Abstract
Allergic rhinitis (AR) is a common inflammation that affects many people globally. Quercetin has anti-allergic biological activity in AR. Here, we aimed to explore the effects of quercetin on type 1 helper T (Th1)/Th2 and regulatory T cells (Treg)/Th17 balance. We established an ovalbumin (OVA)-induced mouse model and orally administered 20, 35, and 50 mg/kg/day quercetin. The nasal symptoms of mice were observed. The immunoglobulin levels, Treg/Th17-related factors, and pro-inflammatory factors were examined by ELISA. The differentiated inflammation cells were visualized using the diff-quick staining assay. The nasal histopathology was evaluated using H&E, periodic acid Schiff (PAS), and Giemsa staining assay. The results showed that quercetin attenuated OVA-induced rubbing and sneezing. Quercetin reduced IgE, IgG1, histamine, and increased IgG2 in serum. The number of differentiated inflammation cells and goblet cells in tissues that elevated by OVA was reduced by quercetin. Moreover, OVA increased the Treg cell percentage, the levels of IL-17, TGF-β, IL-6, TNF-α, and decreased Th17 cell percentage, IL-10 and FOXP3 levels, while quercetin abrogated their levels induced by OVA. Additionally, quercetin inactivated the NF-κB pathway. Taken together, quercetin attenuated AR symptoms by balancing the Th1/Th2, Treg/Th17 ratios, and inactivating the NF-κB pathway. The results suggested that quercetin may use for AR treatment.
Introduction
Allergic rhinitis (AR) is a chronic disease with high prevalence worldwide, resulting in a huge health and economic burden. AR usually occurs in combination with asthma, conjunctivitis, and sinusitis [Citation1,Citation2]. The onset of AR is caused by a response to inhaled allergens mediated by immunoglobulin E (IgE) and characterized by sneezing, nasal discharge, rhinobyon, and rhinocnesmus [Citation1,Citation3]. Avoid of allergens, drug treatment such as antihistamines and corticosteroids, and allergen-specific immunotherapy are the available treatments for AR; however, it is easy to cause adverse reactions and can no cure AR [Citation4]. Thus, it is urgent to understand the pathogenesis of AR and explore new treatments.
The imbalance of innate and adaptive immunity is the important pathogenesis that induces AR, involving antigen-presenting cells, lymphocytes, and specific T cells [Citation5]. The imbalance between type 1 helper T (Th1) and Th2 cells is the immunological basis of AR [Citation6]. Th2 cells are the drivers of IgE-mediated allergic reactions that facilitate the release of IgE, IL-4, and IL-5 [Citation7]. In addition, the dysfunction of regulatory T cells (Treg)/Th17 cells is also involved in the pathogenesis of AR [Citation8]. Th17 cells regulate the pro-inflammatory response, while Treg regulates the anti-inflammatory response [Citation9]. Therefore, it is interesting to explore the Th1/Th2 and Treg/Th17 balance in AR.
Chinese medicine has been widely concerned in the treatment of diseases due to its effectiveness and safety. Quercetin is a hydroxyl phenolic compound, which is an important representative of the flavonoid subclass. It exists in a variety of plants and regulates plant growth and development [Citation10]. Moreover, quercetin has many biological activities, such as antioxidant, antiviral, anti-inflammatory, and anti-allergic effects [Citation11,Citation12]. Accumulating evidence has shown that quercetin is used to treat and prevent diseases, including cancers, cardiac diseases, inflammation, and metabolic diseases [Citation13]. Quercetin plays a central role in a variety of allergic diseases, including AR. It regulates the Th1/Th2 balance and reduces the release of IgE antibodies [Citation12,Citation14]. However, the regulation of quercetin in the Treg/Th17 balance remains largely unknown.
In the current study, we sought to investigate whether quercetin influences AR by affecting the Th1/Th2 and Treg/Th17 balance. The findings suggested that quercetin has the potential to be an effective drug in the clinical treatment of AS.
Materials and methods
Reagents
Quercetin (purity ≥ 95%, ), Ovalbumin (OVA), aluminum hydroxide, phorbol myristate acetate (PMA), ionomycin, and Brefeldin A (BFA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Diff-Quik Stain solution was purchased from Sbjbio (Nanjing, China). Hematoxylin and eosin staining solutions were purchased from Phygene (Fuzhou, China). Periodic acid-Schiff (PAS) staining kit was purchased from Beyotime (Shanghai, China). Glemsa stain working solution was purchased from Leagene (Beijing, China). All ELISA kits were purchased from FineTest (Wuhan, China). TRIzol reagent was purchased from Invitrogen (Carlsbad, CA, USA). PrimeScript RT Master Mix and Realtime PCR Master Mix (SYBR Green) were purchased from KeyGEN biotech (Nanjing, China). CD4+ T cell isolation kit (mouse) was purchased from NovoBiotechnology (Beijing, China). The fixation/permeabilization concentrate was purchased from eBioscience (San Diego, CA, USA). Antibodies used in flow cytometry were all purchased from Biolegend (San Diego, CA, USA). Antibodies used in western blot were all purchased from Abcam (Cambridge, MA, USA). BCA kit and ECL reagent were purchased from Beyotime (Shanghai, China).
Animal study
The animal study protocol was approved by the Ethics Committee of The First Affiliated Hospital of Chongqing Medical University. AR mice model was established by OVA. BALB/c mice (6 weeks old; Vital River, Beijing, China). The mice were housed in an SPF environment with 12 h light/12 h dark, 23 ± 2 °C, 50-60% humidity, and free water and food. The mice were randomly divided into 5 groups: control, OVA, OVA + 20 mg/kg quercetin, OVA + 35 mg/kg quercetin, and OVA + 50 mg/kg quercetin. To establish the model, the mice were intraperitoneally injected with 50 μg OVA and 1 mg aluminum hydroxide which dissolved in 200 μL normal saline on days 0, 7, and 14. From day 15–27, mice were administered with 20, 35, or 50 mg/kg/day quercetin dissolved in normal saline orally once a day. From day 21, mice were given 20 μL 10 mg/mL OVA intranasally until day 27. The nasal symptoms, including the number of rubbing and sneezing, were quantified during 15 min. On day 28, mice were anesthesia with 40 mg/kg pentobarbital sodium and peripheral blood was acquired from the caudal vena cava of mice. Then, all mice were sacrificed. The nasal mucosa tissues and nasal lavage fluid (NALF) were collected.
NALF collection
After the mice were sacrificed, sterile saline was pumped into the nasal cavity from the nasopharynx, and the NALF was collected from the anterior nostril.
Diff-quick staining assay
The differential cells in 200 μL NALF were counted using the Diff-Quick stain solution. Briefly, the NALF smear was fixed with methanol and stained with Diff-Quick I for 10 s and Diff-Quick II for 15 s. The differentiated cells were observed under a microscope.
H&E staining assay
Mouse nasal mucosa was stripped, fixed in formalin, and made into paraffin sections. The sections were dewaxed and rehydrated to water. The sections were stained with hematoxylin for 5 min, followed by staining with eosin for 3 min. The histopathology was observed under a microscope (Olympus, Tokyo, Japan).
Periodic acid Schiff (PAS) staining assay
Mouse nasal mucosa sections were oxidated with periodate acid, and stained with Schiff’s reagent for 10 min and hematoxylin for 3 min. The goblet cells were observed under a microscope.
Giemsa staining assay
The nasal mucosa smear was incubated with Giemsa stain working solution for 30 min at room temperature. The eosinophils were visualized under a microscope.
ELISA
The whole blood was acquired from mice and the serum was isolated. Mouse OVA specific (s)IgE ELISA kit, mouse OVA sIgG1 ELISA kit, mouse OVA IgG2a ELISA kit, and mouse histamine ELISA kit were used to measure the levels of IgE, IgG1, IgG2a, and histamine levels in the serum of mice. Mouse IL-4 kit, mouse IL-5 kit, and mouse IFN-γ kit were used to detect the levels of IL-4, IL-5, and IFN-γ.
The NALF was centrifugated, and the supernatant was collected. The levels of IL-17, TGF-β1, Foxp3, IL-10, IL-6, and TNF-α using their ELISA kit according to the manufacturer’s protocol.
Quantitative real-time PCR (qPCR)
Total RNA was isolated from NALF using the TRIzol reagent. After detecting RNA purity and integrity, total RNA was reversed transcribed to cDNA using the PrimeScript RT Master Mix. The cDNA was used to conduct qPCR using Realtime PCR Master Mix (SYBR Green). The mRNA expression of IL-6 and TNF-α was calculated using the 2−ΔΔCT method. β-actin was used as the normalization. The specific primer sequences were listed as follows: TNF-α sense: 5′-TTGTGGGAAGCCCAGGCACCA-3′, anti-sense: 5′-ATTCCTTAATGTCACGCACGATTTC-3′; IL-6 sense: 5′-AGGAGACTTGCCTGGTGAAA-3′, anti-sense: 5′-CAGGGGTGGTTATTGCATCT-3′; RORγt sense: 5′-TCCATATTTGACTTTTCCCACT-3′, anti-sense: 5′-GATGTTCCACTCTCCTCTTCTC-3′; Foxp3 sense: 5′-CACCCAGGAAAGACAGCAACC-3′, anti-sense: 5′-CTCGAAGACCTTCTCACAACCA-3′; and β-actin sense: 5′-TCACCCACACTGTGCCCATCTACGA-3′, anti-sense: 5′-CAGCGGAACCGCTCATTGCCAATGG-3′
Peripheral blood mononuclear cell isolation
The peripheral blood was diluted with PBS and added on the Ficoll-Hypaque solution to centrifugated at 800 g for 25 min. The peripheral blood mononuclear cells (PBMCs) were collected and cultured at RPMI-1640 supplemented with 10% FBS at 37 °C with 5% CO2.
Flow cytometry
The percentage of Treg and Th17 cells was measured by flow cytometry. To analyze the Treg cell percentage, PBMCs were incubated with the APC anti-mouse CD4 antibody and anti-mouse CD25 antibody complex at 4 °C for 45 min. After cell immobilization and permeabilization with the fixation/permeabilization concentrate, the cells were incubated with PE anti-mouse Foxp3 antibody in the dark for 30 min. For Th17 cell percentage detection, PBMCs were stimulated with 25 ng/mL PMA, 1 mg/mL ionomycin, and 1 μg/mL BFA at 37 °C for 5 h. Then, the samples were incubated with APC anti-mouse CD4 at 4 °C for 45 min. After fixing and permeabilizing, the samples were incubated with PE anti-mouse IL-17A antibody in the dark for 30 min. Finally, all the samples were assessed by a flow cytometer, and the results were analyzed using the FlowJo software.
Western blot
Total protein was isolated from the nasal mucosa tissues using RIPA buffer. After protein concentration was measured by a BCA kit, 25 μg of protein was added to run on SDS-PAGE and transferred to PVDF membranes. The membranes were incubated with primary antibodies COX-2 (ab179800, 1:2000), IkBα (ab32518, 1:10000), p-IkBα (ab133462, 1/10000), p65 (ab16502, 1:2000), histone H3 (ab1791, 1:2000), and GAPDH (ab9485, 1:2500) at 4 °C overnight. Then, goat anti-rabbit IgG antibody (ab6721, 1:3000) was utilized to incubate with the membranes at room temperature for 2 h. The bands were developed using the ECL reagent.
Statistical analysis
At least three independent repetitions were performed per experiment. The data were analyzed using GraphPad Prism 8 software. One-way ANOVA was used to compare the differences among groups. Data are expressed as means ± standard deviation. A p value of <0.05 was considered statistically significant.
Results
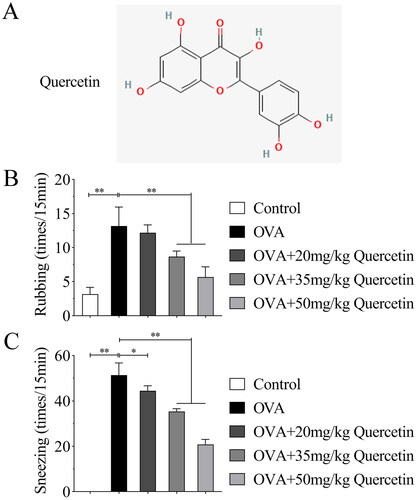
Quercetin inhibits OVA-induced nasal symptoms of AR
First, we established the AR mice model and used multiple differentiations of quercetin to treat. The nasal symptoms were first assessed. The results illustrated that OVA increased the frequency of rubbing and sneezing; however, quercetin reduced the number of rubbing and sneezing in OVA mice in a dose-dependent way ().
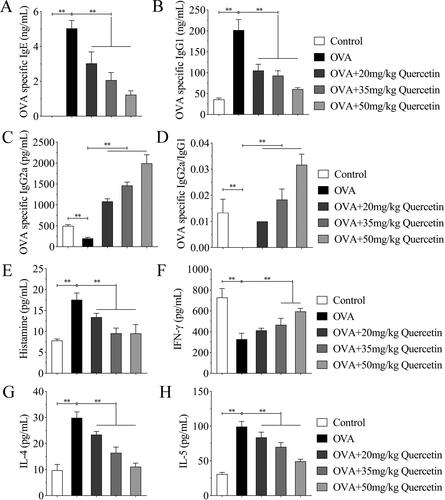
Quercetin promotes Th1/Th2 balance in the serum of OVA-induced mice
IgE, IgG1, and IgG2a mediated immune response during an allergic reaction. Thus, we detected their levels in mice in each group. OVA increased IgE and IgG1 levels, decreased IgG2a levels, and reduced the ratio of IgG2a/IgG1. Quercetin reduced IgE and IgG1 levels, but elevated IgG2a level and IgG2a/IgG1 ratio in OVA mice in a dose-dependent manner (). Moreover, the increase of histamine increased by OVA was abrogated by quercetin along with the increasing concentration (). Additionally, OVA decreased IFN-γ level, and increased IL-4 and IL-5 levels, whereas quercetin reversed OVA-induced effects ().
Quercetin inhibits differentiated inflammatory cells in NALF
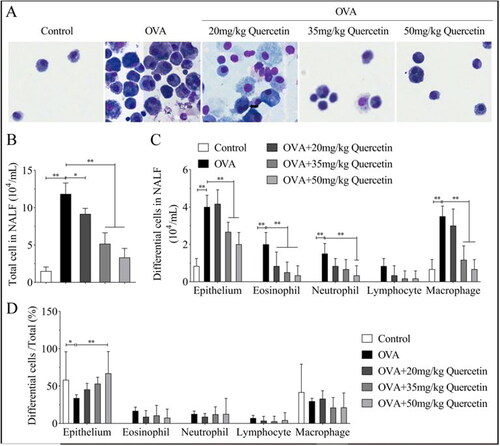
Cell differentiation was observed in NALF acquired from mice. The results showed that the differentiated cells were higher in OVA mice than that in control mice, including epithelial cells, eosinophils, neutrophils, lymphocytes, and macrophages (). Nevertheless, quercetin decreased the number of inflammation-related cells in a dose-dependent manner (). However, only the ratios of differential cells/total cells of the epithelium were changed, while eosinophils, neutrophils, lymphocytes, and macrophages percentage were not regulated by OVA and quercetin ().
Figure 3. Quercetin inhibits differentiated inflammatory cells in NALF. (A) The differentiated inflammation-related cells were observed under a microscope using the diff-quick staining assay. (B) Total differentiated cells in NALF were quantified. (C) Each type of differential cells was quantified. (D) The ratios of differentiated cells/total cells were quantified. **p < 0.01. *p < 0.05.
Quercetin inhibits inflammatory infiltration, goblet cells, and eosinophils induced by OVA
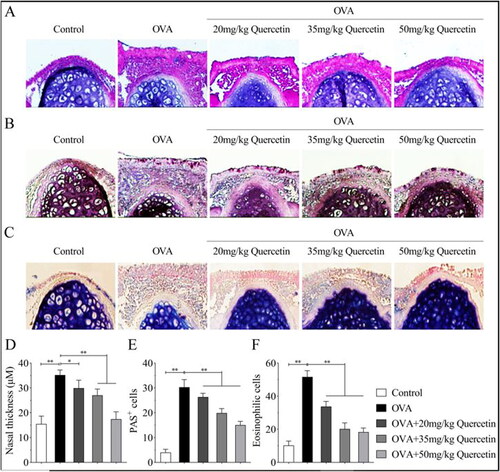
Nasal mucosal tissues were acquired and stained using H&E, PAS, and Giemsa. As shown in , OVA promoted swelling of epithelial cells and inflammatory cell infiltration, while quercetin abrogated the effects induced by OVA. In addition, OVA increased the number of goblet cells, while quercetin suppressed their numbers (). Moreover, quercetin reversed the increase of eosinophils induced by OVA in a dose-dependent way ().
Figure 4. Quercetin inhibits inflammatory infiltration, goblet cells, and eosinophils induced by OVA. The nasal mucosa tissues of mice in each group were collected. (A) Pathological changes of mouse nasal mucosa were visualized using H&E staining assay. (B) The goblet cells were visualized using PAS staining. (C) The eosinophils were visualized using Giemsa staining. (D) The quantification of nasal thickness. (E) The quantification of PAS + cells. (F) The quantification of eosinophilic cells. **p < 0.01. *p < 0.05.
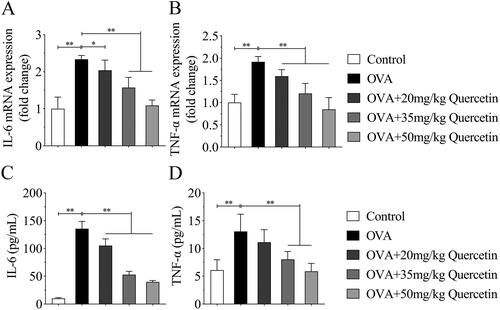
Quercetin inhibits inflammation response in NALF
Subsequently, the pro-inflammatory factors IL-6 and TNF-α were examined. The results of qPCR and ELISA showed that OVA increased IL-6 and TNF-α at mRNA expression and protein levels. However, quercetin abrogated the effects induced by OVA ().
Quercetin promotes the balance of Treg/Th17 cells in NALF
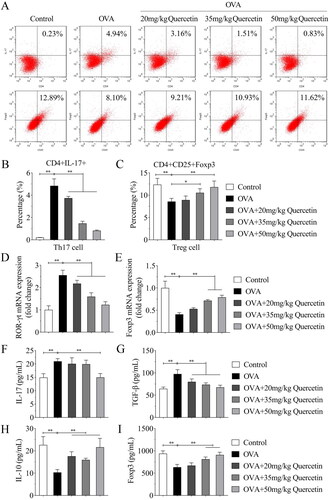
The Treg/Th17 balance was analyzed using flow cytometry. The results illustrated that OVA increased the percentage of Th17 cells and decreased Treg cells, while quercetin decreased Th17 cells and increased Treg cells (). Then, the regulators of Th17 and Treg cells were analyzed. OVA upregulated the mRNA expression of RORγt and downregulated Foxp3 expression, whereas quercetin reversed their expression induced by OVA (). The results of ELISA showed that OVA elevated IL-17 and TGF-β levels compared with the control group; however, quercetin reversed the elevation induced by OVA (). Oppositely, OVA reduced IL-10 and Foxp3 levels, while quercetin increased their levels in OVA mice ().
Figure 6. Quercetin promotes the balance of Treg/Th17 cells in NALF. (A) The percentage of Th17 (CD4 + IL-17+) cells and Treg (CD4+ Foxp3+) cells were measured by flow cytometry and quantified in (B) and (C). The mRNA expression of (D) RORγt and (E) Foxp3 was evaluated by qPCR. The levels of (F) IL-17, (G) TGF-β, (H) IL-10 and (I) Foxp3 were measured using ELISA kits. **p < 0.01. *p < 0.05.
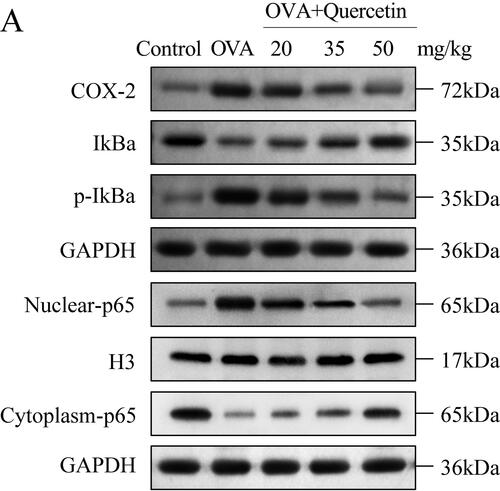
Quercetin inhibits OVA-induced activation the NF-κB pathway
Finally, the main factors of the NF-κB pathway were detected using western blot. The results showed that OVA upregulated the levels of COX-2, p-IkBα, and nuclear-p65, and downregulated the levels of IKBα and cytoplasm-p65; however, quercetin reversed their levels induced by OVA (). The data suggested that quercetin suppressed the activation of the NF-κB pathway and nuclear translocation.
Discussion
In this study, we used OVA to establish the AR mouse model and oral administration with differentiation concentrations of quercetin. We investigated the effects of quercetin on clinical symptoms, inflammation, and Treg/Th17 balance in OVA mice.
Quercetin is a flavonoid that has multiple biological activities. It has been revealed that quercetin is involved in decelerating the progression of human diseases, such as malignancies [Citation15], metabolic syndrome [Citation16], autoimmune diseases [Citation17], and allergic diseases [Citation14]. Ding et al. have reported that quercetin alleviates OVA-induced allergic conjunctivitis by inhibiting inflammation [Citation18]. Quercetin inhibits allergic airway inflammation by suppressing the apoptosis and cytokines of epithelial cells [Citation19]. Additionally, quercetin could alleviate AR by inhibiting neuropeptide production Th1/Th2 cytokine imbalance, and eosinophil activation [Citation20–22]. However, the underlying mechanisms of quercetin were complex, more studies are needed.
An increased number of rubbing and sneezing is a significant clinical feature of AR [Citation23], Thus, we first explore the effects of quercetin on AR symptoms. We found that quercetin inhibited the rubbing and sneezing frequency increased by OVA, suggesting that quercetin could improve AR symptoms.
AR is mediated by IgE, which is driven by Th2 cells. IgG1 antibody, IL-4, and IL-5 levels are the marker of Th2 cells, while IgG2a antibody and IFN-γ levels are the marker of Th1 cells [Citation24,Citation25]. The ratio of IgG2a/IgG1 is related to the immune response that is decreased by sensitization [Citation26]. Additionally, histamine promotes the release of cytokines in Th2 cells and decreases the production of Th1 cells [Citation27]. In this study, the results indicated that quercetin inhibited OVA-induced increase of IgE, IgG1, histamine, IL-4, and IL-5 and decrease of IgG2a, IgG2a/IgG1 ratio, and IFN-γ, suggesting that quercetin prevents Th1/Th2 imbalance.
AR is mediated by inflammation cells. When patients with AR are exposed to an allergen, mast cells are threshed. When AR progresses, the nasal mucosa is infiltrated by eosinophils, neutrophils, lymphocytes, and macrophages, which further promote the development of inflammation [Citation28,Citation29]. Here, we observed that quercetin inhibited the increase of inflammatory cells, including epithelial cells, eosinophils, neutrophils, lymphocytes, and macrophages. Moreover, the nasal mucosa of OVA mice was infiltrated by inflammatory cells and showed more goblet cells and eosinophils, but quercetin alleviated the inflammatory infiltration caused by OVA. The findings suggested that quercetin alleviated inflammation.
The imbalance of Treg/Th17 cells has been proven to be closely associated with inflammation [Citation30]. Foxp3, IL-10, and TGF-β are the important regulators for Treg cells, while IL-17 is the regulator for Th17 cells [Citation31,Citation32]. IL-6 and TNF-α are pro-inflammatory factors that are also regulators of Treg/Th17 balance [Citation33,Citation34]. In the current study, the levels of IL-17, TGF-β, IL-6, and TNF-α were elevated, but the levels of IL-10 and Foxp3 were reduced by OVA. However, quercetin reversed the effects of their levels induced by OVA. The findings suggested that quercetin promotes the balance of Treg/Th17 cells.
The NF-κB pathway is a classic pro-inflammatory signaling pathway that enhance the expression of pro-inflammatory cytokines, chemokines, and adherence factors [Citation35]. Nuclear entry and exit of NF-κB regulates inflammation response. When expose to drugs, the nuclear translocation of p65 showed significant changes [Citation36]. The nuclear translocation was promoted by depredating the phosphorylated IKB proteins, and p65 in the nucleus promotes IKB transcription. COX-2 is a pro-inflammatory chemokine that can be transcriptional regulated by the NF-κB pathway [Citation37]. Previous studies have reported that the NF-κB pathway significantly participates in the progression of AR, particularly the effects of medicine on Treg/Th17 or Th1/Th2 balance [Citation38,Citation39]. In this study, we found that quercetin downregulated COX-2, p-IKBα, nuclear-p65 and upregulated IKBα and cytoplasm-65 levels induced by OVA, suggesting that quercetin inactivated of the NF-κB pathway.
However, the regulation of the NF-κB pathway is complex. One of the limitations of this study has not yet detected the expression of other related factors of the NF-κB pathway, such as MAPK and AP-1. We will further measure their levels in our future work. Moreover, we will also explore whether quercetin was involved in AR progression by NF-κB pathway using the rescue experiments.
In conclusion, oral administration of quercetin attenuated the nasal AR symptoms induced by OVA. In addition, quercetin inhibited inflammation, Th1/Th2 imbalance, Treg/Th17 imbalance, and NF-κB pathway induced by OVA. Thus, we considered that quercetin attenuated the inflammatory reactions of AR by improving the imbalance of Th1/Th2 and Treg/Th17, and inactivating the NF-κB pathway. The findings suggested that quercetin has the potential to be a therapeutic agent for clinical AR treatment.
Authors’ contributions
X K and Z C drafted the work and revised it critically for important intellectual content; X W was responsible for the acquisition, analysis and interpretation of data for the work; H K and S H conceived and designed the project.
Ethical approval
The animal study protocol was approved by the Ethics Committee of The First Affiliated Hospital of Chongqing Medical University.
Data availability statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bousquet J, Anto JM, Bachert C, et al. Allergic rhinitis. Nat Rev Dis Primers. 2020;6(1):1.
- Siddiqui ZA, Walker A, Pirwani MM, et al. Allergic rhinitis: diagnosis and management. Br J Hosp Med (Lond). 2022;83(2):1–9.
- Meng Y, Wang C, Zhang L. Recent developments and highlights in allergic rhinitis. Allergy. 2019;74(12):2320–2328.
- Scadding GK. Optimal management of allergic rhinitis. Arch Dis Child. 2015;100(6):576–582.
- Eifan AO, Durham SR. Pathogenesis of rhinitis. Clin Exp Allergy. 2016;46(9):1139–1151.
- Haitchi HM, Holgate ST. New strategies in the treatment and prevention of allergic diseases. Expert Opin Investig Drugs. 2004;13(2):107–124.
- Bernstein DI, Schwartz G, Bernstein JA. Allergic rhinitis: mechanisms and treatment. Immunol Allergy Clin North Am. 2016;36(2):261–278.
- Huang X, Chen Y, Zhang F, et al. Peripheral Th17/Treg cell-mediated immunity imbalance in allergic rhinitis patients. Braz J Otorhinolaryngol. 2014;80(2):152–155. English, Portuguese.
- Nistala K, Wedderburn LR. Th17 and regulatory T cells: rebalancing pro- and anti-inflammatory forces in autoimmune arthritis. Rheumatology (Oxford). 2009;48(6):602–606.
- Singh P, Arif Y, Bajguz A, et al. The role of quercetin in plants. Plant Physiol Biochem. 2021;166:10–19.
- Di Petrillo A, Orrù G, Fais A, et al. Quercetin and its derivates as antiviral potentials: a comprehensive review. Phytother Res. 2022;36(1):266–278.
- Mlcek J, Jurikova T, Skrovankova S, et al. Quercetin and its anti-allergic immune response. Molecules. 2016;21(5):623.
- Rashidi Z, Khosravizadeh Z, Talebi A, et al. Overview of biological effects of quercetin on ovary. Phytother Res. 2021;35(1):33–49.
- Jafarinia M, Sadat Hosseini M, Kasiri N, et al. Quercetin with the potential effect on allergic diseases. Allergy Asthma Clin Immunol. 2020;16:36.
- Reyes-Farias M, Carrasco-Pozo C. The anti-cancer effect of quercetin: molecular implications in cancer metabolism. IJMS. 2019;20(13):3177.
- Hosseini A, Razavi BM, Banach M, et al. Quercetin and metabolic syndrome: a review. Phytother Res. 2021;35(10):5352–5364.
- Shen P, Lin W, Deng X, et al. Potential implications of quercetin in autoimmune diseases. Front Immunol. 2021;12:689044.
- Ding Y, Li C, Zhang Y, et al. Quercetin as a Lyn kinase inhibitor inhibits IgE-mediated allergic conjunctivitis. Food Chem Toxicol. 2020;135:110924.
- Caglayan Sozmen S, Karaman M, Cilaker Micili S, et al. Effects of quercetin treatment on epithelium-derived cytokines and epithelial cell apoptosis in allergic airway inflammation mice model. Iran J Allergy Asthma Immunol. 2016;15(6):487–497.
- Kashiwabara M, Asano K, Mizuyoshi T, et al. Suppression of neuropeptide production by quercetin in allergic rhinitis model rats. BMC Complement Altern Med. 2016;16:132.
- Tanaka Y, Furuta A, Asano K, et al. Modulation of Th1/Th2 cytokine balance by quercetin in vitro. Medicines (Basel). 2020;7(8):46.
- Sakai-Kashiwabara M, Asano K. Inhibitory action of quercetin on eosinophil activation in vitro. Evid Based Complement Alternat Med. 2013;2013:127105.
- Mims JW. Epidemiology of allergic rhinitis. Int Forum Allergy Rhinol. 2014;4 Suppl 2:S18–S20.
- Ebrahimpoor S, Pakzad SR, Ajdary S. IgG1 and IgG2a profile of serum antibodies to Leishmania major amastigote in BALB/c and C57BL/6Mice. Iran J Allergy Asthma Immunol. 2013;12(4):361–367.
- Waśkiel-Burnat A, Osińska M, Salińska A, et al. The role of serum Th1, Th2, and Th17 cytokines in patients with alopecia areata: clinical implications. Cells. 2021;10(12):3397.
- Mousavi T, Salek Moghadam A, Falak R, et al. Co-administration of CpG oligonucleotides and chenopodium album extract reverse IgG2a/IgG1 ratios and increase IFN-gamma and IL-10 productions in a murine model of asthma. Iran J Allergy Asthma Immunol. 2008;7(1):1–6.
- Packard KA, Khan MM. Effects of histamine on Th1/Th2 cytokine balance. Int Immunopharmacol. 2003;3(7):909–920.
- Gelfand EW. Inflammatory mediators in allergic rhinitis. J Allergy Clin Immunol. 2004;114(5 Suppl):S135–S8.
- Ozaki S, Toida K, Suzuki M, et al. Impaired olfactory function in mice with allergic rhinitis. Auris Nasus Larynx. 2010;37(5):575–583.
- Hanidziar D, Koulmanda M. Inflammation and the balance of Treg and Th17 cells in transplant rejection and tolerance. Curr Opin Organ Transplant. 2010;15(4):411–415.
- Liu B, Zhang H, Li J, et al. Triptolide downregulates Treg cells and the level of IL-10, TGF-β, and VEGF in melanoma-bearing mice. Planta Med. 2013;79(15):1401–1407.
- Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11(10):763–776.
- Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40(7):1830–1835.
- Kosmaczewska A, Ciszak L, Swierkot J, et al. Exogenous IL-2 controls the balance in Th1, Th17, and Treg cell distribution in patients with progressive rheumatoid arthritis treated with TNF-alpha inhibitors. Inflammation. 2015;38(2):765–774.
- Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651.
- Di Z, Herpers B, Fredriksson L, et al. Automated analysis of NF-κB nuclear translocation kinetics in high-throughput screening. PLoS One. 2012;7(12):e52337.
- Picado C, Bioque G, Roca-Ferrer J, et al. Nuclear factor-kappaB activity is down-regulated in nasal polyps from aspirin-sensitive asthmatics. Allergy. 2003;58(2):122–126.
- Van Nguyen T, Piao CH, Fan YJ, et al. Anti-allergic rhinitis activity of α-lipoic acid via balancing Th17/Treg expression and enhancing Nrf2/HO-1 pathway signaling. Sci Rep. 2020;10(1):12528.
- Dong J, Xu O, Wang J, et al. Luteolin ameliorates inflammation and Th1/Th2 imbalance via regulating the TLR4/NF-κB pathway in allergic rhinitis rats. Immunopharmacol Immunotoxicol. 2021;43(3):319–327.