immune system with different infections prevalent in bigger families. However, in Australia, Patrick Holt with co-authors Citation[58] linked the problem with an imbalance of IMB, which is the richest potential immunomodulator in an infant's organism. IMB differences were found in young children living in countries with different degrees of industrialization Citation[59–61], mostly differing in hygienic conditions. In Estonia, infants were more often colonized with lactobacilli during the first year of life than their Swedish counterparts (). However, 5 years after Estonia regained its independence from the Soviet system, the increased income of the people and the improvement of the food hygiene reduced the differences in the prevalence of lactobacilli between the Estonian and Swedish infants Citation[62].
Moreover, prospective studies of an infant's colonization by indigenous microbiota in children developing or not developing allergies showed clear differences, expressed in the lowered colonization resistance of allergic children Citation[39], Citation[40], Citation[63]. The hygiene hypothesis is now revisited concerning the reduction of microbial variety in the environment resulting in fewer signals to immune system. The possibility to prevent or treat an allergy by increasing the richness of microbial communities using probiotics has attracted wide attention from researchers and practitioners Citation[63–65].
Probiotics – evidence-based impact on health
A probiotic is defined as a live microorganism which when administered in adequate amounts confers a health benefit on the host Citation[66]. Widely accepted probiotics contain different lactic acid-producing bacteria of human origin: bifidobacteria, lactobacilli or enterococci. The area of commensal, non-harmful bacteria of human origin serving as probiotics is rapidly expanding and in 2007 more than 3100 scientific publications were cited in the PubMed database.
Widespread agreement in understanding the probiotics area includes that the probiotic strains should be safe, effective and stable in the final product. Internationally accepted criteria have been proposed to consider the selected microbes as probiotics Citation[66].
Yet, more importantly, up to now neither sufficient nor indicative criteria for ‘probiotic status’ have been defined. There are different in vitro and in vivo assays for testing the functional properties and the putative effectiveness of candidate probiotic strains for general health benefits or against specified diseases, recently revised by a project group Joint IDF/ISO Action Team on Probiotics Citation[67]. The search for new effective strains is an expanding process. Still, the main problem is not due to the limited discovery of new strains with new functional properties but in proving their action in vivo. The mechanisms granting the survival and competitiveness of the probiotics in different microbial ecosystems and action sites have not been well explored. The application of different clinical trials was the suggested clue.
The ILSI symposium Citation[68] drafted three different levels of probiotic action: 1) direct interactions with gut microbiota, including pathogens, relying on colonization resistance mechanisms; 2) fortification of the gut barrier function by influencing the quality of tight junctions; 3) modulation of the mucosal immune cells amount and activity and the systemic immune system. Thus, probiotics normalize the composition of the intestinal microbiota and modulate the immune functions of the host. Emerging evidence has revealed that the prevention of GI tract colonization by a variety of pathogens is a primary mechanism of the beneficial effects mediated by probiotics Citation[69–72]. Besides infection control, several gut microbes have been elaborated for use as probiotics in functional food, which aims to prevent and treat various other health problems such as allergy, neoplastic growth and inflammatory bowel diseases. There are also a few sound data about the impact of probiotics on wide metabolic functions of the host. The newer areas include the influence of probiotics on the metabolism of dietary components, like lactose digestion, lipid metabolism, proteins and indigestible dietary compounds. To explore the impact of different probiotics on the cardiovascular system and lipid metabolism, important biomarkers to blood cholesterols and triglycerides, conducted only in a few well-designed clinical studies. The important area of human physiology that is relevant to functional food science according to the ILSI and FUFOSE (the European Commission Concerted Action on Functional Food Science in Europe) is, among others, the modulation of defence against high-grade oxidative stress Citation[56].
Nowadays the concept of functional foods, including probiotic food and dietary supplements, implies their ability to beneficially influence body functions to improve the state of well-being and health and reduce the risk of disease Citation[73]. The provisional regulations of the ILSI and EU Research Commission (FUFOSE and PASSCLAIM: DOI.10.1007/s00394-005-1104-3) require sound evidence of ability to either balance and enhance the particular human functions or to reduce the risk of certain diseases through the use of probiotic microbes. Today, to define the health claims of a new probiotic, primarily two independent double-blinded, placebo-controlled studies are recommended. At the same time, the application of genomics, proteomics and metabolomics has begun to be involved in the elucidation of the mechanisms behind the interventions with useful bacteria Citation[49]. Several supporting and confounding intrinsic, ecological and technological factors may be of importance in the selection of suitable candidates for probiotics: properties of the strain; the metabolic capacity of the strain during passage through the GI tract; acid, bile and heat tolerance; and the ability to grow in milk and to metabolize different substrates, including prebiotics Citation[32], Citation[74–77].
The data depicted in , Citation[68], Citation[69], Citation[78–102] show clearly that the probiotic action, including various health promotions, is strain specific, as among the probiotic preparations there are representatives of all the prevalent species of lactobacilli and their health effects are largely variable.
Table II. Evidence-based probiotic lactobacilli according to randomized double-blind placebo-controlled studies (adapted from 68,69).
Elaboration of probiotic L. fermentum ME-3 at the University of Tartu
Short history of the scientific discovery of L. fermentum ME-3
L. fermentum ME-3 as an antimicrobial and antioxidative probiotic has been aimed at improving the oxidative stress (OxS) status of a consumer's organism and reducing the risk of enteral infections. The history of the discovery of L. fermentum ME-3 began in 1994, when joint research with Sweden started. The University of Linköping (Prof. Bengt Björkstén) initiated a scientific cooperative agreement between the Department of Pediatrics (Dr Kaja Julge, Dr Maire Vasar) and the Department of Microbiology of the University of Tartu (Prof. M. Mikelsaar) aimed at finding associations between allergies and intestinal microbiota. The University of Tartu had long-lasting experience in research into microbial ecology and the lactobacilli of the human organism (Prof. Akivo Lenzner). The faecal samples of Russian astronauts were investigated in Tartu for more than 20 years for detection of the composition of lactobacilli Citation[103], Citation[104]. Prof. B. Björksten has served as a visiting professor at the University of Tartu since 1994, teaching clinical science and advanced research principles to PhD students. For the allergy research he chose two comparative populations: Estonians with a low prevalence and Swedes with a high prevalence of allergy. Glaxo Wellcome Trust supported the study.
In this period more than 200 Lactobacillus strains of different species were collected Citation[38], Citation[105]. These strains have formed the basis of the culture collection of the Department of Microbiology, which is financed according to a national programme of collections in Estonia. On 2 March 1995, five L. fermentum isolates were obtained from a 1-year-old Estonian child (A.M., nr. 822). Paediatricians had previously confirmed the good health of the child, and it continues to be excellent at the age of 12 years. The microbiological work was performed by researchers Epp Sepp (MD, PhD), Paul Naaber (MD, PhD), Krista Lõivukene (MD, PhD), Mall Türi (Pharm. cand. med.), and senior research technician Eha-Mai Laanes. However, in this arena there could be no invention registered, as the strains of the L. fermentum species were also found from some other Estonian children, yet not from Swedes!
In 1996 the Dutch company MONA offered the University of Tartu an opportunity to test their collection of L. acidophilus strains for antioxidativite properties. The Dutch people relied on the research of Japanese authors Citation[106] who had found, with non-standardized methods, some single antioxidative lactobacilli strains among hundreds tested. We were introduced to MONA by docent Seppo Salminen of the University of Helsinki, our research partner in the first stages of the introduction of the probiotic Lactobacillus GG in Finland. In turn, we invited into the research the scientists from the Department of Biochemistry (Prof. Mihkel Zilmer) who had published the first interesting papers on the markers of cellular oxidative stress Citation[107–109]. Great efforts were made to get the antioxidative markers run with bacterial cultures, as the disruption of microbial cells without any enzymatic influence was complicated and quite different from the previous work of biochemists studying the antioxidativity by methods originally developed in blood and blood cells. It was quite hard to achieve the cell-free extracts with no growth, as some intact cells of lactobacilli were always found after disruption. Then we switched to the supernatant of the disrupted lactobacilli cells, comparing this with the response of intact cells.
The cooperative work between the microbiologists and biochemists succeeded in obtaining adequate samples; however, to great disappointment, no strains with good antioxidative properties were found from the Dutch MONA collection. In contrast, among the included lactobacilli of the Estonian and Swedish children from our own culture collection, the biochemist Tiiu Kullisaar found two promising L. fermentum strains (strains 822-1-1 and 822-1-4, used in the laboratory under the acronyms E-3 and E-18).
Now there was a growing hope for invention! The protocols for new research projects were developed with the participation of several other microbiologists (Heidi Annuk, Reet Mändar, Jelena Štšepetova and Epp Songisepp) and biochemists (Kersti Zilmer, Tiiu Vihalemm, Ceslava Kairane and Ann Kilk).
Then, suddenly, we made a substantial mistake: an abstract on the two promising antioxidative lactobacilli strains was presented in Kiel to the congress of the International Dairy Federation. The materials of this congress were published and this paper excluded the novelty of the strains for our patent application Citation[110]. Thus, the priority was shifted for the use of the antioxidative properties in the probiotic preparations of the strains. We had to go further with only one strain to develop an innovative probiotic with tested functional properties, that was safe for consumption and effective in animal and human trials. This work has been performed in close association with clinicians of the University of Tartu and research partners from Finland and Italy. The European Research Council 5th FW offered the possibility of a joint volunteer study by the application of the synbiotic preparation (including our antioxidative strain of L. fermentum) with scientists of Reading University, UK (Prof. Glen Gibson); Wageningen University, The Netherlands (Prof. Willem de Vos); Lund University, Sweden (Prof. Göran Molin) and Turku University, Finland (Prof. Seppo Salminen). The results are presented below.
Development of the probiotic L. fermentum ME-3
The development of the probiotic started according to provisional rules Citation[73], later to some extent comprising the regulations of the FAO/WHO Citation[66]. According to WHO/FAO experts, it is necessary to properly check the strain's systematic, i.e. its identification by phenotypic and genotypic methods, and deposit it in an international culture collection. At this stage the suitable acronym ME-3 was also developed and the strain was deposited in an international culture collection (Deutsche Sammlung für Mikroorganismen und Zellkulturen, DSMZ 14241).
The origin, habitat and species of the Lactobacillus strains have a great impact on their value as probiotics. L. fermentum is a normal inhabitant of the human intestinal tract in the UK Citation[111] and Italy Citation[112]. Some L. fermentum strains are able to metabolize cholesterol Citation[113]. There seems to be an age-dependent colonization by L. fermentum. In Greece, L. fermentum was not detected in children below the age of 3 years and the number of positive samples only increased with aging, reaching 72% in the elderly Citation[114].
In 1995, the prevalence of L. fermentum in the Estonian group of investigated 1-year-old children Citation[105] was also low (11%), whereas in adults and the elderly it reached 55–70% Citation[14].
The low prevalence of L. fermentum as a species in young children was indirectly confirmed with an immunoblot study of 25 healthy children (A-L. Prangli et al., unpublished observations). The different protein domains of the three tested species of indigenous lactobacilli (L. acidophilus, L. fermentum and L. plantarum) induced the production of IgG antibodies with differing prevalence in the sera of control children. IgG antibodies directed to L. fermentum ME-3 showed a lower number of immunoreactive protein MW regions (kDa) than to L. plantarum, whose species has been more frequently detected (33% vs 11%) than the former among the microflora in children Citation[105]. This finding correlates well with the results of previous studies showing that different Lactobacillus spp. could possess different immune system activating properties Citation[26], Citation[115], Citation[116] that may also be related to their colonization ability in different people.
It is well known that the main microbes of the normal microbiota of a child are obtained during labour from the mother's vagina and perineum, and from skin, breast milk and the maternity hospital environment. L. fermentum is a frequent inhabitant of the vagina Citation[14]. The successive colonization with a mainly gram-positive microbiota is speeded up if the child is breast-fed and in close contact with its mother Citation[36]. Afterwards, with the introduction of solid food the microbiota becomes more diverse. However, up to the second year of life an infant's microbiota is still different from an adult's as regards some groups of bacteria and their metabolic activities; yet it starts to more closely resemble the adult's microbiota Citation[117]. Seemingly, the maturation of the host immune and receptor systems and differences in consumed food are responsible for the usual discovery of L. fermentum in adults and not in young children. Thus, a child originally harbouring the ME-3 strain could be considered an exceptional one.
Proper identification
The probiotic strain ME-3 was identified according to its morphological () Citation[41], Citation[118], Citation[119] and cultural properties, negative catalase test, produces lysozyme, gas from glucose and NH3 from arginine, and the acidity in milk is relatively low (1.07%). According to the API 50 CHL kit (BioMerieux, France) analysed by API Lab Plus softwear, very good identification rates were obtained (ID 99.6%, T 0.87, only one test against). The strain is able to ferment ribose, galactose, D-glucose, D-fructose, D-mannose, aesculin, maltose, lactose, melibiose, saccharose, D-raffinose, D-tagatose and gluconate Citation[118], Citation[120].
Figure 2. Lactobacillus fermentum ME-3 (DSM 14241) Citation[118], Citation[119]. (a) Light microscopy, Gram stain, magnification×1000. (b) Fluorescent in situ hybridization (FISH). Probe: Lab 158, Lactobacilli + enterococci according to Franks et al. Citation[41].
![Figure 2. Lactobacillus fermentum ME-3 (DSM 14241) Citation[118], Citation[119]. (a) Light microscopy, Gram stain, magnification×1000. (b) Fluorescent in situ hybridization (FISH). Probe: Lab 158, Lactobacilli + enterococci according to Franks et al. Citation[41].](/cms/asset/232e941a-6c0b-47e9-90a9-712f3a9603f3/zmeh_a_381726_f0002_b.gif)
The internal transcribed spacer polymerase chain reaction (ITS-PCR) followed by enzymatic restriction was used to confirm the species identification of the strain as Lactobacillus fermentumCitation[105], Citation[118]. According to the changes in the previous taxonomy, the II biotype of L. fermentum has now been given distinct species status – L. reuteri with type strain DSM 20016, isolated from humans showing the close relationship of these two species Citation[69], Citation[99]. Further, in the patenting process in the US, it was necessary to prove the difference between ME-3 and the L. reuteri strain RC-5 (previously assessed as L. fermentum RC-5 Citation[69]). We used ITS-PCR followed by restriction by the Taq I enzyme and compared the restriction banding pattern with the reference strains of L. fermentum ATCC 14931 and L. reuteri DSM20016) (Štšepetova, laboratory protocol).
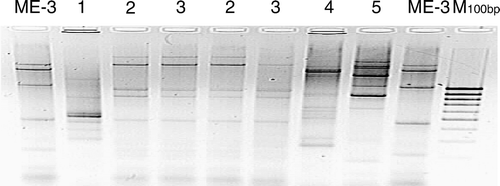
Further, the fingerprints of strain ME-3 (by arbitrarily primed PCR (AP-PCR) with two primers ERIC1 and ERIC2, DNA Technology A/S, Aarhus, Denmark) were compared with some other L. fermentum strains (), important in recognizing the strain in different materials such as faeces and functional food Citation[119–121].
Figure 3. AP-PCR fingerprints for different L. fermentum strains. The two lanes of strain ME-3 have been generated using two DNA samples that were extracted with a time interval of 6 months. Lanes 2 and 3 contain DNA of L. fermentum strains isolated from the same person. Lane M contains a 100 bp DNA ladder.
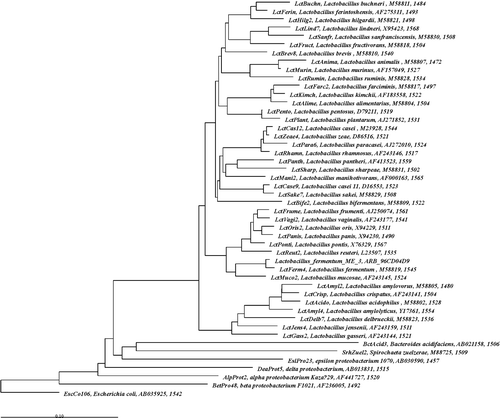
In the Dutch laboratory of Wageningen University (Professor Willem de Vos), the EU Marie Curie stipend Jelena Štšepetova succeeded in showing the position of strain ME-3 in the phylogenetic tree of Lactobacillus spp. () by sequencing the 16S rRNA of strain ME-3, cloning it into the Escherichia coli strain, sequencing using the Sanger method and constructing the phylogenetic tree (J. Štšepetova, unpublished observations).
Metabolic activity
According to the systematics developed by Kandler and Weiss Citation[122], the strain ME-3 belongs to the obligately heterofermentative lactobacilli with a characteristic type of metabolism. We have determined the metabolites of Lactobacillus sp. by the gas chromatographic method using the Hewlett-Packard model 6890 of gas chromatography () Citation[123].
Figure 5. Gas chromatography of polyamines of L. fermentum ME-3 in the decarboxylation medium with ornithine Citation[123].
![Figure 5. Gas chromatography of polyamines of L. fermentum ME-3 in the decarboxylation medium with ornithine Citation[123].](/cms/asset/6b8ff83d-24c9-464a-adc2-b42bfa3eeff2/zmeh_a_381726_f0005_b.gif)
The metabolism () was dependent on the environment, whereas in an anaerobic environment as compared to a microaerobic one, much more ethanol and succinic acids were produced Citation[121].
Table III. Production of short chain fatty acids (SCFAs), ethanol, polyamines, conjugated linoleic acid (CLA) and nitric oxide (NO) by L. fermentum ME-3 DSM 14241 in comparison with Lactobacillus plantarum DSM 21380.
Recently we have discovered that strain ME-3 is able to produce nitric oxide (NO) in MRS medium (). NO production was assessed by the Apollo free radical analyzer 400 in MRS fluid media (Oxoid, UK) after incubation for 48 h. The NO is able to induce among others the protection against inflammation, while in an ischaemic heart the NO can functionally activate the cellular antioxidant defence systems Citation[124].
In the decarboxylation medium Citation[125] containing amino acids such as arginine, ornithine, lysine and histidine, it was possible to assess the production of the polyamine putrescine and minimal amounts of biogenic amine cadaverine (<1 µl/ml) by ME-3. This amount is really minimal compared with E. coli, which produced significantly larger amounts of cadaverine (240 µl/ml) in the lysine media.
The metabolism of the putrescine is closely connected with organic acids metabolism, as in gut putrescine may be converted into the acetylated and oxidated succinate. Besides, it is known that some polyamines can serve as antioxidants Citation[126].
When strain ME-3 was grown in milk media for 4, 10, 20, 30 and 40 days, no biogenic amines were detected, confirming the safety of strain ME-3 for the production of functional food Citation[123].
Acid and bile tolerance
The definition of probiotic implies that the bacteria should be viable at the time of ingestion and after passage through the GI tract. This should be achieved by the ability of a particular probiotic strain to tolerate the highly acidic conditions present in the stomach and the concentrations of intestinal juices and bile salts found in the small intestine. Several in vitro assays, including tolerance to the harsh conditions of the digestive tract and adhesion to the host's gut epithelial cells, have been used to demonstrate the survival of the probiotic strain inside the gut Citation[127]. Usually after oral administration the expected results include faecal recovery of the strain and correction of the imbalance of intestinal microflora by the probiotic Citation[128], Citation[129]. However, it has not been assessed if the improvement of the microbial balance in the upper part of the digestive tract should always be expressed in the abundant microbial communities of the large intestine and faecal samples. In experimental animal studies, it has been shown that there was quite a few association (<30%) between the mucosal flora of the upper parts of the gastrointestinal tract and the fecal microflora Citation[23], Citation[35]. Pereira and Gibson Citation[113] have thoroughly studied the degree of acid and bile tolerance of the human isolate L. fermentum KC and have shown its ability to maintain viability for 2 h at pH 2 and to grow in a medium with 4 mg of bile acids per litre.
L. fermentum ME-3 was found to tolerate the drop of pH values from 4.0 to 2.5 without a loss in viable cell count. Even at pH 2.0 the strain survived for 6 h and only after that the numbers of viable cells fell rapidly Citation[118]. Strain ME-3 tolerated all the tested bile concentrations (0.3–2.0%) similarly well during 24 h without any remarkable loss in viable counts. By the co-action of pH and pepsin followed by bile and pancreatin, a decrease of 0.5 log to 1.5 log of viable count was noticed. Also, the producer of strain ME-3 freeze-dried culture (Probiotical OY, Novara, Italy) has confirmed the tolerance of strain ME-3 to harsh environmental conditions. Besides, the high stress tolerance of strain ME-3 in harsh GI tract conditions after a 3-week consumption of goat milk fermented by the addition of strain ME-3 was shown () by confirmation of strain ME-3 in faecal samples of 100% of volunteers by molecular methods Citation[101].
Lectin typing
The carbohydrate pattern of ME-3 cell surfaces was tested with different lectins in the laboratory of Professor Torkel Wadström (University of Lund, Sweden). Lectins are oligomeric and multimeric plant or animal proteins or glycoproteins with binding specificities toward a particular carbohydrate structure. Multimeric structure gives lectins the ability to agglutinate cells or form precipitates with glycoconjugates Citation[130]. The monosaccharides such as N-acetylglucosamine, N-acetylgalactosamine and mannose are present in the glycocalyx of lactobacilli in significant amounts in the typical gram-positive envelope Citation[131]. These sugars have been linked to adhesive properties of lactobacilli. L. fermentum ME-3 whole cells, similar to other tested lactobacilli strains of the same species, showed specificity to D-Gal, D-GalNAc carbohydrates by lectin Bandeirarea simplifolica I Citation[132]. Harbouring this profile seemingly helps strain ME-3 to compete with E. coli for adhesion to gal-gal receptors in the intestinal tract, where E. coli some virulent strains can be reservoir for recurrent urinary tract infection.
We have seen some effect in a clinical trial for preventing this recurrent infection in children. The data in the literature showed that re-infection with a new uropathogenic E. coli residing in the intestinal tract occurred in nearly 75% of adult women Citation[133]. We wondered if colonization with strain ME-3 could suppress the potentially pathogenic E. coli and decrease the recurrent episodes. In vitro experiments were promising, as the E. coli ATCC strains and several clinical isolates were suppressed on culture media for 2–3 log cfu/g. We hoped that this could decrease the load of uropathogenic E. coli in the intestinal tract to prevent new recurrent episodes of urinary tract infections. Children who had experienced a first attack of acute pyelonephritis consumed either strain ME-3 or a placebo for 3 months: in the placebo group, the recurrence rate was 50% while in the strain ME-3 group it was a somewhat lower 32% (Vainumäe, dissertation in preparation).
Antimicrobial activity
The prevention of GI tract colonization by a variety of pathogens is a primary mechanism of beneficial effects mediated by probiotics Citation[70], Citation[71]. It has been shown that the large spectrum of different metabolites is responsible for the suppression of the growth of pathogens in vitro and for their competitive exclusion in animal models. Many of the metabolites produced by lactic acid bacteria have a broad antimicrobial activity against some other species, especially gram-negative ones, in contrast to particular bacteriocins that usually inhibit only closely related species among other gram-positive bacteria Citation[134].
L. fermentum ME-3 has the ability to suppress mainly gram-negative bacteria but to some extent also enterococci and Staphylococcus aureus. Its antagonistic properties against enteral pathogens (Salmonella Typhimurium and Shigella sonnei), and urinary tract infections caused by E. coli, were assessed by a streak line procedure on plates containing modified (pH 7.2) MRS medium Citation[135–137]. In different environmental conditions (microaerobic and anaerobic milieus) the production of lactic acid by strain ME-3 correlated well with its antagonistic activity.
Beside lactic acid, the acetic and succinic acids and ethanol produced in substantial amounts by strain ME-3 in microaerobic conditions, the production of NO and H2O2 could be responsible for the antimicrobial effect (). In her PhD dissertation, Heidi Annuk was able to construct a diagram showing that the in vitro antagonistic activity, seemingly due to a pH drop and organic acids production, was quite characteristic of particular fermentative groups of lactobacilli (homo-, facultatively heterofermentative and obligately heterofermentative) further identified by ITS-PCR Citation[137].
Recently, we have found by the ROS analyser (APOLLO 4000) that the ratio of signals H2O2:NO was 13.7, produced by strain ME-3 in MRS medium, achieving the first rank among about 30 tested strains of Lactobacillus species. This shows that strain ME-3 is able to manage with both compounds to suppress antagonists and/or initiate signalling using several pathways ().
Remarkably, the modest antimicrobial activity of L. fermentum ME-3 Citation[138] expressing some antimicrobial cationic peptides against the Helicobacter pylori reference strain NCTC 11637 was changed for high activity against clinical H. pylori isolates Citation[139]. So far, the most effective management of H. pylori infection causing gastritis and peptic ulcer disease is combined antimicrobial therapy. Some authors have applied the antagonistic effect of lactic acid bacteria against H. pylori in the prevention of and therapy for H. pylori infection Citation[140], although some failures are also described.
Seemingly, a different tropism of the pathogen and probiotic, besides the inadequacy of the dosage and intervention, survival of the probiotic in the gut and Lactobacillus spp. differences in antagonistic activity and immunomodulating properties, could emerge among putative reasons for the low efficacy of probiotic therapy in GI infections Citation[141]. Moreover, the main mechanisms of probiotic action – such as competing for nutrients, producing antimicrobial substances, blocking the adhesion and toxin receptor sites, removing it by co-aggregation, stimulating the immunity, increasing the barrier function and the attenuation of virulence – have to be differentially expressed in extra- and intracellular infections, and in fact, they are rarely all present in a particular probiotic.
Thus, strain ME-3 possesses several antimicrobial characteristics such as acetic, lactic and succinic acids, putrescine, NO, CO2 and H2O2, produces some cationic peptides, has a suitable lectin profile for competitive adhesion to the epithelium and some immunogenic properties, as assessed in animal experiments and human studies.
Safety
First, lactobacilli and bifidobacteria are historically considered safe for their close association with food. Second, they are inhabitants of the normal indigenous microbiota and their pathogenic potential is low. However, some Lactobacillus spp. strains have been associated with systemic and local infections Citation[142]. They can cause a problem in a world with an increased immunocompromised population. Laboratory investigations, experimental animal studies and volunteer trials were conducted to test the safety of the ME-3 strain. The in vitro experiments confirmed the absence of haemolysins and the suppression of indigenous lactobacilli and bifidobacteria, tested in co-cultivation experiments with particular strains.
The most important feature for safety assurance suggested by a large EU study was the absence of transmissible antibiotic resistance genes/plasmids and the natural resistance to trimethoprim plus sulfamethoxazole, metronidazole, fluoroquinolones and cefoxitin that corresponded to the wild strains of the L. fermentum species Citation[143].
The safety of ME-3 has been tested repeatedly in a mouse model (NIH line conventional male mice, Kuopio Finland) administered commercial diet R-70 (Lactamin, Sweden). The experiments were performed according to European Convention regulations for animal experiments no. 123 from 1986. Freeze-dried L. fermentum ME-3 was added to the tap water in a daily dose of 9.7 log cfu for 30 days. The mice were monitored and the faeces were collected individually for the detection of strain ME-3 daily. All strain ME-3-challenged animals remained in a good state during the feeding trial. Furthermore, safety was also confirmed by feeding mice during several months with a probiotic ME-3 cheese in different doses Citation[136].
The mice were killed by cervical dislocation and autopsies were performed under sterile conditions using a Class II microbiological safety cabinet (Jouan, France). To estimate the content of lactic acid bacteria in the terminal ileum and colon, bacteriological investigations were carried out immediately. No translocation of the probiotic strain into any organs (ileum, liver and spleen) was detected. The increase (from 8.6±0.3 before to 9.1±0.2 log cfu/g after the trial, p=0.003) in total faecal lactic acid bacterial counts was observed only at the end of the 30-day experiment (Truusalu, dissertation in preparation).
Antioxidative effects
Survey of oxidative stress and antioxidative effects
Oxidation is essential to living organisms for energy production. However, it is now well established that abnormal formation of the reactive species (including free radicals) occurs in vivo and can lead to the damage of lipids, proteins, nucleic acids and carbohydrates of cells and tissues Citation[144]. In the human body reactive oxygen species (ROS) and reactive nitrogen species (RNS) are the main players. Both terms involve appropriate free radicals and non-radical species A free radical is defined as an atom or molecule having at least one unpaired electron in its outer orbital; examples include oxygen-centred radicals (superoxide radical, hydroxyl radical), nitrogen-centred radicals (nitric oxide, nitric dioxide radical), sulphur-centred (RS- thiyl), and some others. Principal ROS are superoxide radical, hydroxyl radical, lipid peroxyl radical and non-radical hydrogen peroxide (the latter is produced from superoxide by superoxide dismutase). Principal RNS are nitric oxide and non-radical peroxynitrite. The pathological efficiency of the hydroxyl radical is the most potent and it is rapidly generated via the Fenton cycle where free iron (a very potent prooxidant) reacts with hydrogen peroxide Citation[144].
ROS and RNS are generated within the body from different external reactions (radiation, pollutants, toxins, chemicals and drugs) and internal univalent biochemical redox reactions. Several diseases are associated with the toxic effect of the transition metals (iron, copper and cadmium). An excessive production of reactive species leads to an imbalance in the prooxidants–antioxidants system. Any imbalance in favour of the prooxidants potentially leading to damage was termed ‘oxidative stress’ Citation[145].
There is a large body of evidence that high grade oxidative stress (OxS) has one of the crucial roles in the pathogenesis of several disorders/diseases of the GI tract (inflammatory bowel disease, coeliac disease, etc.), the vascular system (atherosclerosis, myocardial infarction, stroke, vascular dysfunctionality), the nervous system (Alzheimer's disease, Parkinson's disease), the liver (ethanol damage, cirrhosis), the skin (several dermatoses), the pancreas (diabetes mellitus) and the eyes (age-related macular degeneration, retinopathy) Citation[144–152]. Metabolic syndrome, obesity, premature ageing and the development of several tumours also have an OxS-related background Citation[153].
Atherosclerosis is characterized by many potential risk markers such as increased values of low-density lipoprotein (LDL)-cholesterol and elevated blood pressure, while HDL-cholesterol, fasting triacylglycerol and plasma homocysteine are suggested as the diet-related markers Citation[154]. Moreover, the increased inflammatory markers (white blood cells (WBCs), highly sensitive C-reactive protein (hsCRP)) and the oxidative stress indices (oxidized LDL, urine 8-isoprostanes, etc.) have been shown to be characteristic of patients with atherosclerotic lesions of the vascular system, having developed cardiovascular disease (CVD) Citation[146].
For the systemic multi-level control of OxS, the human body has evolved an integrated antioxidant defence system (). It includes both the non-enzymatic antioxidants like reduced glutathione (GSH) and vitamins E, C and Q10, blood albumin, uric acid, bilirubin and enzymatic antioxidants like superoxide dismutase (Cu, Zn-SOD, Mn-SOD), glutathione peroxidase (GSHPx), catalase (CAT) and haem oxygenase Citation[144].
Figure 7. A net of prooxidants and the potency of antioxidant defence system normally balanced in the human body. (a) A summary effect of oxidative stressors and potency of antioxidant defence system of the human body are normally balanced. An imbalance leads to oxidative stress. PUFA, polyunsaturated fatty acids; SOD, superoxide dismutase; GSHPx, glutathione peroxidase; CAT, catalase; HO1, haem oxygenase; GSH, reduced glutathione. (b) Oxidative stress causes the production of oxidized LDL (oxLDL), which is a potent atherogenic and inflammatory agent. Strain ME-3 lowers the level of oxLDL. LDL, low-density lipoprotein; CVD, cardiovascular diseases.
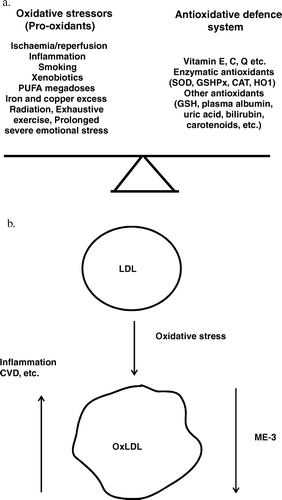
Several antioxidative components that are integrated into the human antioxidant defence system are derived from foodstuffs and/or provided by GI microbiota. However, up to the mid-1990s a few studies Citation[106] had been carried out to screen for the antioxidative properties of indigenous microbiota, including bifidobacteria and lactobacilli. Furthermore, it became apparent that the integrated antioxidant defence system and GI microbiota of the human body are very tightly linked, whereas specific strains with physiologically effective antioxidative properties may have a great impact on the management of the OxS level in the gut lumen, inside mucosa cells and in the blood, to support the functionality of the integrated antioxidant defence system of the human body.
Some new trends for medical bioremediation target the application of microbial catabolic diversity against aging and several major age-related diseases such as atherosclerosis. This follows the historical hypothesis of Metschnikof suggesting the intensive consumption of lactic acid bacteria in order to postpone aging (cited in Vaughan et al. Citation[155]). However, it was not assessed whether the increased content of lactobacilli and temporal colonization with their particular strains could influence the inflammatory and OxS-derived blood indices. We have tried to explore this gap in our probiotic studies.
Studies on the antioxidative effect of L. fermentum ME-3
In vitro studies
In 1996 we started to check the antioxidative characteristics of a large number of Lactobacillus spp. strains. Applying a number of different tests we found that two strains of L. fermentum may have an impact as new probiotics with functional properties towards antimicrobial and antioxidative action. Both strains (E-3 and E-18) and their lysates had physiologically relevant multivalent antioxidativity (TAS and TAA test) to overcome the exogenous and endogeneous OxS of the host Citation[156]. The antioxidative properties of L. fermentum ME-3 and demonstrated effects are summarized in , Citation[100–102], Citation[136], Citation[156–164], Citation[167].
Table IV. Antioxidativity-related properties and effects of strain ME-3.
Researcher Ann Kilk of the Department of Biochemistry performed PCR for the detection of manganese superoxide dismutase (Mn-SOD) in ME-3 cells. Mn-SOD is very important in the control of lipid peroxidation. Manganese and Mn-SOD activity of lactobacilli (not having a usual catalase) is important for their survival in the oxidative milieu (milk, host) created by the production of H2O2Citation[165], Citation[166].
Furthermore, researcher Tiiu Kullisaar designed an original set of experiments showing that strain ME-3 (its previous in-laboratory acronym was E-3) had a good hydroxyl radical scavenging efficiency and was able to survive in high concentrations of hydrogen peroxide content and superoxide anions quite similarly to a highly ROS-resistant Salmonella Typhimurium, although lactobacilli did not have a catalase as compared with salmonellas Citation[156]. The survival in the presence of different ROS was possible due to GSH that is a major non-enzymatic intracellular antioxidant and protector-molecule Citation[144]. Recently it was established that ME-3 cells possess a complete glutathione system for its synthesis, uptake and redox turnover Citation[158].
An independent laboratory confirmed that the in vitro superoxide anion scavenging efficiency of strain ME-3 was more than 80–100 times stronger as compared with trolox or ascorbic acid (Ahotupa, personal communication). That the antioxidant properties of strain ME-3 prevented the oxidative spoilage of soft cheese products has also recently been confirmed by experiments in Finland Citation[159].
Experimental animal infections
Next, an animal model of typhoid fever was developed by the inoculation of mice with Salmonella Typhimurium Citation[161]. S. Typhimurium induces generalized infection in mice with typhoid nodules (granulomas) in the liver and spleen.
In the prophylactic and treatment model with an S. Typhimurium challenging of mice, the application of L. fermentum ME-3 was not able to eradicate salmonellas from organs, yet strain ME-3 suppressed the excessive OxS-related reactions caused by the infectious agent and the inflammation. The enhanced lipid peroxidation and the abnormal glutathione redox ratio were corrected and thus the gut mucosal antioxidative status was improved Citation[161].
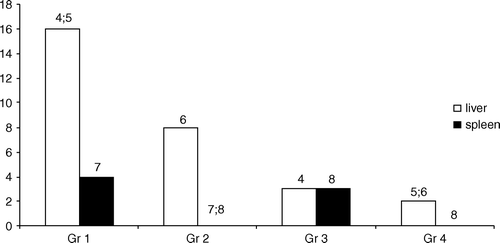
The still wide prevalence of typhoid fever in southern countries and the treatment problems have got our attention. Therefore, we have applied the same experimental typhoid fever model for the elaboration of the treatment principles of S. Typhimurium infection with an antimicrobial quinolone (ofloxacin) combined with the probiotic ME-3. The combinations of antimicrobial preparations with probiotics have been used with good success for the treatment of H. pylori infections, yet mainly to cope with adverse effects Citation[140]. We succeeded in showing the priority of the combination for the eradication of salmonellas from the intestinal tract and liver () and also the reduction of typhoid nodules (granulomas) in the liver ().
Figure 8. The number of mice with viable Salmonella Typhimurium in ileum, blood and liver. Gr1, Salmonella Typhimurium (ST)-challenged mice; Gr2, ST treated with ofloxacin (OFX); Gr3, ST treated with strain ME-3; Gr4, ST treated with OFX + strain ME-3. 1p=0.032 Gr1 vs Gr2 ST in ileum; 2p=0.002 Gr1 vs Gr3 and Gr4 ST in ileum; 3p=0.002 Gr1 vs Gr3 and Gr4 ST in liver.
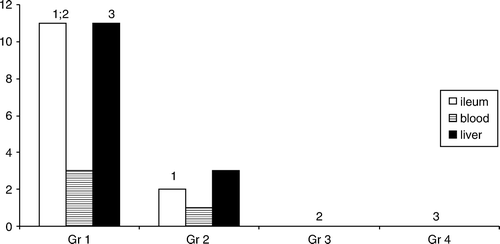
Figure 9. The number of mice with typhoid nodules in liver and spleen. 4p=0.027 Gr1 vs Gr3; 5p<0.001 Gr1 vs Gr4; 6p=0.023 Gr2 vs Gr4; 7p=0.002 Gr1 vs Gr2 and Gr4; 8p=0.048 Gr2 vs Gr3 and Gr3 vs Gr4.
Meanwhile, the indices of lipid peroxidation were reduced () and the glutathione redox ratio (GSSG/GSH) was significantly lower in mice treated with the combination of strain ME-3 and ofloxacin Citation[164].
Table V. Indices of oxidative stress (with standard deviations) in the ileum mucosa in mice challenged with S. Typhimurium and treated with ofloxacin and/or the probiotic L. fermentum ME-3.
Our last in vivo experiments on the NIH mouse model showed that the administration of strain ME-3 induces high levels of anti-inflammatory cytokine interleukin (IL)-10 in both the gut and liver tissue. This could be the reason for the reduced number of typhoid nodules in the liver in mice treated with a combination of ofloxacin and strain ME-3 Citation[164].
Thus, strain ME-3 helps to alleviate OxS- and inflammation-related disorders in the intestinal cells in different ways (cf. discussion about ME-3 action mechanisms).
Volunteers and clinical trials
Strain ME-3 colonization and safety, as well as its antioxidative effects, have been tested in several open placebo-controlled and randomized double-blind placebo-controlled clinical trials Citation[100–102], Citation[162], Citation[167] using capsules with ME-3, goat milk fermented with ME-3, commercial foodstuffs (kefir, cheese) and synbiotics enriched with ME-3. A large spectrum of indices measured in healthy adult volunteers showed that the use of strain ME-3 was safe regarding the physiological values of blood cytokines (including IL-6), inflammatory markers (WBCs, hsCRP), principal markers of carbohydrates and lipids or lipid-like compounds (glycose, triglycerides, cholesterol, LDL, HDL), several metabolites (homocysteine, creatinine, bilirubin) and several other biochemical indices such as blood calcium and iron, and endothelial functionality and arterial stiffness.
The consumption of strain ME-3 had a positive influence on the gut microbiota. The faecal counts of beneficial lactobacilli were increased in several studies () offering protection against colonization by potential pathogens if the volunteers consumed goat milk fermented by strain ME-3 (daily dose 3×1011 cfu) or used freeze-dried capsulated ME-3 (109 cfu per capsule twice a day) as well for 3 weeks. In contrast, in the group of volunteers consuming non-fermented goat milk, a decrease in total lactobacilli counts was seen during the 3-week trial. However, a significant difference was found concerning the applied formulations. In the goat milk trial strain ME-3 was molecularly (ITS-PCR) assessed in all volunteer consumers, yet strain ME-3 was not detectable among ME-3 isolates by bacteriological methods or by AP-PCR in the probiotic capsule trial, although there was a positive shift in blood indices. In capsulate forms higher quantities of bacteria (up to 1010 cfu/g) have been suggested to re-isolate the probiotic bacteria from faecal samples Citation[168], Citation[169].
Figure 10. Increase of total faecal counts of lactobacilli in healthy volunteers consuming strain ME-3 in fermented goat milk and in the DBRP probiotic capsule efficacy trial Citation[118]. *p < 0.005 difference from pretreatment values; ‡p < 0.01 difference between ME-3 and control treatments.
![Figure 10. Increase of total faecal counts of lactobacilli in healthy volunteers consuming strain ME-3 in fermented goat milk and in the DBRP probiotic capsule efficacy trial Citation[118]. *p < 0.005 difference from pretreatment values; ‡p < 0.01 difference between ME-3 and control treatments.](/cms/asset/1116ad06-53f9-4f83-a812-a25f085f47d7/zmeh_a_381726_f0010_b.gif)
At the same time, the consumption of ME-3 bacteria-containing substances showed positive effects on several OxS-related indices such as post-prandial lipid, lipoprotein and OxS profile of blood and urine Citation[162]. Consumption of kefir containing strain ME-3 significantly decreased the post-prandial content of fats, oxLDL and BDC-LDL, enhanced the level of HDL and improved the bioquality of HDL particles.
Synbiotic trial
L. fermentum ME-3 was also applied in the EU Research Commission-funded project ‘EU and Microfunction – Functional assessment of interactions between human microbiota and host’ (QLRT-2001-00135; ISRCTN43435738) using a randomized, double-blind crossover synbiotic intervention study with 53 healthy volunteers Citation[167], Citation[170]. The composition of the synbiotic was as follows: probiotics L. fermentum ME-3, L. paracasei 8700:2, Bifidobacterium longum 46 (both Probi, Sweden) and 5 g of prebiotic Raftilose P95 (Orafti, Belgium). Several shifts in the microbiota composition were assessed by research partners at Reading University (UK), such as an increase in bifidobacteria and clostridia counts, associated with appropriate shifts in profiles of faecal metabolites (short chain fatty acids, SCFAs). An increase in the total antioxidative activity was also registered () accompanied by an improved bioquality of LDL particles (oxLDL and BDC-LDL) of sera of volunteers after consumption of the symbiotic for 3 weeks.
Table VI. Improvement of OxS-related indices of blood sera in the synbiotic DBRP crossover study in healthy volunteers Citation[167].
However, half of the healthy asymptomatic volunteers in this trial were colonized with H. pylori. All applied probiotic strains expressed a high antagonistic activity against the H. pylori reference strain in vitroCitation[138]. Despite the consumption of the symbiotic for 3 weeks, this composition could not eradicate the H. pylori infection Citation[167]. Seemingly the application of the entero-coated probiotic capsules prevented the direct effect of lactobacilli on H. pylori on gastric mucosa. Thus, the antagonistic bacteria, including strain ME-3, were not able to exert any systemic antimicrobial influence on H. pylori colonization, although the same consumed composition improved the antioxidative indices of blood in persons colonized with H. pylori.
Special clinical trials on patients
On the basis of the information about safety and the positive effects of strain ME-3 in healthy volunteers, we also conducted some preliminary clinical pilot trials.
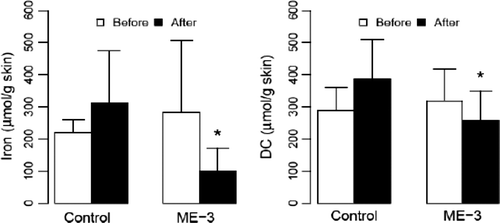
In the first randomized blinded pilot study, patients with mild to moderate atopic dermatitis consumed goat milk fermented with strain ME-3 for 3 months Citation[102]. The clinical SCORAD index decreased from 4.8±3.9 to 1.9±1.8 (p<0.05) in the probiotic group and from 4.8±2.8 to 2.3±0.9 in the control group. A significant amount of oxidized iron (prooxidant) was found in the skin of patients before consumption and the values of iron were decreased. Also, the diene conjugate values (indicating lipid peroxidation) were reduced (). In the same group, the antioxidativity markers of blood also showed an improvement. The levels of oxLDL decreased, and GSH (a protective form of glutathione) levels increased with a concomitant reduction in the GSSG/GSH ratio both in the skin and in the blood level. The results of our study demonstrated that the regular use of probiotics with antioxidative properties decreased inflammation and concomitant OxS in adult patients with mild to moderate atopic dermatitis Citation[102], Citation[162].
Figure 11. Content of iron and diene conjugates in the skin in patients with atopic dermatitis (AD), regularly (3 months) consuming probiotic strain ME-3. *p<0.05 comparing the values before and after consumption.
The second DBRP pilot trial Citation[162] was aimed at evaluation of the antioxidative effect of strain ME-3 on a seriously ill group of patients who had survived a stroke. The 21 patients (80.4±9.9 years), who had survived a brain stroke 12±6.6 days earlier, were randomly distributed into two groups. In addition to regular rehabilitation therapy that included ACE inhibitors, aspirin, diuretics and beta-blockers, the patients were assigned to consume for 3 weeks, twice a day, either capsules (3×per capsule 109 cfu) of freeze-dried ME-3 (ME-3 group, 10 subjects) or placebo capsules (3×250 mg saccharose and microcellulose, control group, 11 subjects), respectively. The functional ability of the stroke patients was assessed before and after the 3-week treatment period using two clinical evaluation scales (). The Functional Independence Measure, FIM, assesses 18 activities of self-care, mobility, locomotion, communication and social cognition on a 7-point scale from fully independent to fully dependent. The Scandinavian Stroke Scale, SSS, is more specific to stroke patients and measures nine items: level of consciousness, mobility of eye, upper and lower limb, orientation, speech, facial tone and gait. The biochemical indices of sera were evaluated twice: before and after treatment Citation[162]. The baseline values of the ME-3 and control group were not statistically different. After rehabilitation and the course and consumption of the antioxidative probiotic, only the ME-3 group showed improved biochemical indices (GSSG, oxLDL, diene conjugates (DC), TAA) and the correlation between improved OxS-related indices and the functional ability scales (between SSS and oxLDL, r= − 0.55, p<0.05) and FIM (between FIM and GSSG/GSH, r= − 0.63, p<0.03). However, in the placebo group the FIM values got even better than in the ME-3 group, seemingly due to the lower starting level of the patients in the ME-3 group. In addition, the application of ME-3 capsules caused a significant decline in the values of inflammatory markers (hsCRP) not assessed in the control group.
Table VII. Clinical and biochemical evaluations of stroke patients: parameters at the baseline (before) and after an application period (after) by means of SSS and FIM scale and biochemical indices (mean±SED) Citation[162].
illustrates the effects of strain ME-3 for increasing the oxiresistance of LDL particles and lowering the level of oxLDL.
Figure 12. Increase of oxiresistance of low-density lipoprotein (LDL) particles (minutes) and lowering oxidized-LDL level (absorbance units) after using strain ME-3. Oxidation of LDL is measured on the basis of conjugated dienes at 234 nm.
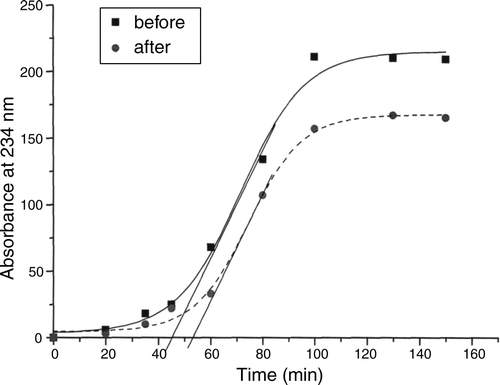
Thus, this pilot trial shows the potential of strain ME-3 to be applied as an adjunct preparation to the ordinary rehabilitation therapy of patients in the course of recovering from a brain stroke.
Possible mechanisms of effects of L. fermentum ME-3
In different experiments and volunteer and clinical trials, the administration of strain ME-3 has led to the improvement of the GI microbial ecology. More than a 10-fold increase of total lactobacilli counts in comparison with the individually different initial count was registered in the collected faecal samples, regardless of the probiotic formulation or daily dose applied. It was supposed that the metabolites secreted by strain ME-3 into the GI tract could be used as a substrate by other lactobacilli. The tolerance to different environmental factors and the successful passage of strain ME-3 through the GI tract has also been confirmed by culture and PCR-based methods Citation[100], Citation[101].
At the same time, by adding L. fermentum strain ME-3 as a probiotic ingredient into a dairy product (yoghurt, cheese, milk), it was able to suppress the putative contaminants of food such as pathogenic Salmonella spp., Shigella spp., urinary tract infections caused by E. coli, Staphylococcus spp. The amounts of secreted SCFAs, the substantial amount of hydrogen peroxide Citation[120], Citation[138], Citation[156] and production of NO by strain ME-3 were seemingly the main antimicrobial operators. The method for the simultaneous suppression of pathogens and the enhancement of the antioxidative activity of food was filed in a US patent application Citation[171]. Thus, the double functional properties of the probiotic strain L. fermentum ME-3 may protect the host against food-derived infections on one hand and help in the prevention of oxidative damage of food on the other hand. The intact cells and cell-free extract of strain ME-3 showed different potent antioxidative effects due to the expression of the antioxidant enzymes such as Mn-SOD and the components of the complete glutathione system (GSH, glutathione peroxidase and glutathione reductase). The antioxidative protection offered by strain ME-3 for the prevention of oxidative spoilage of semi-soft cheeses was assessed in an independent study in Finland Citation[159].
Furthermore, we have looked for the implementation of the functional properties of strain ME-3 to improve antimicrobial defence and some metabolic functions in different hosts.
Experimental animal studies Citation[161], Citation[164] have confirmed that the increase in total lactobacilli counts as much as the specific strain ME-3 antioxidative action in the gut eradicated live salmonellas and prevented the formation of typhoid nodules in experimental Salmonella Typhimurium infections, resembling typhoid fever in humans. For the first time it was shown that the antibiotic therapy of an invasive infection like enteric fever was more effective if administered together with a probiotic. However, it cannot be excluded that beside the antimicrobial and antioxidative effect of strain ME-3, immune enhancement by the probiotic also played a significant role.
Concerning the implementation of the antioxidative properties of strain ME-3 in humans, we have developed a method for enhancing the antioxidative activity of sera, i.e. administering strain ME-3 in a food product to humans comprising increases in TAA, TAS, and the lag phase of LDL and decreases in oxidized glutathione, oxLDL and BCD-LDL of sera Citation[171]. Different trials have shown that the application of strain ME-3 alleviates inflammation- and OxS-related shifts in gut, skin and blood Citation[100–102], Citation[162]. This is based on complicated cross-talk between strain ME-3 and host cells with the integrated influence of several factors such as the ability of strain ME-3 to apply a complete glutathione system, the expression of antioxidative enzymes in strain ME-3, the production of CLA and NO by strain ME-3, etc.
The question was raised as to how it was possible to exert a positive effect such as the reduction of OxS just by consuming a food product? Strain ME-3 of human origin was successful in surviving different fermentation processes of milk due to its good tolerance to low temperature, acid and salt Citation[118], Citation[136], and was capable of the temporal colonization of the intestinal tract of the consumer.
The intestinal surface is an important host organism–environment boundary and the interactions of gut microbes inside the intestinal lumen and mucosal cells are important for the host, as was shown in the characterization of the composition and metabolic acitivities of intestinal microbiota in the first sections of the review. An impaired environment such as the imbalance of GI microbiota, but also the increase of lipid peroxidation and decrease of the reduced GSH both at the intestinal surface and in the intestinal cells, are the mighty modulators causing different unhealthy outcomes in the host. That these modulators of the intestinal mucosal status can be repaired by the administration of strain ME-3 was directly confirmed in a mouse model of experimental S. Typhimurium infection Citation[161], Citation[164]. In this process the involvement of the glutathione system is crucial as GSH, besides its role as a crucial antioxidant, is the principal redox controller for a number of processes in cells. Glutathione-related information has impact for strain ME-3 regarding at least two aspects: a) a recent adapted conception of OxS is advanced as ‘a disruption of redox signalling and control’ Citation[145], Citation[172] that emphasizes an impact of GSH and its redox ratio as good tools for the quantification of OxS and the signalling role of GSH, described also by us Citation[173], Citation[174]; and b) there exists the possibility for the effective participation of strain ME-3 in both enzymatic and non-enzymatic glutathione-driven protection. Besides, the data that just L. fermentum as a species significantly counteracted the depletion of colonic glutathione content induced by some inflammatory processes Citation[175] also supported our understanding. In addition, there exists a correlation between glutathione redox ratio and DNA oxidative damages Citation[176].
Further, our research has shown that the improvement of the intestinal extra- and intracellular environment yielded beneficial changes of some general/systemic biochemical indices of the host. This was proven by the administration of strain ME-3 to healthy volunteers and atopic adults leading to a reduction of lipid peroxidation and a counterbalance of the glutathione system both in blood and in skin. Moreover, in several conducted trials we have seen the positive effect of strain ME-3 on the blood LDL fraction: the prolongation of its resistance to oxidation, the lowering of the content of oxLDL (potent inflammatory and atherogenic factor) and BDC-LDL and the enhancement of the total antioxidative capacity of sera Citation[100], Citation[101], Citation[167].
In our recent investigation of elderly persons over 65 years, the lower content of oxLDL was significantly predicted by the higher count of live lactobacilli in the GI tract (M. Mikelsaar et al., unpublished observations). Seemingly, both the particular antioxidative characteristics of strain ME-3 and the increase in lactobacilli counts induced by its administration could be responsible for the registered impact on the host lipid metabolism.
The status of OxS and blood lipoprotein are both related to the development of different diseases, including inflammation-related diseases and CVD (see above). Recently in CirculationCitation[177] the pathophysiological continuum that traditional cardiovascular risk factors all promote OxS and endothelial dysfunction – the first steps in a cascade of pathological events – was highlighted. OxS leads to the overproduction of oxLDL and the latter has a great impact on the development of atherosclerosis. For example, the higher levels of circulating oxLDL are strongly (much more than LDL-cholesterol) associated with an increased incidence of metabolic syndrome already in people who are currently young and healthy according to a large population-based study Citation[178]. It was previously shown that oxLDL is an important determinant of structural changes of the arteries already in asymptomatic persons Citation[147], Citation[179]. Recent data gathered from the literature suggest that the increased production of atherogenic and inflammatory oxLDL within the vessel wall suppresses several immunity-related cells, including regulatory T cells Citation[180] exerting antiatherogenic and antiallergic effects. In addition, it is widely accepted that post-prandial abnormal events are crucial as regards the development of CVD Citation[181].
The systemic influence of strain ME-3 on host OxS indices has also been assessed by the decline of the values of isoprostanes and 8-OHdG in urine Citation[100], Citation[101], Citation[162]. Both indices are accepted as very informative markers for human systemic OxS burden Citation[144], Citation[145]. Evidently the systemic antioxidative effect of strain ME-3 starts from the alleviation of the OxS- and inflammation-related abnormalities in the intestinal cells that lead to the assembling of particles of chylomicrons, LDL and HDL with a higher bioquality with lower levels of harmful oxidation products and higher concentrations of antioxidant enzymes in their particles. Furthermore, the increased bioquality of assembled lipoprotein particles is bound to aid the improvement of their metabolism/circulation in the host body. This is one of the possible explanations why strain ME-3 exerted the prolonged resistance of the blood lipoprotein fraction to oxidation, lowered the level of oxLDL and enhanced the total antioxidative capacity of sera in both healthy and diseased strain ME-3 consumers Citation[100], Citation[101], Citation[162], Citation[163]. Our recent data have shown that administration of strain ME-3 alleviates the post-prandial elevation of triglyceride levels in the blood, and improves HDL bioquality (elevation of antioxidative enzyme level in HDL particles) Citation[162], Citation[163]. This new HDL-related antioxidative information is supported both by our findings of anti-inflammatory effects of strain ME-3 on the liver Citation[164] and by a hepato-protective role for paraoxonase against inflammation, fibrosis and liver disease mediated by OxS Citation[182].
The necessity for new approaches in global cardiovascular risk reduction has become widely accepted Citation[183]. In the prevention of cardiovascular risk the anti-inflammatory agents and antioxidants are considered as a possible ‘third great wave’ Citation[184]. The prevention complexes of several diseases could become more successful by also including probiotics with multivalent biopotency. Hopefully the antimicrobial and antioxidative probiotic L. fermentum ME-3 has earned its place in this putative list of health promoters.
Summary and conclusions
The probiotics aimed at stabilizing the microbial communities and the health-promoting effects have an important position in the medical health care of different age groups and diseased persons. This paper describes the process of the discovery, identification and molecular typing of the strain of human origin, Lactobacillus fermentum ME-3 (DSM-14241), elaborated according to the regulations of WHO/FAO (2002). In this review the authors have attempted to compile the available information concerning the ability of L. fermentum ME-3 to protect the host from different diseases induced by pathogenic bacteria (Salmonella spp., Shigella spp. and urinary tract infections cause by E. coli), inflammation and oxidative stress.
Safety and health-promoting studies of the probiotic strain cited in this report were carried out by assessing a large number of microbiological, biochemical and clinical indices. This strain is still unique among Lactobacillus species, having both antimicrobial and physiologically effective antioxidative properties. Tartu University has patented this probiotic strain in Estonia (priority June 2001, patent in 2006), Europe (pending), Russia (patent in 2006) and USA (patent in 2007).
The functional efficacy of this multipotential probiotic has been proven by the eradication of pathogenic microbes and the reduction of liver and spleen granulomas in Salmonella Typhimurium-infected mice treated by the combination of ofloxacin and L. fermentum strain ME-3. When used in capsules or foodstuffs (yoghurt, kefir, cheese) L. fermentum ME-3 expresses several health-promoting effects. It has been shown in different subjects (healthy volunteers, patients) with different clinical study designs (including double-blind, placebo-controlled, crossover studies) that this probiotic increased the counts of lactobacilli in the intestinal tract, lowered the 8-isoprostanes content in urine, increased the antioxidative activity, lowered the content of atherogenic oxLDL, and improved post-prandial lipid as well as oxidative stress status in sera, thus demonstrating an anti-atherogenic effect.
The elaboration of the probiotic L. fermentum strain ME-3 has drawn on wide international cooperative research and has taken more than 12 years altogether. The new probiotic products containing strain ME-3 have been successfully marketed and sold in Baltic countries and Finland.
Acknowledgements
The authors wish to express their sincerest appreciation to the many organizations such as the University of Tartu, the Estonian Technology Agency, Enterprise Estonia, the Ministry of Science and Education of Estonia, TERE AS, FoodWest OY, Maitokolmio OY, Probiotical OY and inviduals S. Salminen, B. Björkstén, T. Wadström, W. de Vos, G. Gibson, M. Ahotupa, E. Songisepp, H. Annuk, E. Sepp, M. Laanes, M. Türi, K. Lõivukene, J. Stsepetova, I. Smidt, I. Roots, K. Julge, T. Voor, K. Truusalu, P. Hütt, R. Mändar, P. Naaber, S. Kõljalg, H. Kolk, H. Andreson, A-L. Prangli, M. Utt, R. Uibo, K. Kilk, T. Kullisaar, K. Zilmer, A. Rehema, J. Kals, P. Kampus, T. Vihalemm, J. Maaroos, A. Lukman, S. Kaur, M. Reiman, J. Saatre, R. Adamsoo, S. Kahu and all the personnel of the Department of Microbiology and the Department of Biochemistry of Tartu University whose contributions and collaborations have helped to discover, carefully investigate and apply, as a probiotic, this microbial species to protect and promote human and animal health. Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Mackie RI. Mutualistic fermentative digestion in the gastrointestinal tract: diversity and evolution. Integ Comp Biol 2002; 42: 319–26
- McFarland LV. Normal flora: diversity and functions. Microb Ecol Health Dis 2000; 12: 193–218
- Dubos R, Schaedler RW. Some biological effects of the digestive flora. Am J Med Sci 1962; 244: 265–71
- Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol 1977; 31: 107–33
- Savage DC. The normal human microflora composition. The regulatory and protective role of the normal microflora, R Grubb, T Midtvedt, E Norin. N-Stockton Press, New York 1989; 3–18
- Bhat P, Albert MJ, Rajan D, Ponniah J, Mathan VI, Baker SJ. Bacterial flora of the jejunum: a comparison of luminal aspirate and mucosal biopsy. J Med Microbiol 1980; 13: 247–56
- Croucher SC, Houston AP, Bayliss CE, Turner RJ. Bacterial populations associated with different regions of the human colon wall. Appl Environ Microbiol 1983; 45: 1025–33
- Mikelsaar, ME, Tjuri, ME, Valjaots, ME, Lencner, AA. [Anaerobic lumen and mucosal microflora of the gastrointestinal tract.]. Nahrung 1984;28:727–33, (in German).
- Mikelsaar, M, Turi, M, Lencner, H, Kolts, K, Kirch, R, Lencner, A. Interrelations between mucosal and luminal microflora of gastrointestine. Nahrung 1987;31:449–56, 637–8.
- Zoetendal EG, von Wright A, Vilpponen-Salmela T, Ben-Amor K, Akkermans AD, de Vos WM. Mucosa-associated bacteria in the human gastrointestinal tract are uniformly distributed along the colon and differ from the community recovered from feces. Appl Environ Microbiol 2002; 68: 3401–7
- Nielsen DS, Moller PL, Rosenfeldt V, Paerregaard A, Michaelsen KF, Jakobsen M. Case study of the distribution of mucosa-associated Bifidobacterium species, Lactobacillus species, and other lactic acid bacteria in the human colon. Appl Environ Microbiol 2003; 69: 7545–8
- Lepage P, Seksik P, Sutren M, de la Cochetiere MF, Jian R, Marteau P, et al. Biodiversity of the mucosa-associated microbiota is stable along the distal digestive tract in healthy individuals and patients with IBD. Inflamm Bowel Dis 2005; 11: 473–80
- van der Waaij LA, Harmsen HJ, Madjipour M, Kroese FG, Zwiers M, van Dullemen HM, et al. Bacterial population analysis of human colon and terminal ileum biopsies with 16S rRNA-based fluorescent probes: commensal bacteria live in suspension and have no direct contact with epithelial cells. Inflamm Bowel Dis 2005; 11: 865–71
- Mikelsaar M, Mändar R. Development of individual lactic acid microflora in the human microbial ecosystem. Lactic acid bacteria, S Salminen, A von Wright. Marcel Dekker, New York 1993; 237–93
- Zoetendal EG, Akkermans AD, De Vos WM. Temperature gradient gel electrophoresis analysis of 16S rRNA from human fecal samples reveals stable and host-specific communities of active bacteria. Appl Environ Microbiol 1998; 64: 3854–9
- Holdeman LV, Good IJ, Moore WE. Human fecal flora: variation in bacterial composition within individuals and a possible effect of emotional stress. Appl Environ Microbiol 1976; 31: 359–75
- Bochkov, IA, Lizko, NN, Semina, NA, Borunov, NL, Iurko, LP. [Peculiarities of cosmonauts faecal flora.]. Aviakosm Ekolog Med 1998;32:25–8, (in Russian).
- Finegold SM, Sutter VL, Sugihara PT, Elder HA, Lehmann SM, Phillips RL. Fecal microbial flora in Seventh Day Adventist populations and control subjects. Am J Clin Nutr 1977; 30: 1781–92
- Bry L, Falk PG, Midtvedt T, Gordon JI. A model of host-microbial interactions in an open mammalian ecosystem. Science 1996; 273: 1380–3
- Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 2006; 124: 837–48
- Li M, Wang B, Zhang M, Rantalainen M, Wang S, Zhou H, et al. Symbiotic gut microbes modulate human metabolic phenotypes. Proc Natl Acad Sci U S A 2008; 105: 2117–22
- Zoetendal EG, Akkermans AD, Akkermans-van der Vliet VM, De Visser JA, De Vos WM. The host genotype affects the bacterial community in the human gastrointestinal tract. Microb Ecol Health Dis 2001; 13: 129–34
- Mikelsaar M., Mändar R., Sepp E. Lactic acid microflora in the human microbial ecosystem and its development. Lactic acid bacteria. Marcel Dekker, New York 1998; 279–342
- Van de Merwe JP, Stegeman JH, Hazenberg MP. The resident faecal flora is determined by genetic characteristics of the host. Implications for Crohn's disease?. Antonie Van Leeuwenhoek 1983; 49: 119–24
- Apperloo-Renkema HZ, Jagt TG, Tonk RH, van der Waaij D. Healthy individuals possess circulating antibodies against their indigenous faecal microflora as well as against allogenous faecal microflora: an immunomorphometrical study. Epidemiol Infect 1993; 111: 273–85
- Kimura K, McCartney AL, McConnell MA, Tannock GW. Analysis of fecal populations of bifidobacteria and lactobacilli and investigation of the immunological responses of their human hosts to the predominant strains. Appl Environ Microbiol 1997; 63: 3394–8
- Egert M, De Graaf AA, Smidt H, De Vos WM, Venema K. Beyond diversity: functional microbiomics of the human colon. Trends Microbiol 2006; 14: 86–91
- McFarland LV. Beneficial microbes: health or hazard?. Eur J Gastroenterol Hepatol 2000; 12: 1069–71
- O'Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep 2006; 7: 688–93
- Tannock GW. A special fondness for lactobacilli. Appl Environ Microbiol 2004; 70: 3189–94
- van der Waaij D, Berghuis JM. Determination of the colonization resistance of the digestive tract of individual mice. J Hyg (Lond) 1974; 72: 379–87
- Naaber, P, Mikelsaar, M. Interactions between lactobacilli and antibiotic-associated diarrhea, In: AI Laskin, Bennett, JW, Gadd, GM. Advances in applied microbiology. vol 54. New York: Elsevier; 2004. p. 231–60.
- Bäckhed F, Ley RF, Sonnenberg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science 2005; 307: 1915–20
- Banasaz M, Alam M, Norin E, Midtvedt T. Gender, age and microbial status influence upon intestinal cell kinetics in a compartmentalized manner: an experimental study in germfree and conventional rats. Microb Ecol Health Dis 2000; 12: 208–18
- Mikelsaar, M . Evaluation of the gastrointestinal microbial ecosystem in health and disease. Dis Med Univ Tartuensis, Tartu, 1992.
- Sepp E, Naaber P, Voor T, Mikelsaar M, Björkstén B. Development of intestinal microflora during the first month of life in Estonian and Swedish infants. Microb Ecol Health Dis 2000; 12: 22–6
- Mikelsaar M, Siigur U, Lenzner A. Evaluation of the quantitative composition of fecal microflora. Laboratornoje Delo 1990; 5: 62–6
- Sepp E, Julge K, Vasar M, Naaber P, Bjorksten B, Mikelsaar M. Intestinal microflora of Estonian and Swedish infants. Acta Paediatr 1997; 86: 956–61
- Sepp E, Julge K, Mikelsaar M, Bjorksten B. Intestinal microbiota and immunoglobulin E responses in 5-year-old Estonian children. Clin Exp Allergy 2005; 35: 1141–6
- Björkstén B, Sepp E, Julge K, Voor T, Mikelsaar M. Allergy development and the intestinal microflora during the first year of life. J Allergy Clin Immunol 2001; 108: 516–20
- Franks AH, Harmsen HJ, Raangs GC, Jansen GJ, Schut F, Welling GW. Variations of bacterial populations in human feces measured by fluorescent in situ hybridization with group-specific 16S rRNA-targeted oligonucleotide probes. Appl Environ Microbiol 1998; 64: 3336–45
- Zoetendal EG, Vaughan EE, de Vos WM. A microbial world within us. Mol Microbiol 2006; 59: 1639–50
- Rajilić-Stojanović M, Smidt H, de Vos WM. Diversity of the human gastrointestinal tract microbiota revisited. Environ Microbiol 2007; 9: 2125–36
- Ben-Amor K, Heilig H, Smidt H, Vaughan EE, Abee T, de Vos WM. Genetic diversity of viable, injured, and dead fecal bacteria assessed by fluorescence-activated cell sorting and 16S rRNA gene analysis. Appl Environ Microbiol 2005; 71: 4679–89
- Midtvedt T, Bjorneklett A, Carlstedt-Duke B, Gustafsson BE, Hoverstad T, Lingaas E, et al. The influence of antibiotics upon microflora-associated characteristics in man and mammals. Prog Clin Biol Res 1985; 181: 241–4
- Falk PG, Hooper LV, Midtvedt T, Gordon JI. Creating and maintaining the gastrointestinal ecosystem: what we know and need to know from gnotobiology. Microbiol Mol Biol Rev 1998; 62: 1157–70
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol 2003; 21: 335–76
- Tamm A, Siigur U, Mikelsaar M, Vija M. Output of bacterial metabolites as a diagnostic means. Nahrung 1987; 31: 485–92
- Marco ML, Pavan S, Kleerebezem M. Towards understanding molecular modes of probiotic action. Curr Opin Biotechnol 2006; 17: 204–10
- De Vos WM, Bron PA, Kleerebezem M. Post-genomics of lactic acid bacteria and other food-grade bacteria to discover gut functionality. Curr Opin Biotechnol 2004; 15: 86–93
- Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JL. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A 2005; 102: 11070–5
- Sonnenburg JL, Xu J, Leip DD, Chen CH, Westover BP, Weatherford J, et al. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science 2005; 307: 1955–9
- Lay C, Rigottier-Gois L, Holmstrom K, Rajilic M, Vaughan EE, de Vos WM, et al. Colonic microbiota signatures across five northern European countries. Appl Environ Microbiol 2005; 71: 4153–5
- Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Diversity of the human intestinal microbial flora. Science 2005; 308: 1635–8
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006; 444: 1027–31
- ILSI Europe. Scientific concepts of functional foods in Europe: consensus document. Br J Nutr. 1999;81:1S–27S.
- Strachan DP, Golding J, Anderson HR. Regional variations in wheezing illness in British children: effect of migration during early childhood. J Epidemiol Community Health 1990; 44: 231–6
- Holt PG., Macaubas C, Prescott SL, Sly PD. Microbial stimulation as an aetiologic factor in atopic disease. Allergy 1999; 54: 12–16
- Adlerberth, I . The establishment of normal intestinal microflora in the newborn infants. PhD Dissertation, Göteborg, 1996.
- Bennet R, Eriksson M, Nord CE, Zetterström R. Suppression of aerobic and anaerobic fecal flora in newborns receiving parenteral gentamicin and ampicillin. Acta Paediatr Scand 1982; 71: 559–62
- Stsepetova J, Sepp E, Julge K, Vaughan E, Mikelsaar M, de Vos WM. Molecularly assessed shifts of Bifidobacterium ssp. and less diverse microbial communities are characteristic of 5-year-old allergic children. FEMS Immunol Med Microbiol 2007; 51: 260–9
- Sepp E, Voor T, Julge K, Lõivukene K, Björkstén B, Mikelsaar M. Is intestinal microbiota bound up with changing lifestyle?. Modern multidisciplinary applied microbiology: exploiting microbes and their interactions, A Mendez-Vilas. Wiley-VCH Verlag, WeinheimGermany 2006; 708–12
- Björkstén B, Graninger G, Jacobsson Ekman G. New ideas in asthma and allergy research: creating a multidisciplinary graduate school. J Clin Invest 2003; 112: 816–20
- Kirjavainen PV, Salminen SJ, Isolauri E. Probiotic bacteria in the management of atopic disease: underscoring the importance of viability. J Pediatr Gastroenterol Nutr 2003; 36: 223–7
- Kirjavainen PV, Apostolou E, Arvola T, Salminen SJ, Gibson GR, Isolauri E. Characterizing the composition of intestinal microflora as a prospective treatment target in infant allergic disease. FEMS Immunol Med Microbiol 2001; 32: 1–7
- Food Agriculture Organization : Guidelines for the evaluation of probiotics in food. Available at: http://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf.
- Mercenier A, Lenoir-Wijnkoop I, Sanders ME. Physiological and functional properties of probiotics. Bull Int Dairy Fed 2008; 429: 2–6
- Rijkers, GT , Watzl, B , Rabot, S , Rafter, J , Antoine, JM , Haller, D , . ILSI Europe Workshop in association with the International Dairy Federation, et al . Guidance for assessing the probiotics beneficial effects: how to fill GAP. 22–24, May, 2008, Montreaux, Switzerland.
- Reid G, Kim SO, Kohler GA. Selecting, testing and understanding probiotic microorganisms. FEMS Immunol Med Microbiol 2006; 46: 149–57
- Lu L, Walker WA. Pathologic and physiologic interactions of bacteria with the gastrointestinal epithelium. Am J Clin Nutr 2001; 73: 1124S–1130S
- Ljungh A, Wadstrom T. Lactic acid bacteria as probiotics. Curr Issues Intest Microbiol 2006; 7: 73–89
- Marteau PR, de Vrese M, Cellier CJ, Schrezenmeir J. Protection from gastrointestinal diseases with the use of probiotics. Am J Clin Nutr 2001; 73: 430S–436S
- Salminen S. Human studies on probiotics: aspects of scientific documentation. Scand J Nutr 2001; 45: 8–12
- Charteris WP, Kelly PM, Morelli L, Collins JK. Development and application of an in vitro methodology to determine the transit tolerance of potentially probiotic Lactobacillus and Bifidobacterium species in the upper human gastrointestinal tract. J Appl Microbiol 1998; 84: 759–68
- Ross RP, Desmond C, Fitzgerald GF, Stanton C. Overcoming the technological hurdles in the development of probiotic foods. J Appl Microbiol 2005; 98: 1410–17
- Klaver FA, van der Meer R. The assumed assimilation of cholesterol by Lactobacilli and Bifidobacterium bifidum is due to their bile salt-deconjugating activity. Appl Environ Microbiol 1993; 59: 1120–4
- Gibson GR. Dietary modulation of the human gut microflora using prebiotics. Br J Nutr 1998; 80: S209–212
- Wang KY, Li SN, Liu CS, Perng DS, Su YC, Wu DC, et al. Effects of ingesting Lactobacillus- and Bifidobacterium-containing yogurt in subjects with colonized Helicobacter pylori. Am J Clin Nutr 2004; 80: 737–41
- Anderson JW, Gilliland SE. Effect of fermented milk (yoghurt) containing Lactobacillus acidophilus L1 on serum cholesterol in hypercholesterolemic humans. J Am Coll Nutr 1999; 18: 43–50
- Cruchet S, Obregon MC, Salazar G, Diaz E, Gotteland M. Effect of the ingestion of a dietary product containing Lactobacillus johnsonii La1 on Helicobacter pylori colonization in children. Nutrition 2003; 19: 716–21
- Sakamoto I, Igarashi M, Kimura K, Takagi A, Miwa T, Koga Y. Suppressive effect of Lactobacillus gasseri OLL 2716 (LG21) on Helicobacter pylori infection in humans. J Antimicrob Chemother 2001; 47: 709–10
- Koebnick C, Wagner I, Leitzmann P, Stern U, Zunft HJ. Probiotic beverage containing Lactobacillus casei Shirota improves gastrointestinal symptoms in patients with chronic constipation. Can J Gastroenterol 2003; 17: 655–9
- De Preter V, Vanhoutte T, Huys G, Swings J, De Vuyst L, Rutgeerts P, et al. Effects of Lactobacillus casei Shirota, Bifidobacterium breve, and oligofructose-enriched inulin on colonic nitrogen-protein metabolism in healthy humans. Am J Physiol Gastrointest Liver Physiol 2007; 292: G358–368
- Turchet P, Laurenzano M, Auboiron S, Antoine JM. Effect of fermented milk containing the probiotic Lactobacillus casei DN-114001 on winter infections in free-living elderly subjects: a randomised, controlled pilot study. J Nutr Health Aging 2003; 7: 75–7
- Pereg D, Kimhi O, Tirosh A, Orr N, Kayouf R, Lishner M. The effect of fermented yogurt on the prevention of diarrhea in a healthy adult population. Am J Infect Control 2005; 33: 122–5
- Tiollier E, Chennaoui M, Gomez-Merino D, Drogou C, Filaire E, Guezennec CY. Effect of a probiotics supplementation on respiratory infections and immune and hormonal parameters during intense military training. Mil Med 2007; 172: 1006–11
- Peng GC, Hsu CH. The efficacy and safety of heat-killed Lactobacillus paracasei for treatment of perennial allergic rhinitis induced by house-dust mite. Pediatr Allergy Immunol 2005; 16: 433–8
- Hilton E, Kolakowski P, Singer C, Smith M. Efficacy of Lactobacillus GG as a diarrheal preventive in travelers. J Travel Med 1997; 4: 41–3
- Szajewska H, Kotowska M, Mrukowicz JZ, Armanska M, Mikolajczyk W. Efficacy of Lactobacillus GG in prevention of nosocomial diarrhea in infants. J Pediatr 2001; 138: 361–5
- Viljanen M, Kuitunen M, Haahtela T, Juntunen-Backman K, Korpela R, Savilahti E. Probiotic effects on faecal inflammatory markers and on faecal IgA in food allergic atopic eczema/dermatitis syndrome infants. Pediatr Allergy Immunol 2005; 16: 65–71
- Kalliomaki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet 2001; 357: 1076–9
- Kalliomaki M, Salminen S, Poussa T, Isolauri E. Probiotics during the first 7 years of life: a cumulative risk reduction of eczema in a randomized, placebo-controlled trial. J Allergy Clin Immunol 2007; 119: 1019–21
- Di Caro S, Tao H, Grillo A, Franceschi F, Elia C, Zocco MA, et al. Bacillus clausii effect on gene expression pattern in small bowel mucosa using DNA microarray analysis. Eur J Gastroenterol Hepatol 2005; 17: 951–60
- Rosenfeldt V, Michaelsen KF, Jakobsen M, Larsen CN, Moller PL, Tvede M, et al. Effect of probiotic Lactobacillus strains on acute diarrhea in a cohort of nonhospitalized children attending day-care centers. Pediatr Infect Dis J 2002; 21: 417–19
- Sheih YH, Chiang BL, Wang LH, Liao CK, Gill HS. Systemic immunity-enhancing effects in healthy subjects following dietary consumption of the lactic acid bacterium Lactobacillus rhamnosus HN001. J Am Coll Nutr 2001; 20(2 Suppl)149–56
- Rayes N, Seehofer D, Hansen S, Boucsein K, Muller AR, Serke S, et al. Early enteral supply of lactobacillus and fiber versus selective bowel decontamination: a controlled trial in liver transplant recipients. Transplantation 2002; 74: 123–7
- Linsalata M, Russo F, Berloco P, Caruso ML, Matteo GD, Cifone MG, et al. The influence of Lactobacillus brevis on ornithine decarboxylase activity and polyamine profiles in Helicobacter pylori-infected gastric mucosa. Helicobacter 2004; 9: 165–72
- Valeur N, Engel P, Carbajal N, Connolly E, Ladefoged K. Colonization and immunomodulation by Lactobacillus reuteri ATCC 55730 in the human gastrointestinal tract. Appl Environ Microbiol 2004; 70: 1176–81
- Casas IA, Dobrogosz WJ. Validation of the probiotic concept: Lactobacillus reuteri confers broad-spectrum protection against disease in humans and animals. Microb Ecol Health Dis 2000; 12: 247–85
- Kullisaar T, Songisepp E, Mikelsaar M, Zilmer K, Vihalemm T, Zilmer M. Antioxidative probiotic fermented goats’ milk decreases oxidative stress-mediated atherogenicity in human subjects. Br J Nutr 2003; 90: 449–56
- Songisepp E, Kals J, Kullisaar T, Mandar R, Hutt P, Zilmer M, et al. Evaluation of the functional efficacy of an antioxidative probiotic in healthy volunteers. Nutr J 2005; 4: 22
- Kaur S, Kullisaar T, Mikelsaar M, Eisen M, Rehema A, Vihalemm T, et al. Successful management of mild atopic dermatitis in adults with probiotics and emollients. Cent Eur J Med 2008; 3: 215–20
- Lencner, AA , Lencner, CP , Mikelsaar, ME , Tjuri, ME , Toom, MA , Valjaots, ME , , et al . [The quantitative composition of the intestinal lactoflora before and after space flights of different lengths.]. Nahrung 1984;28:607–13, (in German).
- Lencner, A , Lencner, H , Brilis, V , Brilene, T , Mikelsaar, M , Turi, M . [The defense function of the digestive tract lactoflora.]. Nahrung 1987;31:405–11, (in German).
- Mikelsaar MH, Annuk A, Shchepetova J, Mändar R, Sepp E, Björkstén B. Intestinal lactobacilli of Estonian and Swedish children. Microb Ecol Health Dis 2002; 14: 75–80
- Kaizu H, Sasaki M, Nakajima H, Suzuki Y. Effect of antioxidative lactic acid bacteria on rats fed a diet deficient in vitamin E. J Dairy Sci 1993; 76: 2493–9
- Parik T, Allikmets K, Teesalu R, Zilmer M. Oxidative stress and hyperinsulinaemia in essential hypertension: different facets of increased risk. J Hypertens 1996; 14: 407–10
- Kaur S, Zilmer M, Eisen M, Kullisaar T, Rehema A, Vihalemm T. Patients with allergic and irritant contact dermatitis are characterized by striking change of iron and oxidized glutathione status in nonlesional area of the skin. J Invest Dermatol 2001; 116: 886–90
- Karelson E, Bogdanovic N, Garlind A, Winblad B, Zilmer K, Kullisaar T, et al. The cerebrocortical areas in normal brain aging and in Alzheimer's disease: noticeable differences in the lipid peroxidation level and in antioxidant defense. Neurochem Res 2001; 26: 353–61
- Mikelsaar M, Kullisaar T, Zilmer M. Antagonistic and antioxidative activity of lactobacilli and survival in oxidative milieu. Am J Clin Nutr 2001; 73: 495S–495S
- Woodmansey EJ, McMurdo ME, Macfarlane GT, Macfarlane S. Comparison of compositions and metabolic activities of fecal microbiotas in young adults and in antibiotic-treated and non-antibiotic-treated elderly subjects. Appl Environ Microbiol 2004; 70: 6113–22
- Silvi S, Verdenelli MC, Orpianesi C, Cresci A. EU project Crownalife: functional foods, gut microflora and healthy ageing. Isolation and identification of lactobacillus and Bifidobacterium strains from faecal samples of elderly subjects for a possible probiotic use in functional foods. J Food Engin 2003; 56: 195–200
- Pereira DI, Gibson GR. Cholesterol assimilation by lactic acid bacteria and bifidobacteria isolated from the human gut. Appl Environ Microbiol 2002; 68: 4689–93
- Vassos D, Maipa V, Voidarou C, Alexopoulos A, Bezirtzoglou E. Development of human lactic acid (LAB) gastrointestinal microbiota in a Greek rural population. Cent Eur J Biol 2008; 3: 55–60
- Matsuguchi T, Takagi A, Matsuzaki T, Nagaoka M, Ishikawa K, Yokokura T, et al. Lipoteichoic acids from Lactobacillus strains elicit strong tumor necrosis factor alpha-inducing activities in macrophages through Toll-like receptor 2. Clin Diagn Lab Immunol 2003; 10: 259–66
- Vinderola CG, Medici M, Perdigon G. Relationship between interaction sites in the gut, hydrophobicity, mucosal immunomodulating capacities and cell wall protein profiles in indigenous and exogenous bacteria. J Appl Microbiol 2004; 96: 230–43
- Midtvedt AC, Midtvedt T. Production of short chain fatty acids by the intestinal microflora during the first 2 years of human life. J Pediatr Gastroenterol Nutr 1992; 15: 395–403
- Songisepp, E . Evaluation of technological and functional properties of the new probiotic Lactobacillus fermentum ME-3. Diss Med Univ Tartuensis, 2005.
- Štšepetova J, Sepp E, Hütt P, Mikelsaar M. Estimation of lactobacilli, enterococci and bifidobacteria in faecal samples by quantitative bacteriology and FISH. Microecol Therapy 2002; 29: 51–9
- Mikelsaar, M , Zilmer, M , Kullisaar, M , Annuk, H , Songisepp, E . Strain of microorganism Lactobacillus fermentum ME-3 as novel antimicrobial and antioxidative probiotic. Patent application no. P200100356 29.06.2001.
- Annuk H, Shchepetova J, Kullisaar T, Songisepp E, Zilmer M, Mikelsaar M. Characterization of intestinal lactobacilli as putative probiotic candidates. J Appl Microbiol 2003; 94: 403–12
- Kandler, O, Weiss, N. Regular gram positive nonsporing rods. In: PHA Sneath, Mair, NS, Sharp, MS, Holt, JG, Bergey's manual of systematic bacteriology, vol 2. Baltimore: Williams & Wilkins, 1986;208–34.
- Smidt, I , Stsepetova, J , Hütt, P , Sepp, E , Mikelsaar, M . Evaluation of biogenic amines in urine after synbiotic consumption. Probiotics, Synbiotics and Functional Foods: Scientific and Clinical Aspects, 15–16 May 2007, Peterburg, Russia: Medline-Medina, 2007;1-2:A13-A14.
- Songisepp, E , Mikelsaar, M , Rätsep, M , Zilmer, M , Hütt, P , Utt, M , , et al . Isolated microorganism strain Lactobacillus plantarum Tensia DSM 21380 as antimicrobial and antihypertensive probiotic, food product and composition comprising said microorganism and use of said microorganism for production of antihypertensive medicine and method for suppressing non-starter lactobacilli in food product. Estonian Patent Office. 13.05.2008. P200800026.
- Bover-Cid S, Holzapfel WH. Improved screening procedure for biogenic amine production by lactic acid bacteria. Int J Food Microbiol 1999; 53: 33–41
- Kidata M, Igarashi K, Hirose S, Kitagawa H. Inhibition by polyamines of lipid peroxide formation in rat liver microsomes. Biochem Biophys Res Commun 1979; 87: 388–94
- Fernandez MF, Boris S, Barbes C. Probiotic properties of human lactobacilli strains to be used in the gastrointestinal tract. J Appl Microbiol 2003; 94: 449–55
- Alander M, Satokari R, Korpela R, Saxelin M, Vilpponen-Salmela T, Mattila-Sandholm T, et al. Persistence of colonization of human colonic mucosa by a probiotic strain, Lactobacillus rhamnosus GG, after oral consumption. Appl Environ Microbiol 1999; 65: 351–4
- Cesana BM. Comments on ‘Sustained reduction in circulating cholesterol in adult hypopituitary patients given low dose titrated growth hormone replacement therapy: a two-year study’ by Florakis et al.(2000). Clin Endocrinol 2001; 55: 695–7
- Liener IE, Sharon N, Goldstein IJ. The lectins. Properties, functions, and applications in biology and medicine. Academic Press, Orlando, FL 1986
- Onyshchenko, AM , Korobkova, KS , Kovalenko, NK , Kasumova, SO , Skrypal, IH . [The role of the carbohydrate composition of the glycocalyx in some species of lactobacilli in the manifestation of their adhesive properties.]. Mikrobiol Z 1999;61:22–8, (in Ukrainian).
- Annuk H, Hynes SO, Hirmo S, Mikelsaar M, Wadstrom T. Characterisation and differentiation of lactobacilli by lectin typing. J Med Microbiol 2001; 50: 1069–74
- Kärkkainen UM, Ikaheimo R, Katila ML, Siitonen A. Recurrence of urinary tract infections in adult patients with community-acquired pyelonephritis caused by E. coli: a 1-year follow-up. Scand J Infect Dis 2000; 32: 495–9
- Ouwehand AC, Derrien M, de Vos W, Tiihonen K, Rautonen N. Prebiotics and other microbial substrates for gut functionality. Curr Opin Biotechnol 2005; 16: 212–17
- Annuk H, Hirmo S, Turi E, Mikelsaar M, Arak E, Wadstrom T. Effect on cell surface hydrophobicity and susceptibility of Helicobacter pylori to medicinal plant extracts. FEMS Microbiol Lett 1999; 172: 41–5
- Songisepp E, Kullisaar T, Hutt P, Elias P, Brilene T, Zilmer M, et al. A new probiotic cheese with antioxidative and antimicrobial activity. J Dairy Sci 2004; 87: 2017–23
- Annuk, H . Selection of medicinal plants and intestinal lactobacilli as antimicrobial components for functional foods. Diss Med Univ Tartuensis, Tartu, 2002.
- Hütt P, Shchepetova J, Lõivukene K, Kullisaar T, Mikelsaar M. Antagonistic activity of probiotic lactobacilli and bifidobacteria against entero- and uropathogens. J Appl Microbiol 2006; 100: 1324–32
- Hütt, P , Lõivukene, K , Utt, M . Antagonistic activity of probiotic Lactobacillus fermentum ME-3 against H. pylori strains. 6th International Workshop on Pathogenesis and Host Response in Helicobacter Infections, 23–26. June, 2004, Helsingör, Denmark.
- Gotteland M, Brunser O, Cruchet S. Systematic review: are probiotics useful in controlling gastric colonization by Helicobacter pylori?. Aliment Pharmacol Ther 2006; 23: 1077–86
- Marchand J, Vandenplas Y. Micro-organisms administered in the benefit of the host: myths and facts. Eur J Gastroenterol Hepatol 2000; 12: 1077–88
- Salminen MK, Rautelin H, Tynkkynen S, Poussa T, Saxelin M, Valtonen V, et al. Lactobacillus bacteremia, species identification, and antimicrobial susceptibility of 85 blood isolates. Clin Infect Dis 2006; 42: 35–44
- Vankerckhoven V, Huys G, Vancanneyt M, Vael C, Klare I, Romond MB, et al. Biosafety assessment of probiotics used for human consumption: recommendations from the EU-PROSAFE project. Trends Food Sci Technol 2008; 19: 102–14
- Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. Oxford University Press, New York 1999
- Sies, H . Oxidative stress: oxidants and antioxidants. London, Academic Press, 1991.
- Stocker R, Keaney JF, Jr. Role of oxidative modifications in atherosclerosis. Physiol Rev 2004; 84: 1381–478
- Kals J, Kampus P, Kals M, Zilmer K, Kullisaar T, Teesalu R, et al. Impact of oxidative stress on arterial elasticity in patients with atherosclerosis. Am J Hypertens 2006; 19: 902–8
- Stojiljkovic V, Todorovic A, Radlovic N, Pejic S, Mladenovic M, Kasapovic J, et al. Antioxidant enzymes, glutathione and lipid peroxidation in peripheral blood of children affected by coeliac disease. Ann Clin Biochem 2007; 44(Pt 6)537–43
- Krzystek-Korpacka M, Neubauer K, Berdowska I, Boehm D, Zielinski B, Petryszyn P, et al. Enhanced formation of advanced oxidation protein products in IBD. Inflamm Bowel Dis 2008; 14: 794–802
- Tsukahara H. Biomarkers for oxidative stress: clinical application in pediatric medicine. Curr Med Chem 2007; 14: 339–51
- Suzuki M, Kamei M, Itabe H, Yoneda K, Bando H, Kume N, et al. Oxidized phospholipids in the macula increase with age and in eyes with age-related macular degeneration. Mol Vis 2007; 13: 772–8
- Castellani RJ, Lee HG, Zhu X, Perry G, Smith MA. Alzheimer disease pathology as a host response. J Neuropathol Exp Neurol 2008; 67: 523–31
- Vincent HK, Innes KE, Vincent KR. Oxidative stress and potential interventions to reduce oxidative stress in overweight and obesity. Diabetes Obes Metab 2007; 9: 813–39
- Mensink RP, Aro A, Den Hond E, German JB, Griffin BA, ten Meer HU, et al. PASSCLAIM – Diet-related cardiovascular disease. Eur J Nutr 2003; 42(Suppl 1)I6–27
- Vaughan EE, Heilig HG, Ben-Amor K, de Vos WM. Diversity, vitality and activities of intestinal lactic acid bacteria and bifidobacteria assessed by molecular approaches. FEMS Microbiol Rev 2005; 29: 477–90
- Kullisaar T, Zilmer M, Mikelsaar M, Vihalemm T, Annuk H, Kairane C, et al. Two antioxidative lactobacilli strains as promising probiotics. Int J Food Microbiol 2002; 72: 215–24
- Mikelsaar M, Zilmer M. Probiotics series (4) Probiotics biotherapeutics & health: microbial, biochemical and probiotical history (ME-3), C Schiavi. Mofin Alce, NovaraItaly 2005; 114–37
- Kullisaar, T , Songisepp, E , Aunapuu, M , Kilk, K , Arend, A , Mikelsaar, M , , et al . Complete glutathione system in probiotic L. fermentum ME-3. Appl Biochem Microbiol 2009; 45, (in press).
- Jarvenpaa S, Tahvonen RL, Ouwehand AC, Sandell M, Jarvenpaa E, Salminen S. A probiotic, Lactobacillus fermentum ME-3, has antioxidative capacity in soft cheese spreads with different fats. J Dairy Sci 2007; 90: 3171–7
- Teemu H, Seppo S, Jussi M, Raija T, Kalle L. Reversible surface binding of cadmium and lead by lactic acid and bifidobacteria. Int J Food Microbiol 2008; 125: 170–5
- Truusalu K, Naaber P, Kullisaar T, Tamm H, Mikelsaar R-H, Zilmer K, et al. The influence of antibacterial and antioxidative probiotic lactobacilli on gut mucosa in mouse model of Salmonella infection. Microb Ecol Health Dis 2004; 16: 180–7
- Kullisaar, T , Mikelsaar, M , Kaur, S , Songisepp, E , Zilmer, K , Hütt, P , . Probiotics, oxidative stress and diseases. In: D Ćurić, et al. Proceedings of the 2008 Joint Central European Congress: 4th Central European Congress on Food and 6th Croatian Congress of Food Technologists, Biotechnologists and Nutritionists, 15–17 May 2008, Cavtat, Croatia, 2008;1:367–73. ISBN 978-953-6207-87-9.
- Kullisaar, T , Zilmer, K , Vihalemm, T , Zilmer, M . Consumption of antioxidative probiotic probiotic L. fermentum ME-3 and its impact on post-prandial lipid profile in human volunteers. Third Central European Congress on Food, May 2006, Sofia, Bulgaria: p. 208.
- Truusalu K, Mikelsaar RH, Naaber P, Karki T, Kullisaar T, Zilmer M, et al. Eradication of Salmonella Typhimurium infection in a murine model of typhoid fever with the combination of probiotic Lactobacillus fermentum ME-3 and ofloxacin. BMC Microbiol 2008; 8: 132
- Igarashi T, Kono Y, Tanaka K. Molecular cloning of manganese catalase from Lactobacillus plantarum. J Biol Chem 1996; 271: 29521–4
- Rochat T, Gratadoux JJ, Gruss A, Corthier G, Maguin E, Langella P, et al. Production of a heterologous nonheme catalase by Lactobacillus casei: an efficient tool for removal of H2O2 and protection of Lactobacillus bulgaricus from oxidative stress in milk. Appl Environ Microbiol 2006; 72: 5143–9
- Mikelsaar M, Hütt P, Kullisaar T, Zilmer K, Zilmer M. Double benefit claims for antimicrobial and antioxidative probiotic. MEHD 2008; 20(4)184–88
- Mattila-Sandholm T, Blum S, Collins JK, Crittenden R, de Vos W, Dunne C, et al. Probiotics: towards demonstrating efficacy. Trends Food Sci Technol 1999; 10: 393–9
- Brigidi P, Vitali B, Swennen E, Bazzocchi G, Matteuzzi D. Effects of probiotic administration upon the composition and enzymatic activity of human fecal microbiota in patients with irritable bowel syndrome or functional diarrhea. Res Microbiol 2001; 152: 735–41
- Saulnier DMA, Hutt P, Mikelsaar M, Bosscher D, Gibson G, Kolida S. Effects of a symbiotic on biomarkers of oxidative stress and fecal microbiota in healthy adults: results of a cross-over double-blind placebo controlled trial. Proc Nutr Soc 2007; 66: 101A
- Mikelsaar, M , Zilmer, M , Kullisaar, T , Annuk, H , Songisepp, E . Strain of microorganism Lactobacillus fermentum ME-3 as novel antimicrobial and antioxidative probiotic. United States Patent 7, 244, 424 B2. Approved July 17, 2007.
- Jones DP. Redefining oxidative stress. Antioxid Redox Signal 2006; 8: 1865–79
- Karelson E, Mahlapuu R, Zilmer M, Soomets U, Bogdanovic N, Langel U. Possible signaling by glutathione and its novel analogue through potent stimulation of fontocortical G proteins in normal aging and in Alzheimer's disease. Ann N Y Acad Sci 2002; 973: 537–40
- Zilmer M, Soomets U, Rehma A, Langel Ü. Drug Design Reviews – Online 2005; 2: 121–7
- Peran L, Sierra S, Comalada M, Lara-Villoslada F, Bailon E, Nieto A, et al. A comparative study of the preventative effects exerted by two probiotics, Lactobacillus reuteri and Lactobacillus fermentum, in the trinitrobenzenesulfonic acid model of rat colitis. Br J Nutr 2007; 97: 96–103
- de la Asuncion JG, Millan A, Pla R, Bruseghini L, Esteras A, Pallardo FV, et al. Mitochondrial glutathione oxidation correlates with age-associated oxidative damage to mitochondrial DNA. FASEB J 1996; 10: 333–8
- Dzau VJ, Antman EM, Black HR, Hayes DL, Manson JE, Plutzky J, et al. The cardiovascular disease continuum validated: clinical evidence of improved patient outcomes: part I: Pathophysiology and clinical trial evidence (risk factors through stable coronary artery disease). Circulation 2006; 114: 2850–70
- Holvoet P, Lee DH, Steffes M, Gross M, Jacobs DR, Jr. Association between circulating oxidized low-density lipoprotein and incidence of the metabolic syndrome. JAMA 2008; 299: 2287–93
- Kampus P, Kals J, Ristimae T, Muda P, Ulst K, Zilmer K, et al. Augmentation index and carotid intima-media thickness are differently related to age, C-reactive protein and oxidized low-density lipoprotein. J Hypertens 2007; 25: 819–25
- George J. Mechanisms of disease: the evolving role of regulatory T cells in atherosclerosis. Nat Clin Pract Cardiovasc Med 2008; 5: 531–40
- Lopez-Miranda J, Perez-Martinez P, Marin C, Moreno JA, Gomez P, Perez-Jimenez F. Postprandial lipoprotein metabolism, genes and risk of cardiovascular disease. Curr Opin Lipidol 2006; 17: 132–8
- Marsillach J, Camps J, Ferré N, Beltran R, Rull A, Mackness B, et al. Paraoxonase-1 is related to inflammation, fibrosis and PPAR delta in experimental liver disease. BMC Gastroenterol 2009; 9: 3–10
- Elliott WJ. Renovascular hypertension: an update. J Clin Hypertens (Greenwich) 2008; 10: 522–33
- Bhatt DL. Anti-inflammatory agents and antioxidants as a possible “third great wave” in cardiovascular secondary prevention. Am J Cardiol 2008; 101(10A)4D–13