Abstract
Gastric cancer (GC) is a type of the most common cancers. Autoimmune gastritis (AIG) and infection with Helicobacter pylori (HP) are the risk factors of triggering GC. With the emphasis on the treatment of HP, the incidence and prevalence of HP infection in population is decreasing. However, AIG lacks accurate diagnosis and treatment methods, which occupies high cancer risk factors. AIG is controlled by the immune environment of the stomach, including immune cells, inflammatory cells, and infiltrating intercellular material. Various immune cells or cytokines play a central role in the process of regulating gastric parietal cells. Abnormal expression levels of cytokines involved in immunity are bound to face the risk of tumorigenesis. Therefore, it is particularly important for preventing or treating AIG and avoiding the risk of gastric cancer to clarify the confirmed action mode of immune cells and cytokines in the gastric system. Herein, we briefly reviewed the role of the immune environment under AIG, focussing on describing these double-edged effects between immune cells and cytokines, and pointing out potential research challenges.
Introduction
Gastric cancer (GC) is a tumour of the stomach, which is the most common cancer contributing to 5.5% of all new cases of cancers [Citation1]. GC accounts for about 800,000 deaths (7.7% of all cancer deaths), making it the fourth leading cause of cancer death in humans [Citation2]. According to the gradual evolution model of “normal gastric mucosa–chronic superficial gastritis–chronic atrophic gastritis (CAG)–intestinal metaplasia–dysplasia–GC” mentioned by Correa, CAG is a precursor disease of gastric cancer [Citation3]. It is well known that CAG is associated with Helicobacter pylori (HP) infection, showing atrophy of the gastric mucosal epithelium and glands, thinning of the gastric mucosa, thickening of the mucosal basal layer, or with metaplasia from the pyloric and intestinal glands [Citation4, Citation5]. Because HP infection has made an important progress in epidemiology, pathology, treatment plans, and strategies, the incidence and prevalence of HP infection in Western populations are decreasing [Citation6, Citation7]. Gradually, humans realised that gastric inflammation may be formed through the action of immune cells, cytokines, chemokines, and other mediators, which together drive the development of cancer. This immune-induced CAG is called autoimmune gastritis (AIG). Survey data show the prevalence of AIG is increasing nowadays [Citation8]. AIG is a non-self-limiting, chronic inflammatory disorder defined by affecting oxygenated (acid secreting gastric compartment) mucosa and causing gastric mucosal atrophy [Citation9]. Different from non-AIG, the immune inflammatory reaction site only appears in the corpus and fundus glands of the stomach, as shown in . The reason is that the site of oxyntic secretion by the parietal cells triggers the immune response [Citation10, Citation11]. Gastric atrophy leads to impairment of the proton pump to oxygenate the mucosa, resulting in reduced gastric acid secretion, lack of intrinsic factors (here mainly refers to the combination of vitamin b12 and glycoprotein), and is prone to digestive tract abnormalities and pernicious anaemia [Citation11, Citation12]. Unfortunately, AIG frequently manifests as corpus dominant advanced atrophy (reverse atrophy) due to lack of early diagnosis [Citation7]. Allowing AIG to expand arbitrarily leads to hypergastrinemia, intestinal chromaffin cell-like cell proliferation, and even tumours. In addition, AIG is strongly associated with autoimmune diseases in extra gastric glandular tissues, including the thyroid, parathyroid and pancreas [Citation13, Citation14]. Some experts have called for AIG to be treated as a systemic disease [Citation13].
Figure 1. Inflammatory location and deterioration of AIG. The stomach is mainly divided into four parts, namely the cardia, fundus, corpus, and antrum glands. The area of inflammation of AIG is concentrated in the fundus and corpus glands of the stomach. Allowing the development of AIG disease will eventually lead to cancer.
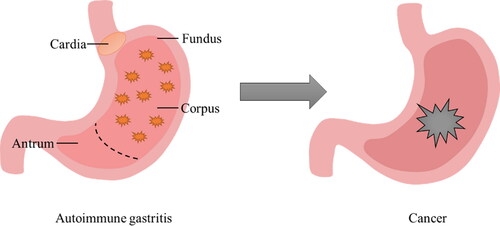
Figure 2. Interaction of immune cells and cytokines in AIG. The gastric mucosa consists of many cells, including chief cells, mucous neck cells and parietal cells. As the major target autoantigen on parietal cells, the gastric proton pump, H+/K+ ATPase is recognised by anti-parietal cell antibodies (PCAs). AIG is attributed to the abnormal mode of the immune system, and the mode of action of cytokines and immune cells leads to changes in AIG, including positive and negative regulation.
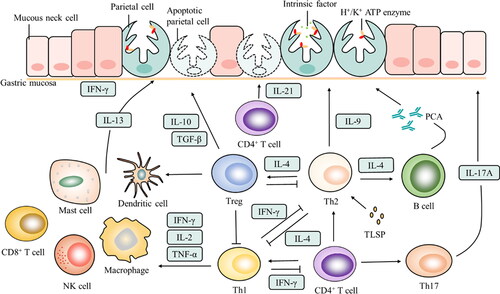
A variety of cytokines or immune cells play a central role in the process of regulating gastric parietal cells. AIG is controlled by the immune environment of the stomach, including immune cells, inflammatory cells, and infiltrating intercellular material. These cell-to-cell interactions allow cytokines from families of interleukins, interferons, and growth factors to induce immune or inflammatory responses, as shown in [Citation12, Citation15, Citation16]. Once gastric parietal cells have been deeply trapped in inflammatory mediators and abnormal cytokine expression levels, it is bound to be at risk of tumorigenesis. In AIG, immune cells that have been found so far include T helper (Th) 1, Th2, Th17, T regulatory cells (Tregs), CD4+ cells, B cells, mast cells, dendritic cells (DCs), macrophages, natural killer cells (NK cells), and CD8+ cells. The cytokines secreted by these cells include interleukin (IL)-9, IL-10, IL-13, IL-17, IL-21, IL-27, interferon (IFN)-γ, transforming growth factor (TGF)-β, and thymic stromal lymphopoietin (TSLP). With the deepening of research, in order to further explore the mechanism of action and corresponding treatment strategy of AIG in the immune environment, it is particularly critical to clearly explain the mode of action of the above-mentioned immune cells and cytokines on the gastric system. Therefore, based on briefly describing the role of the immune environment under AIG, this review focuses on summarising the interaction and mode of action between important immune cells and cytokines in regulating the development of AIG and points out possible challenges.
Immune environment
Human immune system consists of innate immunity and adaptive immunity. Innate immunity is also known as non-specific immunity. Innate immunity involves some innate immune cells (macrophages, DCs, NK cells) that quickly respond to foreign microorganisms entering the human body. It is a natural initial line of defense that prevents viruses, microorganisms, or tissue damage. Adaptive immunity is also called specific immunity, which generally occurs after innate immunity. Adaptive immunity generally refers to a series of immune responses mediated by T cells and B cells [Citation17]. However, the immune system plays a dual role in the progression of AIG. On the one hand, innate immunity and acquired immunity defend against inflammatory substances and maintain the stability of the body. On the other hand, immune cells and cytokines continue to accumulate in the immune environment of the stomach, and a large number of recruited immune cells participate in suppressing the immune microenvironment, promoting inflammatory progression and leading to immune escape of tumour cells [Citation18]. Once the benign immune response process is out of balance, it is easy to provoke immune diseases.
Despite immune mechanism is closely related to diagnose and treat autoimmune diseases, the immune environment of AIG is still in the exploratory stage at present. During the gastrointestinal endoscopy in the 1960s, it was found that the destruction of parietal cells was caused by the complement-dependent cytotoxic effect of anti-parietal cell antibodies (PCA) on gastric parietal cells [Citation12]. The main target is the autoantigen gastric proton pump H+/K+ ATPase whose major epitope is located between residues 360 and 525 on the cytosolic side of the secretory membrane [Citation19]. PCA is usually detected in serum of AIG patients and excess IgG4-positive plasma cells are detected in gastric biopsy, which may be caused by local production of PCA by partial B cell-dependent activation of T cells [Citation20]. In fact, the disease of AIG is attributed to autoreactive T cells. Mice expressing a transgenic T-cell receptor that recognises the neonatal thymocyte antigen H+/K+ ATPase develop severe AIG [Citation21]. Although T cells are divided into CD4+ T cells and CD8+ T cells based on the expression of CD4 and CD8 molecules, studies have shown that T cell-mediated autoimmunity mainly depends on the former rather than the latter [Citation22]. Studies have proved in neonataly thymectomized BALB/c AIG mice and some human samples that the gastric proton pump H+/K+ ATPase is involved in inducing the proliferation of gastric mucosal CD4+ T cells in vitro [Citation23]. Most of CD4+ T cells specific proliferating clones, predominantly of the Th1 subtype, express perforin-mediated cytotoxicity against antigen-presenting cells and induce Fas-Fas ligand-mediated apoptosis in T cells [Citation24]. In addition, Th2, Th17, and Treg, which are generated by CD4+ T cell differentiation, are also closely associated with AIG.
Immune cells
T helper cells
T helper cells (Th) are regarded as the main players in the response against infectious organisms and the promotion of systemic and organ-specific autoimmune diseases. Th secretes a variety of cytokines, which are divided into three different types according to their cytokine characteristics: Th1 that secretes IFN-γ and TNF-α; Th2 that secretes IL-4, IL-5 and IL-13; and Th17 that secretes IL-17 [Citation25].
Th cells differentiation mainly produce Th1 and Th2 subgroups, and only the sum of the two accounts for about 80% of all activated Th cells. Th1 enhances cellular immunity and promotes the phagocytosis of macrophages and the killing ability of NK cells. The secreted cytokines IFN-γ, IL-2 and TNF-α promote the proliferation, differentiation and maturation of CD8+ T cells (CD8+ T cells exhibit a cytotoxicity) [Citation20]. Th1 induces gastric epithelial cell death through Fas-Fas ligand and perforin/granzyme B pathway, which is closely related to the Th1 cytokine IFN-γ [Citation20]. Excessive secretion of IFN-γ causes gastric epithelial cells to be infiltrated by inflammation, which will further activate professional antigen-presenting cells, leading to the activation of autoreactive T cells with gastric H+/K+ ATPase peptide exceeding the threshold level [Citation26]. IFN-γ directly induces CD4+ T cell apoptosis through IFNγR1 and induces autophagy in gastric epithelial cells by up-regulating Beclin-1 [Citation27].
The above seemed to prove that AIG is mainly mediated by Th1, but autoantigen-specific Th2 was detected in the gastric mucosa [Citation28]. In nu/nu recipient animals, Th2 leads to AIG with a mixed infiltrate composed of polymorphonuclear leukocytes and eosinophils [Citation29]. Th2 enhances humoral immunity and secretes cytokines IL-4 and IL-13 to promote the proliferation, differentiation and maturation of B cells. Under IL-4-/- DEREG mice, IL-4 and Th2 responses are critical to mediate gastric mucosal damage after Treg consumption, and are the main contributors to the pathogenesis of AIG [Citation30]. Although both IL-4 and IL-13 are related to Th2 subgroups and are involved in allergic reactions, the AIG molecular mechanism for the production and progression of IL-13 is still complicated, and it seems that it is secreted by mast cells, etc., and has nothing to do with Th2 [Citation31].
Th1 and Th2 inhibit each other’s differentiation in immunity. IL-4 strongly inhibits the development of Th1 cells even at high levels of IFN-γ, thereby resisting Th1-activated macrophages and inhibiting the secretion of a variety of effective pro-inflammatory mediators. IFN-γ, secreted by Th1, inhibits the differentiation of naïve T cells into Th2 cells [Citation32]. It seems that Th1 and Th2 are like rivals, but in fact they are in a dynamic equilibrium relationship in the body. Th2 cytokines directly inhibit the development of Th1/Th17 through IL-4/IL-13, or mediate protection by inhibiting Th1-mediated inflammation [Citation25]. Excessive Th1 differentiation damages tissues, and excessive Th2 differentiation triggers hypersensitivity reactions. The long-term imbalance of Th1/Th2 is an important cause of autoimmune diseases such as AIG, and even severely leads to the production of tumours [Citation25].
The role of Th17 in the entire immune system cannot be ignored. Th17 plays a role in the activation of neutrophils and bacterial immunity, especially on the mucosal surface. In an animal model experiment designed for the ATPase of gastric parietal cells, each injection of Th1, Th2 and Th17 cells can induce AIG [Citation33]. IL-17A is directly involved in the loss of gastric mucosal parietal cells and this finding can explain the pathogenicity of Th17 cells in chronic atrophic gastritis [Citation34]. Th17 cells are important for host defense against invading bacteria and are key effector T cells involved in multiple sclerosis, rheumatoid arthritis, respiratory diseases, systemic lupus erythematosus and chronic inflammatory bowel disease. Th17 cells promote tissue damage by secreting IL-17, which is an important pathogenic factor in experimental autoimmune diseases [Citation35]. This finding defies the long-held belief that AIG has been mediated by Th1. In addition, TGF-β1, secreted by Treg cells or Th17, seems to be essential for the differentiation of Th17, by inhibiting the expression of interleukin-12 receptor β2 and interleukin-27 receptor α to maintain the stability of Th17 [Citation36]. Under homeostasis, inhibition of kynurenine 3-monooxygenasethat is preferentially expressed by Th17 cells significantly aggravates the disease severity of Th17-driven AIG model [Citation37]. However, Th17 cells also exhibit anti-inflammatory function, produce effective anti-inflammatory cytokine IL-10, and reduce diseases [Citation38].
Regulatory T cells
The lack of Tregs has been proved to be closely related to the triggering of various immune diseases. Treg is a type of CD4+ T cell subset that can inhibit autoimmune response, specifically express Foxp3, secrete IL-10 and TGF-β and other intracellular factors. Among them, the transcription factor Foxp3 is necessary for the development and function of Tregs. The main phenotype of Tregs is CD4+CD5+ T cells, which inhibit the immune response of other cells to self and foreign antigens, that is, immune tolerance. The presence of CD4+CD25+ T cells sustains to inhibit the DC activation of autoreactive T cells, and DCs may exhibit H+/K+ ATPase in a tolerant manner [Citation39]. In a model of AIG induced by injection of Th1 and Th2, Tregs (IL-10 and TGF-β secretion) reduce the incidence and severity of this autoimmune disease, which is strangely not in the Th17 induced group [Citation33]. In RAG-2−/− mice, it was confirmed that both the induction and prevention of AIG disease require CD4+CD25+T cells [Citation40]. Similarly, Jessica Harakal’s research also proved that transient Treg deletion leads to long-term AIG, which is related to H+/K+ ATPase and intrinsic factor autoantibody reaction [Citation30]. However, unlike juvenile neonatal mice, in normal adult mice without CD4+CD25+ regulatory T cells, the activation rate of AIG is less than 30%. It is speculated that other factors are involved, and the role of Treg in adult mice may be to ensure that the autoimmune response after a local inflammatory event is suppressed [Citation41].
It is worth noting that natural regulatory T cells (nTregs) and TGF-β-induced Tregs (iTregs) are both important for regulating immune response and maintaining immune health. nTregs inhibit the differentiation of naïve T cells into Th1 in the early stages of the disease, preventing the migration of effector cells to target organs. Because nTregs possess specific killing of antigen-presenting B cells, Shevach et al. also speculated that nTreg-mediated killing induction of antigen-presenting cells is a way of long-term inhibition of autoimmune diseases [Citation42]. Significantly, iTregs help repair and restore the function of severely damaged gastric tissue in advanced AIG. iTregs secrete a large number of chemokines, recruits additional iTregs into the gastric mucosa, and has maintained the function of Foxp3, participating in the inhibition of effector T cells from entering the gastric mucosa [Citation43]. The transfer of iTregs inhibits the ongoing inflammation and prevents the destruction of parietal cells. Although iTregs promote the apoptosis of naïve T cells, there are still self-reactive T cells or memory T cells in the body. Once iTregs are lost again, AIG will make a comeback [Citation44].
Cytokine signalling
The immune system is essentially similar to a huge network microenvironment, and cytokines are the bridges for communication between cells. Cytokines mainly secreted by immune cells, mediate the fate, function and pathology of immune cells [Citation45]. The cytokine environment in homeostasis or disease is a key factor that determines the role of immune function. Polymorphisms of genes encoding immunomodulatory molecules may affect the secretion or function of corresponding proteins, thereby affecting immune response, inflammation and tissue damage. Once AIG is activated, the relationship between inflammatory response and cytokines has been fully established. As a specific cytokine binds to specific immune cells, the intracellular signal is transduced by the second messenger, leading to the activation of the transcription factor [Citation46]. According to analysing cytokines, the occurrence of AIG is accompanied by significant changes in the expression of many different cytokines, including interleukins, interferons, and growth factors [Citation35, Citation47, Citation48].
Interleukin-10
As an anti-inflammatory factor, produced by CD4+ T cells, CD8+ T cells, Th cells, activated monocytes and B cells, IL-10 is an important regulator of immune response which can inhibit the synthesis of cytokines in IFN-γ and IL-2 produced by T cells in vivo and vitro [Citation49, Citation50]. IL-10 inhibits the expression of many pro-inflammatory cytokines, chemokines and chemokine receptors, and mediates allergen tolerance in allergen-specific immunotherapy. Studies have shown that in AIG mice regulatory dendritic cells can induce regulatory T cells and IL-10 in vitro, induce immunogenic tolerance, and participate in the process of protecting murine AIG [Citation51]. However, there seem to be different voices. According to the IL-10 gene knockout mouse model of gastritis, in the absence of IL-10, the mouse gastritis was cured [Citation40, Citation52]. Therefore, IL-10 is not the only factor, there are other influencing factors that affect the AIG.
Interleukin-13
IL-13 is a pro-inflammatory cytokine involved in allergic responses and IgE by IL4Ra and IL-13Ra1 heterodimeric receptor signalling, produced by innate lymphoid cells type 2, macrophages, and mast cells. Research proves the IL-4/IL-13 signal pathway mainly involves up-regulation of inflammation [Citation53]. In addition, IL-33/IL-13 signalling has been identified as an important immune pathway for M2 macrophage polarisation and metaplasia progression after drug-induced macrowall cell atrophy [Citation54, Citation55]. In response to infection and cellular stress, various investigations have uncovered the role of IL-13 in SPEM development during AIG [Citation56–58]. Clinical data shows that IL-13 levels in peripheral blood of patients with GC are much higher than normal [Citation54]. According one latest research, in the gastric mucosa of mice with AIG and inflammation-induced metaplasia, mast cells are the largest producers of IL-13 among B cells, macrophages, ILC2s, CD4+ and CD8+ T cells during AIG [Citation31]. IL-13 acts directly on gastric epithelial cells, avoiding surrounding immune cells, to increase organoid size, viability, and increasing MUC6-expressing by affecting epithelial cell growth, survival, and differentiation [Citation31]. Due to the involvement of many immune cell subsets, the mechanism of action of these subsets cannot be determined.
Interleukin-17
IL-17 is an inflammatory cytokine secreted by a distinct lineage of CD4+ Th called Th17 that over-expression of IL-17 plays a crucial role in the onset of several autoimmune diseases including AIG [Citation23, Citation59]. According to research, IL-23/IL-17 axis is involved in the development of inflammatory and autoimmune diseases such as long-lasting autoimmune disease, inflammation of the kidney and intestinal inflammation [Citation60, Citation61]. IL-17 mediated by CD4+ T cell self-immune response can recognise H+/K+ ATPase and activate the NLRP3 inflammasome-reactive oxygen species pathway, which causes parietal cell death and AIG [Citation62–64]. Based on the mouse model of autoimmune-mediated atrophic gastritis (TXA23 mouse model), the correlation between the degree of gastric atrophy and the pro-inflammatory cytokine IL-17 was proved [Citation65]. Similarly, IL-17 and IFN-γ aggravate the deterioration of AIG, although the lack of IL-17 or IFN-γ will not prevent the occurrence [Citation59]. In the IL-17 family, IL-17A and IL-17F as a novel inflammatory cytokine’s subset of CD4+ Th play the main role in stomach inflammation [Citation66]. However, elevated IL-17A expression levels frequently appear in patients with AIG or gastric cancer [Citation67]. Studies have confirmed that cells that secrete IL-17A more actively exist in the gastric mucosa, suggesting a close connection between IL-17A and gastric mucosal inflammation [Citation68]. In the AIG mice model, IL-17A is closely related to the degree of inflammation of its own gastric mucosa, which can directly act on the gastric epithelium to induce caspase-dependent apoptosis including parietal cell apoptosis in vivo [Citation34].
Interleukin-21
IL-21 as a multifunctional cytokine, produced by CD4+ T and NK cells is well-known for regulating the proliferation and function of other immune cells [Citation69]. IL-21 with IL-21R can effect T and B cell via the JAK/STAT, MAPK, and PI3K pathways [Citation70]. The study confirms IL-21/IL-21R pathway activates JAK/STAT signalling participating in the pathogenesis of disease including immune disease or GC [Citation71–73]. In NTx-PD-1-/- mice, IL-21 was highly expressed in the inflamed gastric mucosa during AIG. After vivo administration of anti-IL-21, the disease deterioration of AIG is suppressed in NTx-PD-1-/- mice [Citation74]. Though IL-21 plays a critical role for the development of AIG, whether IL-21 overexpression will trigger AIG still needs to be studied.
Interleukin-27
IL-27, belonging to the IL-12 family, is a relatively new cytokine produced by DCs, monocytes, and macrophages including CD4+ T cells, which simultaneously regulates adaptive and innate immune responses [Citation75–77]. In the detection of human HP infection, the expression level of IL27 is generally high [Citation78]. IL-27 regulates IFN-γ and IL-17A that directly act on the gastric epithelium and promote parietal cell atrophy, indicating that IL-27 may play a key role in the process of gastritis [Citation79, Citation80]. Kevin A. Bockerstett’s team successfully identified IL-27 secreted by macrophages and dendritic cells in TxA23 AIG mice [Citation80]. In contrast, mice with IL-27 gene defects developed more severe gastritis symptoms and even atrophy with SPEM. Furthermore, supplement of IL-27 could hinder the progression of the disease by acting on the effector CD4+ T cells during AIG [Citation80].
Interferon-γ
IFN-γ is a critical proinflammatory mediator in immunity and inflammation most secreted by CD4+ or CD8+ T cells, which mediate responses to bacterial infections and autoimmune diseases [Citation81, Citation82]. However, research data proves the triggering of AIG is caused by H+/K+ ATPase-specific CD4+ T cells, not CD8+ T cells [Citation40, Citation83]. CD8+ T cells need the help of CD4+ T cells to enter the target tissue [Citation84]. A high proportion of IFN-γ+ CD4+ T cells were found in mice in the mid-stage of gastritis infection. It is speculated that this phenomenon may be caused by the production of high levels of IFN-γ to promote the recruitment of T cells into the stomach tissue and result in single cells Infiltration. The reason why IFN-γ produced by CD8+ T cells is increased is because the number of T cells produced by total IFN-γ increases significantly [Citation59]. Highly expressed IFN-γ may inhibit the regulatory function of Treg cells in the gastrointestinal environment. IFN-γ has always played an important role in the progression of AIG to SPEM including AIG trigger [Citation32]. In TXA23 mouse model, IFN-γ is directly involved in the death of gastric epithelial cells, leading to the development of chronic atrophic gastritis and SPEM, which is a key determinant of gastric cancer risk [Citation85]. In addition, in NTx-PD-1-/- mice or human, administration of IFN-γ inhibitors hinders the development of AIG [Citation86]. However, the loss of anti-T cell proliferation capacity due to the reduction of IFN-γ results in increased hepatic T cell infiltration in autoimmune hepatitis [Citation87].
Transforming growth factor-β
TGF-β plays an important role in maintaining immune homeostasis, as an effective immunosuppressant, especially in regulating T cells [Citation88]. TGF-β plays a key role in Foxp3+ regulatory T cell-mediated immune suppression [Citation89]. TGF-β has the ability of Tregs to regulate continuous inflammation. On the one hand, it uses antigen-specific TGF-β-induced regulatory T cells to secrete chemokines to regulate T cell transport. The transfer of iTregs prevents and reduces the development of AIG [Citation43]. According to the characteristics of autoantigen-specific TGF-induced Foxp3+ regulatory T cells, AIG is prevented by inhibiting DCs from activating autoreactive T cells in TXA23 mice [Citation90]. On the other hand, the co-transfer of Ag-specific iTregs induced by TGF-β completely prevents tissue destruction in the gastric mucosa, reduces the number of transgenic effector T cells in gastric lymph nodes, and inhibits Th17-mediated AIG [Citation91]. In addition, Studies have confirmed that TGF-β1 can prevent the benign growth of gastric glands and activate myofibroblasts to transform into smooth muscle cells. This process has an important effect on the triggering and aggravation of CAG into SPEM [Citation92]. Therefore, TGF-β is a concerned homeostasis regulator in the stomach and plays an important role in the regulation of gastric diseases.
Thymic stromal lymphopoietin
TSLP is an important cytokine in IL-2 family, which stimulates thymocytes and promote B cell lymphopoiesis like IL-7 [Citation93, Citation94]. TSLP is mainly expressed by epithelial cells and activates many haematopoietically derived cells, including DCs, mast cells, T cells, and B cells [Citation95, Citation96]. TLSP is a master regulator participated in Th2-type inflammation immune responses [Citation97]. However, this cytokine also is involved in Th17-mediated autoimmune responses [Citation98]. Depending on the context and tissues in which it is expressed, TSLP exhibit dual effects including promoting inflammation aggravation or maintaining homeostasis [Citation99]. Similarly, in the gastric system, TLSP has been used as both a tumour-promoting gene and a tumour suppressor [Citation100]. GC is inextricably linked to the patient’s immune system. In the AIG model induced by thymus resection in BALB/c mice 3 days after birth, defects of TSLPR are more likely to produce anti-parietal cell antibodies, gastric mucosal inflammation occurs earlier, and AIG worsens [Citation101]. Contrary to allergic skin disorders, respiratory diseases, intestinal inflammation, etc., TSLP negatively regulates the development of AIG. In AIG mice, TSLP negatively regulates the production of IL-12/23p40 through DCs to promote Th1-type autoimmunity in the stomach [Citation102]. However, given that the induction of TSLP did not dispel inflammation after AIG inflammation was triggered, it is speculated that AIG still has other unknown regulatory effects [Citation101].
Cytokines potentially involved in AIG
Evidence have shown that AIG is caused by the destruction of T cell tolerance to gastric H+/K+ ATPase [Citation103]. IL-2 is a vital growth factor for T cells, and it is the most critical cytokine that promotes the clonal expansion of T cells that have recently been activated by antigens [Citation104]. In addition, for the immune response, IL-2 inhibits the main target of unnecessary immune response and resists immune diseases. In mouse models, IL-2 deficiency can produce a fatal lymphoproliferative inflammatory disease called "IL-2 deficiency syndrome," which manifests as inflammatory bowel disease, autoimmune anaemia, splenomegaly, and lymphadenopathy and lymphocyte infiltration of multiple organs [Citation105]. IL-2 is also necessary for Treg cell function activation. However, it is not clear that IL-2 is necessary for thymogenesis or peripheral expansion and survival of natural Treg. CD25low CD4+ T cells, which seem to include self-reactive T cells, maintain the proliferation and survival of CD25+ CD4+ natural T cells by secreting IL-2. Loss of IL-2 disrupts this feedback control and reduces natural T cells for a certain time. Negative feedback loops through IL-2 impairment may be a cause and predisposing factor of autoimmune diseases [Citation106].
IL-4 has a unique anti-inflammatory effect, which is secreted by Th2 and significantly inhibits the expression of TNF-α, IL-1 and Prostaglandin E2 in human monocytes at mRNA and protein levels. In chronic gastritis, IL-4 is induced by activating the JAK/STAT6 and PI3K signalling pathway, which contributes to the activation of macrophages [Citation107]. This process improves the endocytosis capacity of macrophages, but reduces the motility and cytotoxic activity, showing anti-inflammatory activity and tissue repair function [Citation108]. However, most studies involve HP induction and lack of systematic studies due to autoimmune factors. Recently, the AIG developed in the adult DEREG model exhibits unique antibody response and immunopathological features, indicating that IL-4 is dependent on the powerful Th2 dominant autoimmune response of IL-4, IL-4 is involved in the destruction of gastric parietal cells, and is the main contribution to the pathogenesis of AIG [Citation30].
IL-9 is mainly a product of Th2 cells, which is an important regulatory cytokine of the lung and gastrointestinal tract [Citation109]. Because it is related to Th2, IL-9 is a key pathogenic factor of diseases. However, IL-9 has different activity characteristics, including both anti-inflammatory and pro-inflammatory [Citation109, Citation110]. Other studies have shown that Th17 cells are an important source of IL-9 in humans and mice. IL-9 limits the pathogenic activity of organ-specific autoimmune diseases including AIG. This process relies on activating tolerance to promote mucosal mast cells to inhibit Th17 cells from causing disease [Citation111]. Although the pathway of IL-9-mediated action is still unclear, the future biological exploration of IL-9 in AIG and autoimmune-mediated diseases still has significance.
In mouse models, adrenomedullin induces IL-12 produced by macrophages, promotes the T cell response that produces IFN-γ, and accelerates the development of HP-related gastritis [Citation112]. The expression of IL-12 derived from epithelial cells in the gastric mucosa of patients with gastritis was confirmed by immunohistochemistry [Citation113]. Although epithelial cells have a major impact on the type of immune response through epithelial-derived mediators, the exact function is still unclear. Here we speculate that these gastric epithelial-derived cytokine IL-12 may drive T cells to differentiate into Th1 or Th17 type immune response.
Challenge
As we all know, GC has always been a terrible disease that threatens human life and health. Due to the imbalance of the immune microenvironment, the triggering of AIG has always been a risk factor leading to CAG and even cancer. The gastric mucosal immune microenvironment involves a wide range of immune cells. Research is not only limited to the mainstream Th1, Th2, Th17 and Treg, it is necessary to further study the interaction mode between a variety of immune cells. Explaining clearly the mechanism is very complicated and challenging. However, one point that cannot be ignored is that the risk factors of HP are equally important. In these two disease environments, the induction of immune cells is the main and consistent pathological process of the two, showing extremely similarities [Citation114]. The appropriate HP cross-reactive peptides are as powerful as the specific H+/K+ ATPase peptides in inducing gastric T cells to proliferate and to express their Th1 functional profile [Citation115]. Both expressed perforin-mediated cytotoxicity and Fas-Fas ligand-mediated pro-apoptotic activity, causing gastric atrophy [Citation24, Citation115]. The relationship between HP infection and the development of AIG remains controversial. Ohana et al. found that the degree of gland atrophy, hyperplasia, gastric pH value and serum gastrin level in HP infected AIG mice were significantly lower than those in non-Hp infected AIG mice. They hypothesised that HP infection inhibits the development of AIG in mice [Citation116]. A recent meta-analysis showed that HP infection can prevent the development of AIG by stimulating inflammation in the antrum [Citation117]. Therefore, more research data are needed to confirm the relationship between HP and AIG.
Dysregulation of other gastrointestinal microbes, not only HP, is associated with a large number of inflammatory and autoimmune diseases [Citation118]. More and more attention has been focussed on the composition of gastric microbiota in the context of precancerous lesions, but no specific gastric microbiota has been identified in HP gastritis, chronic atrophic gastritis, or AIG [Citation119]. A total of 266 gastric microbial genera were identified [Citation120]. Helicobacter, Streptococcus, and Prevotella were the most abundant genera [Citation120]. The gastric microbial abundance and diversity in HP-related atrophic gastritis were significantly lower than those in normal stomach, while the gastric microbial diversity in AIG was just opposite [Citation119]. Most scholars speculate that the reason for this phenomenon may be due to differences in acid secretion between these conditions or different immune characteristics. Gastric acid secretion, which has a defensive barrier function, is reduced, leading to possible overgrowth of other microbial groups other than Hp [Citation121]. This assumption is contradictory and lacks extensive data support. In one study, Streptococcaceae (38%) was the most predominant bacterial group found in the stomach of AIG patients [Citation119]. However, there is no conclusive evidence that the non-HP community in the stomach is directly involved in the carcinogenesis [Citation122]. Thus, longitudinal studies tracking changes in the gastric microbiome during disease evolution are needed.
Selective and non-selective immune defects, combined with the overlapping effects of genetic, environmental, and hormonal factors like an "Autoimmune Mosaic," leave many of the processes involved in the pathogenesis of AIG poorly understood [Citation9].The author found here that important information for studying the pathogenic mechanism and therapeutic effect of AIG is basically derived from transgenic mice. Immune diseases of the gastric system in these transgenic animal models are induced by exposure to splenocytes from neonatal thymectomized mice or other highly artificial manipulations of the animal’s immune system. Although animal models have pointed out controversial roles for different Th subpopulations and their cytokines, AIG is a Th1/Th17 sustained autoimmune disease in humans.
Conclusion
AIG is an organ-specific immune-mediated chronic progressive inflammation characterised by hypoxic mucosal atrophy, which leads to the production of anti-apical cell antibodies, parietal cell atrophy and replacement of metaplastic mucosa. This review aims to emphasise the important role of AIG’s cytokines and immune cells in regulating gastric mucosal inflammation, atrophy and AIG progression, and more work will still be needed to clarify the complex mechanisms of how immune cells and cytokines form AIG. The analysis of the important cytokines will also be conducive to develop preventive and therapeutic therapies to reduce the development of AIG induced by inflammation-induced parietal cell apoptosis and avoid the risk of GC.
Disclosure statement
The authors declare no conflict of interest.
Data availability statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Additional information
Funding
References
- Seidlitz T, Koo B-K, Stange DE. Gastric organoids—an in vitro model system for the study of gastric development and road to personalized medicine. Cell Death & Differentiation. 2021;28(1):1–10.
- Ilic M, Ilic I. Epidemiology of stomach cancer. World J Gastroenterol. 2022;28(12):1187–1203.
- Correa P. Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol. 1995;19:s37–s43.
- Dilaghi E, Baldaro F, Pilozzi E, et al. Pseudopyloric metaplasia is not associated with the development of gastric cancer. Am J Gastroenterol. 2021;116(9):1859–1867.
- Shah SC, Piazuelo MB, Kuipers EJ, et al. AGA clinical practice update on the diagnosis and management of atrophic gastritis: expert review. Gastroenterology. 2021;161(4):1325–1332.e7.
- Sousa C, Ferreira R, Azevedo NF, et al. Helicobacter pylori infection: from standard to alternative treatment strategies. Crit Rev Microbiol. 2022;48(3):376–396.
- Terao S, Suzuki S, Yaita H, et al. Multicenter study of autoimmune gastritis in Japan: clinical and endoscopic characteristics. Dig Endosc. 2020;32(3):364–372.
- Coati I, Fassan M, Farinati F, et al. Autoimmune gastritis: pathologist’s viewpoint. World J Gastroenterol. 2015;21(42):12179–12189.
- Neumann WL, Coss E, Rugge M, et al. Autoimmune atrophic gastritis–pathogenesis, pathology and management. Nat Rev Gastroenterol Hepatol. 2013;10(9):529–541.
- Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest. 2007;117(1):60–69.
- Rustgi SD, Bijlani P, Shah SC. Autoimmune gastritis, with or without pernicious anemia: epidemiology, risk factors, and clinical management. Therap Adv Gastroenterol. 2021;14:17562848211038771.
- Lenti MV, Rugge M, Lahner E, et al. Autoimmune gastritis. Nat Rev Dis Primers. 2020;6(1):56.
- Kamada T, Maruyama Y, Monobe Y, et al. Endoscopic features and clinical importance of autoimmune gastritis. Dig Endosc. 2022;34(4):700–713.
- Panimolle F, Tiberti C, Granato S, et al. Evidence of increased humoral endocrine organ-specific autoimmunity in severe and classic X-chromosome aneuploidies in comparison with 46, XY control subjects. Autoimmunity. 2018;51(4):175–182.
- Troilo A, Grassi A, Petrone L, et al. Intrinsic factor recognition promotes T helper 17/T helper 1 autoimmune gastric inflammation in patients with pernicious anemia. Oncotarget. 2019;10(30):2921–2929.
- Pikkarainen S, Martelius T, Ristimaki A, et al. A High prevalence of gastrointestinal manifestations in common variable immunodeficiency. Am J Gastroenterol. 2019;114(4):648–655.
- Mafi S, Mansoori B, Taeb S, et al. mTOR-Mediated regulation of immune responses in cancer and tumor microenvironment. Front Immunol. 2021;12:774103.
- Li L, Yu R, Cai T, et al. Effects of immune cells and cytokines on inflammation and immunosuppression in the tumor microenvironment. Int Immunopharmacol. 2020;88:106939.
- Alderuccio F, Toh B, Tan S-S, et al. An autoimmune disease with multiple molecular targets abrogated by the transgenic expression of a single autoantigen in the thymus. J Exp Med. 1993;178(2):419–426.
- Di Sabatino A, Lenti MV, Giuffrida P, et al. New insights into immune mechanisms underlying autoimmune diseases of the gastrointestinal tract. Autoimmun Rev. 2015;14(12):1161–1169.
- Shevach EM, McHugh RS, Piccirillo CA, et al. Control of T‐cell activation by CD4+ CD25+ suppressor T cells. Immunol Rev. 2001;182(1):58–67.
- Katakai T, Mori KJ, Masuda T, et al. Differential localization of Th1 and Th2 cells in autoimmune gastritis. Int Immunol. 1998;10(9):1325–1334.
- Karlsson F, Burman P, Lööf L, et al. Major parietal cell antigen in autoimmune gastritis with pernicious anemia is the acid-producing H+, K+-adenosine triphosphatase of the stomach. J Clin Invest. 1988;81(2):475–479.
- D’elios MM, Bergman MP, Azzurri A, et al. H+, K+-ATPase (proton pump) is the target autoantigen of Th1-type cytotoxic T cells in autoimmune gastritis. Gastroenterology. 2001;120(2):377–386.
- Raphael I, Nalawade S, Eagar TN, et al. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine. 2015;74(1):5–17.
- Lopez-Diaz L, Hinkle KL, Jain RN, et al. Parietal cell hyperstimulation and autoimmune gastritis in cholera toxin transgenic mice. Am J Physiol Gastrointest Liver Physiol. 2006;290(5):G970–G979.
- Tu SP, Quante M, Bhagat G, et al. IFN-gamma inhibits gastric carcinogenesis by inducing epithelial cell autophagy and T-cell apoptosis. Cancer Res. 2011;71(12):4247–4259.
- Candon S, McHugh RS, Foucras G, et al. Spontaneous organ-specific Th2-mediated autoimmunity in TCR transgenic mice. J Immunol. 2004;172(5):2917–2924.
- Suri-Payer E, Amar A, McHugh R, et al. Post-thymectomy autoimmune gastritis: fine specificity and pathogenicity of anti-H/K ATPase-reactive T cells. Eur J Immunol. 1999;29(2):669–677.
- Harakal J, Rival C, Qiao H, et al. Regulatory T cells control Th2-dominant murine autoimmune gastritis. J Immunol. 2016;197(1):27–41.
- Noto CN, Hoft SG, Bockerstett KA, et al. IL13 Acts directly on gastric epithelial cells to promote metaplasia development during chronic gastritis. Cell Mol Gastroenterol Hepatol. 2022;13(2):623–642.
- Barrett SP, Gleeson PA, De Silva H, et al. Interferon‐γ is required during the initiation of an organ‐specific autoimmune disease. Eur J Immunol. 1996;26(7):1652–1655.
- Stummvoll GH, DiPaolo RJ, Huter EN, et al. Th1, Th2, and Th17 effector T cell-induced autoimmune gastritis differs in pathological pattern and in susceptibility to suppression by regulatory T cells. J Immunol. 2008;181(3):1908–1916.
- Bockerstett KA, Osaki LH, Petersen CP, et al. Interleukin-17A promotes parietal cell atrophy by inducing apoptosis. Cell Mol Gastroenterol Hepatol. 2018;5(4):678–690 e1.
- Vojdani A, Lambert J. The Role of Th17 in neuroimmune disorders: target for CAM therapy. Part I. Evid Based Complement Alternat Med. 2011;2011:927294.
- Choi G, Park Y-J, Cho M, et al. A critical role for Th17 cell-derived TGF-β1 in regulating the stability and pathogenicity of autoimmune Th17 cells. Exp Mol Med. 2021;53(5):993–1004.
- Stephens GL, Wang Q, Swerdlow B, et al. Kynurenine 3‐monooxygenase mediates inhibition of T h17 differentiation via catabolism of endogenous aryl hydrocarbon receptor ligands. Eur J Immunol. 2013;43(7):1727–1734.
- Xu J, Yang Y, Qiu G, et al. c-Maf regulates IL-10 expression during Th17 polarization. J Immunol. 2009;182(10):6226–6236.
- McHugh R. Autoimmune gastritis is a well-defined autoimmune disease model for the study of CD4+ CD25+ T cell-mediated suppression. Curr Top Microbiol Immunol. 2005;293:153–177.
- Suri-Payer E, Cantor H. Differential cytokine requirements for regulation of autoimmune gastritis and colitis by CD4+ CD25+ T cells. J Autoimmun. 2001;16(2):115–123.
- Laurie KL, Van Driel IR, Gleeson PA. The role of CD4+ CD25+ immunoregulatory T cells in the induction of autoimmune gastritis. Immunol Cell Biol. 2002;80(6):567–573.
- Shevach EM, DiPaolo RA, Andersson J, et al. The lifestyle of naturally occurring CD4+ CD25+ Foxp3+ regulatory T cells. Immunol Rev. 2006;212(1):60–73.
- Nguyen T-LM, Sullivan NL, Ebel M, et al. Antigen-Specific TGF-β–induced regulatory T cells secrete chemokines, regulate T cell trafficking, and suppress ongoing autoimmunity. J Immunol. 2011;187(4):1745–1753.
- Tu E, Bourges D, Gleeson PA, et al. Pathogenic T cells persist after reversal of autoimmune disease by immunosuppression with regulatory T cells. Eur J Immunol. 2013;43(5):1286–1296.
- Bockerstett KA, DiPaolo RJ. Regulation of gastric carcinogenesis by inflammatory cytokines. Cell Mol Gastroenterol Hepatol. 2017;4(1):47–53.
- Gaffen SL. An overview of IL-17 function and signaling. Cytokine. 2008;43(3):402–407.
- Oksanen AM, Haimila KE, Rautelin HI, et al. Immunogenetic characteristics of patients with autoimmune gastritis. World J Gastroenterol. 2010;16(3):354–358.
- Munari F, Fassan M, Capitani N, et al. Cytokine BAFF released by Helicobacter pylori-infected macrophages triggers the Th17 response in human chronic gastritis. J Immunol. 2014;193(11):5584–5594.
- Bodger K, Wyatt J, Heatley R. Gastric mucosal secretion of interleukin-10: relations to histopathology, Helicobacter pylori status, and tumour necrosis factor-alpha secretion. Gut. 1997;40(6):739–744.
- Kindlund B, Sjöling Å, Yakkala C, et al. CD4+ regulatory T cells in gastric cancer mucosa are proliferating and express high levels of IL-10 but little TGF-β. Gastric Cancer. 2017;20(1):116–125.
- Torisu M, Murakami H, Akbar F, et al. Protective role of interleukin-10-producing regulatory dendritic cells against murine autoimmune gastritis. J Gastroenterol. 2008;43(2):100–107.
- Asseman C, Mauze S, Leach MW, et al. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190(7):995–1004.
- Bridgewood C, Newton D, Bragazzi N, et al. Unexpected connections of the IL-23/IL-17 and IL-4/IL-13 cytokine axes in inflammatory arthritis and enthesitis. Semin Immunol. 2021;58:101520.
- Petersen CP, Meyer AR, De Salvo C, et al. A signalling cascade of IL-33 to IL-13 regulates metaplasia in the mouse stomach. Gut. 2018;67(5):805–817.
- Duffen J, Zhang M, Masek-Hammerman K, et al. Modulation of the IL-33/IL-13 axis in obesity by IL-13Ralpha2. J Immunol. 2018;200(4):1347–1359.
- Amo-Aparicio J, Garcia-Garcia J, Francos-Quijorna I, et al. Interleukin-4 and interleukin-13 induce different metabolic profiles in microglia and macrophages that relate with divergent outcomes after spinal cord injury. Theranostics. 2021;11(20):9805–9820.
- Conde E, Bertrand R, Balbino B, et al. Dual vaccination against IL-4 and IL-13 protects against chronic allergic asthma in mice. Nat Commun. 2021;12(1):2574.
- May RD, Fung M. Strategies targeting the IL-4/IL-13 axes in disease. Cytokine. 2015;75(1):89–116.
- Tu E, Ang DK, Bellingham SA, et al. Both IFN‐γ and IL‐17 are required for the development of severe autoimmune gastritis. Eur J Immunol. 2012;42(10):2574–2583.
- Jones CM, Callaghan JM, Gleeson PA, et al. The parietal cell autoantibodies recognized in neonatal thymectomy-induced murine gastritis are the α and β subunits of the gastric proton pump. Gastroenterology. 1991;101(2):287–294.
- Zandi F, Bagheri N, Rahimian G, et al. Evaluation of H. pylori infection and IL23R gene polymorphism in dyspeptic subjects. Life Sci J. 2014;11(SPEC. I):40–46.
- Toh B-H, Sentry JW, Alderuccio F. The causative H+/K+ ATPase antigen in the pathogenesis of autoimmune gastritis. Immunol Today. 2000;21(7):348–354.
- Hu Z, Chai J. Structural mechanisms in NLR inflammasome assembly and signaling. Curr Top Microbiol Immunol. 2016;397:23–42.
- Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–1141.
- McHugh RS, Shevach EM, Margulies DH, et al. At cell receptor transgenic model of severe, spontaneous organ‐specific autoimmunity. Eur J Immunol. 2001;31(7):2094–2103.
- Azadegan-Dehkordi F, Abbasi A, Abadi ATB, et al. From genes polymorphisms to mucosal expression of cytokines: evaluating IL-23/IL-17 axis in adult patients with gastritis. Afr Health Sci. 2020;20(3):1452–1462.
- Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity. 2004;21(4):467–476.
- Moseley T, Haudenschild DR, Rose L, et al. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14(2):155–174.
- Long D, Chen Y, Wu H, et al. Clinical significance and immunobiology of IL-21 in autoimmunity. J Autoimmun. 2019;99:1–14.
- Davis MR, Zhu Z, Hansen DM, et al. The role of IL-21 in immunity and cancer. Cancer Lett. 2015;358(2):107–114.
- Huang Y, Zhang J, Wang G, et al. Oxymatrine exhibits anti-tumor activity in gastric cancer through inhibition of IL-21R-mediated JAK2/STAT3 pathway. Int J Immunopathol Pharmacol. 2018;32:2058738418781634.
- Ren HM, Lukacher AE, Rahman ZS, et al. New developments implicating IL-21 in autoimmune disease. J Autoimmun. 2021;122:102689.
- Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57–79.
- Nishiura H, Iwamoto S, Kido M, et al. Interleukin‐21 and tumor necrosis factor‐α are critical for the development of autoimmune gastritis in mice. J Gastroenterol Hepatol. 2013;28(6):982–991.
- Meka RR, Venkatesha SH, Dudics S, et al. IL-27-induced modulation of autoimmunity and its therapeutic potential. Autoimmun Rev. 2015;14(12):1131–1141.
- Wojno EDT, Hunter CA, Stumhofer JS. The immunobiology of the interleukin-12 family: room for discovery. Immunity. 2019;50(4):851–870.
- Hunter CA, Kastelein R. Interleukin-27: balancing protective and pathological immunity. Immunity. 2012;37(6):960–969.
- Rocha GA, de Melo FF, Cabral MM, et al. Interleukin‐27 is abrogated in gastric cancer, but highly expressed in other Helicobacter pylori‐associated gastroduodenal diseases. Helicobacter. 2020;25(1):e12667.
- Lucas S, Ghilardi N, Li J, et al. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and-independent mechanisms. Proc Natl Acad Sci. 2003;100(25):15047–15052.
- Bockerstett KA, Petersen CP, Noto CN, et al. Interleukin 27 protects from gastric atrophy and metaplasia during chronic autoimmune gastritis. Cell Mol Gastroenterol Hepatol. 2020;10(3):561–579.
- Billiau A, Heremans H, Vermeire K, et al. Immunomodulatory Properties of interferon‐γ: an update a. Ann N Y Acad Sci. 1998;856(1):22–32.
- Kelchtermans H, Billiau A, Matthys P. How interferon-γ keeps autoimmune diseases in check. Trends Immunol. 2008;29(10):479–486.
- Silva D, Driel V, Gruta L. CD4+ T cells, but not CD8+ T cells, are required for the development of experimental autoimmune gastritis. Immunology. 1998;93(3):405–408.
- Nakanishi Y, Lu B, Gerard C, et al. CD8+ T lymphocyte mobilization to virus-infected tissue requires CD4+ T-cell help. Nature. 2009;462(7272):510–513.
- Osaki LH, Bockerstett KA, Wong CF, et al. Interferon-gamma directly induces gastric epithelial cell death and is required for progression to metaplasia. J Pathol. 2019;247(4):513–523.
- Fabbri C, Jaboli MF, Giovanelli S, et al. Gastric autoimmune disorders in patients with chronic hepatitis C before, during and after interferon-alpha therapy. World J Gastroenterol. 2003;9(7):1487–1490.
- Iwamoto S, Kido M, Aoki N, et al. IFN-γ is reciprocally involved in the concurrent development of organ-specific autoimmunity in the liver and stomach. Autoimmunity. 2012;45(2):186–198.
- Shevach EM, Davidson TS, Huter EN, et al. Role of TGF-β in the induction of Foxp3 expression and T regulatory cell function. Journal of Clinical Immunology. 2008;28(6):640–646.
- Marie JC, Letterio JJ, Gavin M, et al. TGF-β1 maintains suppressor function and Foxp3 expression in CD4+ CD25+ regulatory T cells. J Exp Med. 2005;201(7):1061–1067.
- DiPaolo RJ, Brinster C, Davidson TS, et al. Autoantigen-specific TGFβ-induced Foxp3+ regulatory T cells prevent autoimmunity by inhibiting dendritic cells from activating autoreactive T cells. J Immunol. 2007;179(7):4685–4693.
- Huter EN, Stummvoll GH, DiPaolo RJ, et al. Cutting edge: antigen-specific TGFβ-induced regulatory T cells suppress Th17-mediated autoimmune disease. J Immunol. 2008;181(12):8209–8213.
- Tong Y, Wang R, Liu X, et al. Zuojin Pill ameliorates chronic atrophic gastritis induced by MNNG through TGF-β1/PI3K/akt axis. J Ethnopharmacol. 2021;271:113893.
- Roan F, Bell BD, Stoklasek TA, et al. The multiple facets of thymic stromal lymphopoietin (TSLP) during allergic inflammation and beyond. J Leukoc Biol. 2012;91(6):877–886.
- Leonard WJ. TSLP: finally in the limelight. Nat Immunol. 2002;3(7):605–607.
- Ohno T, Nakamura T, Nakae S, et al. TSLP is a negative regulator of RANKL-induced osteoclastogenesis. Biochem Biophys Res Commun. 2020;530(3):508–512.
- Marković I, Savvides SN. Modulation of signaling mediated by TSLP and IL-7 in inflammation, autoimmune diseases, and cancer. Front Immunol. 2020;11:1557.
- Adhikary PP, Tan Z, Page BD, et al. TSLP as druggable target-a silver-lining for atopic diseases? Pharmacol Ther. 2021;217:107648.
- Ziegler SF, Roan F, Bell BD, et al. The biology of thymic stromal lymphopoietin (TSLP). Adv Pharmacol. 2013;66:129–155.
- Shubin NJ, Clauson M, Niino K, et al. Thymic stromal lymphopoietin protects in a model of airway damage and inflammation via regulation of caspase-1 activity and apoptosis inhibition. Mucosal Immunol. 2020;13(4):584–594.
- Barooei R, Mahmoudian RA, Abbaszadegan MR, et al. Evaluation of thymic stromal lymphopoietin (TSLP) and its correlation with lymphatic metastasis in human gastric cancer. Med Oncol. 2015;32(8):1–8.
- Nishiura H, Kido M, Aoki N, et al. Increased susceptibility to autoimmune gastritis in thymic stromal lymphopoietin receptor-deficient mice. J Immunol. 2012;188(1):190–197.
- Taylor BC, Zaph C, Troy AE, et al. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J Exp Med. 2009;206(3):655–667.
- Tu E, Ang DK, Hogan TV, et al. A convenient model of severe, high incidence autoimmune gastritis caused by polyclonal effector T cells and without perturbation of regulatory T cells. PLoS One. 2011;6(11):e27153.
- Malek TR. The main function of IL‐2 is to promote the development of T regulatory cells. J Leukoc Biol. 2003;74(6):961–965.
- Taguchi O, Takahashi T. Administration of anti‐interleukin‐2 receptor α antibody in vivo induces localized autoimmune disease. Eur J Immunol. 1996;26(7):1608–1612.
- Setoguchi R, Hori S, Takahashi T, et al. Homeostatic maintenance of natural Foxp3+ CD25+ CD4+ regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201(5):723–735.
- Jiménez‐Garcia L, Herránz S, Luque A, et al. Critical role of p38 MAPK in IL‐4‐induced alternative activation of peritoneal macrophages. Eur J Immunol. 2015;45(1):273–286.
- Yang T, Wang R, Liu H, et al. Berberine regulates macrophage polarization through IL-4-STAT6 signaling pathway in Helicobacter pylori-induced chronic atrophic gastritis. Life Sci. 2021;266:118903.
- Nowak EC, Weaver CT, Turner H, et al. IL-9 as a mediator of Th17-driven inflammatory disease. J Exp Med. 2009;206(8):1653–1660.
- Kar S, Gupta R, Malhotra R, et al. Interleukin-9 facilitates osteoclastogenesis in rheumatoid arthritis. Int J Mol Sci. 2021;22(19):10397.
- Stephens GL, Swerdlow B, Benjamin E, et al. IL‐9 is a Th17‐derived cytokine that limits pathogenic activity in organ‐specific autoimmune disease. Eur J Immunol. 2011;41(4):952–962.
- Kong H, You N, Chen H, et al. Helicobacter pylori-induced adrenomedullin modulates IFN-γ-producing T-cell responses and contributes to gastritis. Cell Death Dis. 2020;11(3):1–15.
- Al-Sammak F, Kalinski T, Weinert S, et al. Gastric epithelial expression of IL-12 cytokine family in Helicobacter pylori infection in human: is it head or tail of the coin? PLoS One. 2013;8(9):e75192.
- Okazaki K, Ohana M, Oshima C, et al. Interaction of Helicobacter pylori-induced follicular gastritis and autoimmune gastritis in BALB/c mice with post-thymectomy autoimmune gastritis. J Gastroenterol. 2003;38(12):1131–1137.
- Amedei A, Bergman MP, Appelmelk BJ, et al. Molecular mimicry between Helicobacter pylori antigens and H+, K+–adenosine triphosphatase in human gastric autoimmunity. J Exp Med. 2003;198(8):1147–1156.
- Ohana M, Okazaki K, Oshima C, et al. Inhibitory effects of Helicobacter pylori infection on murine autoimmune gastritis. Gut. 2003;52(8):1102–1110.
- Youssefi M, Tafaghodi M, Farsiani H, et al. Helicobacter pylori infection and autoimmune diseases; is there an association with systemic lupus erythematosus, rheumatoid arthritis, autoimmune atrophy gastritis and autoimmune pancreatitis? A systematic review and meta-analysis study. J Microbiol Immunol Infect. 2021;54(3):359–369.
- Karczewski J, Poniedziałek B, Adamski Z, et al. The effects of the microbiota on the host immune system. Autoimmunity. 2014;47(8):494–504.
- Parsons BN, Ijaz UZ, D’Amore R, et al. Comparison of the human gastric microbiota in hypochlorhydric states arising as a result of Helicobacter pylori-induced atrophic gastritis, autoimmune atrophic gastritis and proton pump inhibitor use. PLoS Pathog. 2017;13(11):e1006653.
- Rajilic‐Stojanovic M, Figueiredo C, Smet A, et al. Systematic review: gastric microbiota in health and disease. Aliment Pharmacol Ther. 2020;51(6):582–602.
- Conti L, Annibale B, Lahner E. Autoimmune gastritis and gastric microbiota. Microorganisms. 2020;8(11):1827.
- Engstrand L, Graham DY. Microbiome and gastric cancer. Dig Dis Sci. 2020;65(3):865–873.

