Abstract
Introduction: To explore the value of serum sirtuin-1 (SIRT1) in the diagnosis and evaluation of joint mobility of rheumatoid arthritis (RA). Materials and Methods: Serum was randomly obtained from 212 RA patients,210 non-RA patients and 58 healthy controls in a large tertiary first-class hospital in Jiangxi province from November 2021 to June 2022. The level of serum Sirt1,anti-cyclic citrulline polypeptide antibody (anti-CCP), anti-mutant citrulline vimentin antibody (anti-MCV), rheumatoid factor (RF),high-mobility group box 1 (HMGB1), collagen triple helix repeat containing 1 (CTHRC1), erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were detected by ELISA, to explore the correlation between them and their value in the diagnosis and evaluation of joint range of motion of RA and statistically analyse their diagnostic efficiency. Results: ① The level of all markers was higher in the RA group than in the non-RA group and the healthy controls (p < 0.05). ② The AUC of the SIRT1 was 0.882, second only to the anti-MCV and anti-CCP. ③ The anti-CCP showed the highest sensitivity to RA diagnosis of 0.948. The specificity and positive predictive value of SIRT1 for the diagnosis of RA were the highest, which are 0.959 and 0.934 respectively. ④ In serial combination, SIRT1/anti-CCP、SIRT1/anti-MCV showed the highest specificity.SIRT1/anti-CCP in parallel combination had the highest sensitivity. ⑤ SIRT1 showed a significant correlation with other markers and DAS28 scores (p < 0.01). Conclusion: SIRT1 can be used as a new serological marker for RA diagnosis, which has a significant correlation with RA joint mobility and has a certain reference value in RA differential diagnosis, providing a new detection basis for RA differential diagnosis.
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease that mainly causes synovial inflammation and joint injury, it can occur at early ages, and often occurs in elderly people over 60 years old. The disease can occur at any age, but it is most common among those aged 40–70 years. RA’s aetiology is unclear and may be related to many factors. Currently, it is believed that its pathogenesis is related to immune cells and cytokines in vivo. The existing markers do not seem to make us particularly satisfied in the accurate diagnosis of RA patients, and those with atypical symptoms are easy to be missed and misdiagnosed [Citation1]. Although anti-cyclic peptide containing citrulline (anti-CCP) has high specificity and good sensitivity, there are still 20–30% of RA patients whose anti-CCP are negative. Sirtuins (SIRT) is a class III histone deacetylase family, which is highly conserved in evolution. SIRT1 was first discovered in the human body in 1999 and its complementary DNA sequence contains an open reading frame of about 2.4 kb and nine exons, which has an antioxidant and anti-apoptotic effect [Citation2]. By reviewing the literature, we found that SIRT1 has a certain relationship with the occurrence and development of RA, and can be used as a new biomarker to improve the diagnostic efficiency of RA [Citation3–5].
Materials and methods
Subjects
The serum of 212 RA patients, 210 non-RA patients, and 58 healthy controls was collected randomly in a large tertiary first-class hospital in Jiangxi province from November 2021 to June 2022. The level of serum SIRT1, anti-CCP, anti-mutant citrulline vimentin (anti-MCV), RF, high-mobility group box 1 (HMGB1), collagen triple helix repeat containing 1 (CTHRC1), erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were detected, exploring the application value of SIRT1 and other markers in the diagnosis of RA [Citation3–5].
Diagnostic criteria
(1) Patients in the RA group meet the latest diagnostic classification criteria for RA as revised by the European Union against Rheumatism (EULAR) [Citation6–8]; (2) Patients with other autoimmune diseases diagnosed as non-RA were classified as a non-RA group, including osteoarthritis (OA), ankylosing spondylitis (AS), Sjogren’s syndrome (SS) and systemic lupus erythematosus (SLE). The clinical diagnosis of the patients in this group meets the corresponding diagnostic criteria [Citation9–13].
Inclusion criteria
(1) All specimens were obtained with patients’ informed consent and the study was approved by the hospital ethics committee of the hospital. (2) There is no abnormality in the routine examination of each system of the healthy controls.
Exclusion criteria
(1) Suffer from other arthritis or joint diseases; (2) Combined with severe liver and kidney function damage or metabolic diseases (such as thyroid and parathyroid diseases), affecting bone metabolism; (3) Combined with serious cardiovascular and cerebrovascular diseases or chronic kidney diseases; (4) Combined with tumour and bone metastasis; (5) Taking anticancer drugs or other drugs that affect bone metabolism; (6) Pregnant or lactating women; (7) Exclude other autoimmune diseases.
Instruments, reagents, and methods
Instruments and methods
Three ml of fasting venous blood from patients and healthy controls were collected, separated by centrifugation at 1026 g for 15 min, and various markers were tested within 4h after collection. SIRT1 (Laer Biotechnology Co. LTD, Hefei, China),anti-CCP (Yonghe Luo Biotechnology Co. LTD, Shenzhen, China),anti-MCV (Kexin Biotechnology Co. LTD, Shanghai, China), HMGB1 (Hengyuan Biotechnology Co. LTD, Shanghai, China) and CTHRC1 (Huzhen Industrial Co. LTD, Shanghai, China) were measured by commercial ELISAs. RF and CRP were detected by rate-scattering turbidimetry by Beckman IMMAGE800 automatic specific protein analysis system and supporting reagents. ESR was detected by automatic rapid erythrocyte sedimentation rate analyser Roller20 and supporting reagents made by ALIFAX Company of Italy. All operations are carried out in strict accordance with the reagent, instrument instructions, and the hospital’s standardised operating procedures (SOP).
Scoring of disease activity
The joint swelling and tenderness of RA patients were recorded by two experienced rheumatic immunologists. The range of motion of RA patients was evaluated by the DAS28 method, and the DAS28 score was calculated.
Statistical analysis
SPSS 26.0 was used for data analysis. Data that satisfy normal distribution are represented by mean and standard deviation, otherwise, quartile spacing are used. If the two groups of data satisfy the normal distribution, an independent sample t-test was used, otherwise, the Mann-Whitney U test was used. The ROC curve, area under the curve (AUC) and youden index were measured, comprehensively evaluating their application effect in the diagnosis and prediction of RA. In addition, combined detection evaluated the reference value of SIRT1 in joint diagnosis. Using Spearman correlation analysis to test whether there is a correlation between each marker. p<0.05 indicated that the difference was statistically significant.
Results
General data and differential analysis
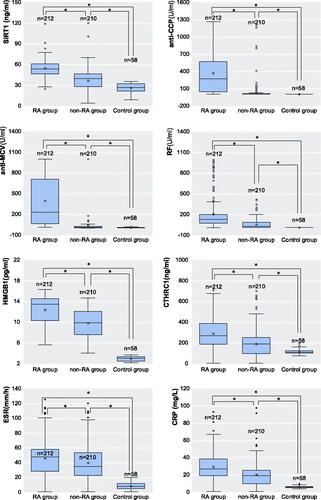
There was no significant difference in age, sex, and other general data among the RA group, non-RA group, and healthy control group (p < 0.05). The data of the eight markers (SIRT1, anti-CCP, anti-MCV, RF, HMGB1, CTHRC1, ESR, and CRP) of the three groups do not satisfy the normal distribution and homogeneity of variance. Pairwise analysis of three groups by U test, there are significant differences in the eight markers (p < 0.05, and ).
Table 1. General information of each group.
ROC curve drawing and comparison of the area under the curve
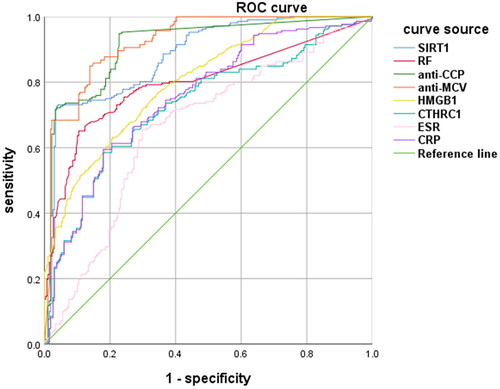
Established the ROC curve of 8 detection markers, and then we obtained the AUC. The area under the curve of anti-MCV was the largest, followed by anti-CCP, and the area under the SIRT1 curve was 0.882, which was second only to the first two markers ( and ).
Table 2. Regions under the ROC curve of the eight test markers.
Diagnostic value and significance of SIRT1
According to the results, the best cut-off value, sensitivity, specificity, youden index, negative likelihood ratio, positive likelihood ratio, negative predictive value, and positive predictive value were calculated. Among them, anti-CCP had the highest sensitivity (0.948). The specificity and positive predictive value of SIRT1 were the highest (0.959 and 0.934 respectively). For joint detection of SIRT1/anti-CCP、SIRT1/anti-MCV and anti-CCP/anti-MCV, the series combination of SIRT1/anti-CCP and SIRT1/anti-MCV had the highest specificity (0.959), the parallel combination of SIRT1/anti-CCP had the highest sensitivity (0.972) ( and ).
Table 3. Clinical evaluation of the eight test markers.
Table 4. Clinical evaluation of the combined test.
Correlation analysis between various markers
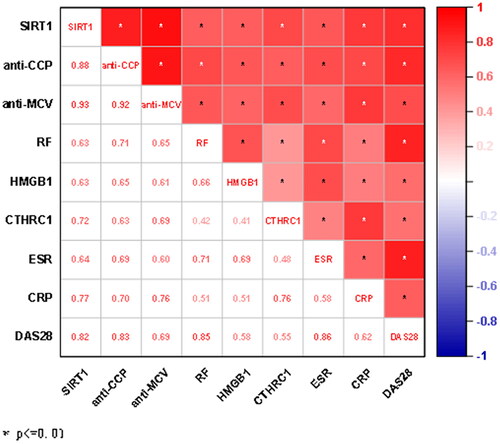
Using Spearman to analyse the data, there was a significant correlation among the 8 markers and between the 8 markers and the DAS28 score (p < 0.05), the correlation coefficients between SIRT1 and anti-CCP, SIRT1 and anti-MCV, SIRT1 and CTHRC1, SIRT1 and CRP, SIRT1 and DAS28, anti-CCP and anti-MCV, anti-CCP and RF, anti-CCP and CRP, anti-CCP and DAS28, anti-MCV and CRP, RF and ESR, RF and DAS28, CTHRC1 and CRP, ESR and DAS28 were all greater than 0.7, showing a very significant strong positive correlation ().
Discussion
The incidence of RA in China is about 0.3–0.5%. In clinical practice, patients often fail to get timely and effective treatment due to misdiagnosis and other reasons, which seriously affects patients’ quality of life. At present, the common clinical diagnostic markers for RA are mainly anti-CCP, anti-MCV and RF. In addition, elevated HMGB1, CTHRC1, ESR and CRP can also be used as auxiliary diagnostic markers for RA [Citation4].
The distribution of seven members of the SIRT family is different in cells, SIRT3-SIRT5 protein is concentrated in organelles, SIRT2 is located in the cytoplasm, and SIRT1 is distributed in nucleus and cytoplasm [Citation14]. Activation of SIRT in mammals can effectively prevent the further development of neurological diseases, cardiovascular diseases, inflammatory diseases, diabetes, cancer and other diseases. In recent years, the role of SIRT has attracted more and more attention from researchers, among which SIRT1 has attracted much attention [Citation14–16]. SIRT1 can affect the activity of mitogen-activated protein kinase (MAPK), nuclear factor kappa-B (NF- κ B), activator protein1 (AP-1), hypoxia-inducible factor-1 α (HIF-1 α) and other protein substrates through self-deacetylation and regulate the inflammatory signal transduction of macrophages and T cells, and then affect the stability of the internal environment of the body [Citation2,Citation17]. Other studies have shown that activation of MAPK can induce the expression of matrix metalloproteinases and M1 polarisation of macrophages, accelerate the proliferation and invasion of rheumatoid arthritis fibroblast-like synoviocytes (RA-FLS), and increase the secretion of inflammatory mediators, thus aggravating the injury of RA bones and joints [Citation18]. In addition, the overexpression of SIRT1 can inhibit apoptosis, extracellular matrix degradation and inflammatory cell invasion in OA.
At present, the correlation and mechanism of SIRT1 on RA are still unclear, and the related research reports are still relatively few. This study concluded that SIRT1 can be used as a new serological index for the diagnosis of RA, which has a significant correlation with the range of motion of RA joint, and has a certain reference value in the differential diagnosis of RA, which provides a new detection basis for the differential diagnosis of RA.
In this study, the activity level of SIRT1, anti-CCP, anti-MCV, RF, HMGB1, CTHRC1, ESR and CRP were significantly higher than those in the non-RA group and healthy group (p < 0.05), and the results were consistent with previous studies [Citation3–5]. There was a significant correlation between SIRT1 and the other 7 markers and DAS28 scores (p < 0.01). Among them, the correlation coefficient among SIRT1,anti-CCP and anti-MCV is more than 0.7, which is a very significant positive correlation, suggesting that SIRT1 is a pro-inflammatory factor and can aggravate the disease of RA. There is a high correlation between SIRT1 and DAS28 scores, indicating that there is a strong correlation between SIRT1 activity and disease activity in patients with RA. It has been reported in the literature [Citation19], SIRT1 activity was found to be lower in the peripheral blood of RA patients than in healthy controls (p < 0.05), and the correlation analysis showed a negative correlation of SIRT1 activity with tumour necrosis factor α (TNF-α), NF-kB and other markers (p < 0.05), suggesting that SIRT1 plays a role as an anti-inflammatory factor in RA. However, other studies found that overexpression of SIRT1 in RA synovial cells promoted the occurrence and development of chronic inflammation by stimulating the production of inflammatory factors and inhibiting cell apoptosis, resulting in bone mass decline and bone loss, which was somewhat contradictory to the results of this study [Citation20]. Different pieces of literature have obtained different results for the same indicator of SIRT1, it is considered that the effect of SIRT1 on the disease in RA patients will change with the occurrence and development of RA and the continuous accumulation of metabolites.
HMGB1 is a highly conserved DNA-binding protein, which exists widely in the nucleus of eukaryotic cells. HMGB1 secretion increases after inflammation, and migrates extracellular, activating the NF-kB signal pathway, triggering the transcription of various inflammatory factors, and then aggravating the inflammatory response [Citation21]. Previous studies have shown that HMGB1 is associated with the progression of immune diseases such as RA [Citation22]. In this experiment, the AUC of HMGB1 is 0.802, second only to anti-CCP, anti-MCV and SIRT1. The correlation between HMGB1 and anti-CCP, anti-MCV, RF and SIRT1 is greater than 0.6, showing a moderate positive correlation.
This study shows that the area under the ROC curve of SIRT1 is second only to anti-CCP and anti-MCV, indicating that its diagnostic efficacy is good, and the results are similar to previous experiments [Citation3–5]. The specificity of SIRT1 is the highest, indicating that SIRT1 is a specific diagnostic index of RA and has an important recognition value. The positive likelihood ratio of SIRT1 is the highest, suggesting that it can be used as a relatively independent and more clinical evaluation index for the diagnosis of RA. In the serial detection, the specificity of the combined detection of SIRT1/anti-CCP and SIRT1/anti-MCV is the highest, and in the parallel detection, the youden index of SIRT1/anti-CCP antibody is the highest, suggesting that the combined detection can improve the sensitivity of RA diagnosis, which is consistent with the view of Li et al [Citation3].There are still some limitations in this experiment: this study is limited to the cases screened in our hospital, which is not enough to represent the test results in this region or even the whole country; The sample size of this experiment is relatively small, so there is inevitably bias in statistical analysis, such as the high correlation of some data; SIRT1 is not very clear about the pathogenic mechanism of RA, and it is still necessary to further verify its role in the early diagnosis, occurrence, development and prognosis of RA.
In conclusion, SIRT1 may be involved in chronic inflammation in RA patients, and the pathogenesis of RA remains unclear, and further studies are needed to determine. SIRT1 is one of the important diagnostic markers of RA, with good specificity. Combined testing with other RA diagnostic markers can improve the diagnostic rate of RA, which is important for the differential diagnosis and prognostic evaluation of RA, further research is needed in large patient data to establish it as a biomarker.
Data availability statement
The data that support the findings of this study are available from the corresponding author, [LT], upon reasonable request.
Disclosure statement
All authors have no conflicts of interest.
Additional information
Funding
References
- Zhao J, Li ZG. The challenges of early diagnosis and therapeutic prediction in rheumatoid arthritis. Int J Rheum Dis. 2018;21(12):1–6.
- Livshits G, Kalinkovich A. Inflammaging as a common ground for the development and maintenance of sarcopenia, obesity, cardiomyopathy and dysbiosis. Ageing Res Rev. 2019;56:100980.
- Li X, Li X, Zeng T, et al. The clinical value of serum sirtuin-1 in the diagnosis of rheumatoid arthritis: a pilot study. Br J Biomed Sci. 2021;78(4):191–194.
- Hu T, Liu Y, Tan L, et al. Value of serum collagen triple helix repeat containing-1(CTHRC1) and 14-3-3eta protein compared to anti-CCP antibodies and anti-MCV antibodies in the diagnosis of rheumatoid arthritis. Br J Biomed Sci. 2021;78(2):67–71.
- Huang J, Zeng T, Zhang X, et al. Clinical diagnostic significance of 14-3-3eta protein, high-mobility group box-1, anti-cyclic citrullinated peptide antibodies, anti-mutated citrullinated vimentin antibodies and rheumatoid factor in rheumatoid arthritis. Br J Biomed Sci. 2020;77(1):19–23.
- Roodenrijs NMT, van der Goes MC, Welsing PMJ, et al. Difficult-to-treat rheumatoid arthritis: contributing factors and burden of disease. Rheumatology (Oxford). 2021;60(8):3778–3788.
- Mankia K, Siddle H, Di Matteo A, et al. A core set of risk factors in individuals at risk of rheumatoid arthritis: a systematic literature review informing the EULAR points to consider for conducting clinical trials and observational studies in individuals at risk of rheumatoid arthritis. RMD Open. 2021;7(3):e001768.
- Nikiphorou E, Santos EJF, Marques A, et al. 2021 EULAR recommendations for the implementation of self-management strategies in patients with inflammatory arthritis. Ann Rheum Dis. 2021;80(10):1278–1285.
- van Middelkoop M, Bennell KL, Callaghan MJ, et al. International patellofemoral osteoarthritis consortium: consensus statement on the diagnosis, burden, outcome measures, prognosis, risk factors and treatment. Semin Arthritis Rheum. 2018;47(5):666–675.
- Aringer M, Costenbader K, Daikh D, et al. European league against rheumatism/American college of rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 2019;71(9):1400–1412.
- Ward MM, Deodhar A, Gensler LS, et al. 2019 Update of the American college of rheumatology/spondylitis association of america/spondyloarthritis research and treatment network recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Care Res (Hoboken). 2019;71(10):1285–1299.
- Combe B, Landewe R, Daien CI, et al. 2016 Update of the EULAR recommendations for the management of early arthritis. Ann Rheum Dis. 2017;76(6):948–959.
- Shiboski CH, Shiboski SC, Seror R, et al. 2016 American College of Rheumatology/European League against rheumatism classification criteria for primary Sjogren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis. 2017;76(1):9–16.
- Li G, Xia Z, Liu Y, et al. SIRT1 inhibits rheumatoid arthritis fibroblast-like synoviocyte aggressiveness and inflammatory response via suppressing NF-kappaB pathway. Biosci Rep. 2018;38(3):BSR20180541.
- Chen WG, Zhang SS, Pan S, et al. alpha-Mangostin treats early-stage adjuvant-induced arthritis of rat by regulating the CAP-SIRT1 pathway in macrophages. Drug Des Devel Ther. 2022;16:509–520.
- Pei B, Chen K, Zhou S, et al. IL-38 restrains inflammatory response of collagen-induced arthritis in rats via SIRT1/HIF-1alpha signaling pathway. Biosci Rep. 2020;40(5):BSR20182431.
- Hao L, Wan Y, Xiao J, et al. A study of Sirt1 regulation and the effect of resveratrol on synoviocyte invasion and associated joint destruction in rheumatoid arthritis. Mol Med Rep. 2017;16(4):5099–5106.
- Sujitha S, Rasool M. MicroRNAs and bioactive compounds on TLR/MAPK signaling in rheumatoid arthritis. Clin Chim Acta. 2017;473:106–115.
- Wu YJ, Fang WJ, Pan S, et al. Regulation of Sirt1 on energy metabolism and immune response in rheumatoid arthritis. Int Immunopharmacol. 2021;101(Pt A):108175.
- Pasquereau S, Totoson P, Nehme Z, et al. Impact of glucocorticoids on systemic sirtuin 1 expression and activity in rats with adjuvant-induced arthritis. Epigenetics. 2021;16(2):132–143.
- Chen X, Xu Y, Xiong P, et al. Effects of mimicked acetylated HMGB1 on macrophages and dendritic cells. Mol Med Rep. 2018;18(6):5527–5535.
- Taniguchi N, Kawakami Y, Maruyama I, et al. HMGB proteins and arthritis. Hum Cell. 2018;31(1):1–9.