?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
LncRNA OIP5-AS1 has a common gene imbalance in various cancers and tumours, which plays an important role in regulating its biological function. However, there are few studies on lncRNA OIP5-AS1 in rheumatoid arthritis (RA). The purpose of the present study was to investigate the role of lncRNA OIP5-AS1 in the pathogenesis of RA. In the present study, we established an adjuvant arthritis (AA) rat model to obtain primary fibroblast-like synoviocytes (FLSs);The subcellular localisation of lncRNA OIP5-AS1 was detected by fluorescence in situ hybridisation (FISH) assay; Cell proliferation of FLSs was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide(MTT) assay;IL-1β, IL-6 and TNF-α concentrations were measured by enzyme-linked immunosorbent assay (ELISA);Quantitative real-time PCR (qRT-PCR), Western blots(WB) and immunofluorescence were used to detect the expression of lncRNA OIP5-AS1/miR-410-3p/wnt7b signal axis and Wnt/β-catenin signal pathway related indicators in FLSs. FISH assay confirmed the presence of lncRNA OIP5-AS1 in the cytoplasm, suggesting that it acts as a competing endogenous RNA (ceRNA). qRT-PCR showed that the expression of lncRNA OIP5-AS1 was upregulated in FLSs, while the expression of miR-410-3p was downregulated in FLSs. We also found that lncRNA OIP5-AS1 knockdown inhibited the proliferation and inflammation of FLSs. Moreover, the expression of Wnt7b, the downstream target gene of miR-410-3p, and the activation of the Wnt/β-catenin signalling pathway were also inhibited by lncRNA OIP5-AS1 knockdown. These results suggested that lncRNA OIP5-AS1 promotes the activation of the Wnt/β-catenin signalling pathway by regulating the miR-410-3p/Wnt7b signalling axis, thereby participating in the occurrence and development of RA.
Introduction
Rheumatoid arthritis (RA) is a common chronic autoimmune-mediated inflammatory disease characterised by persistent synovitis, systemic inflammation, and joint destruction [Citation1]. Due to the high rate of disability, easy recurrence, and long treatment cycle of RA, which causes severe inconvenience and heavy economic burden on the life of patients, there is an urgent need to develop effective methods to treat RA [Citation2]. At present, the pathogenesis of RA is not clear, but immune genetics and environment are considered to be the main causes of RA. Accumulating evidence suggests that fibroblast-like synoviocytes (FLSs) play an important role in the pathogenesis of RA. Under an inflammatory environment, FLSs are continuously activated to exhibit tumour-like hyperplasia and transform into an aggressive inflammatory and invasive phenotype [Citation3], and the activation of FLSs releases a variety of inflammatory cytokines and chemokines, including interleukin-6 (IL-6), tumour necrosis factor-α (TNF-α), and matrix metalloproteinases (MMP), which stimulate joint destruction, leading to bone invasion and articular cartilage damage [Citation4–6].
Long noncoding RNAs (LncRNAs) are RNA transcripts with a length of more than 200 nucleotides without protein-coding function [Citation7]. LncRNAs were initially regarded as “transcriptional noise” and have received little attention [Citation8]. In recent years, it has been found that lncRNAs are widely involved in the biological processes of many diseases. Studies have found that lncRNAs not only regulate cell proliferation, differentiation, and apoptosis but also mediate inflammatory and immune processes in diseases. LncRNA OIP5-AS1 is ubiquitously expressed in mammals, highly conserved during vertebrate evolution, and highly expressed in various diseases [Citation9]. Many studies have focussed on lncRNA OIP5-AS1 in cancer and reported that it regulates proliferation, apoptosis, migration, and invasion of cancer cells [Citation10,Citation11]. The pathogenesis of RA is mainly characterised by excessive proliferation of FLSs. In recent years, it has been proposed that lncRNA OIP5-AS1 plays an important role in the regulation of cell proliferation, apoptosis, and inflammation in osteoarthritis [Citation12,Citation13]. Therefore, we focussed on the potential mechanism of lncRNA OIP5-AS1 in the pathogenesis of RA.
MicroRNAs (miRNAs) are small non-coding RNAs with a length of 20-24 nucleotides, and miRNAs can block or promote the translation or degradation of their targeted mRNAs after binding to their targeted mRNAs [Citation14]. Competing endogenous RNA (ceRNA) is a novel regulatory mechanism of RNA-RNA interaction. miR-410-3p has been studied as a tumour suppressor in cancer, and it has been shown that upregulation of miR-410-3p expression promotes apoptosis and inhibits cell proliferation [Citation15]. Recent studies have found that miR-410-3p also plays an important role in cell proliferation, apoptosis, and anti-inflammatory in RA [Citation16–18]. A previous study has demonstrated that lncRNA OIP5-AS1, miR-410-3p, and Wnt7b have direct binding effects with each other according to a dual-luciferase reporter assay [Citation19]. Wnt7b is upstream of the Wnt/β-catenin signalling pathway, and upregulation of Wnt7b activates the Wnt/β-catenin signalling pathway [Citation20,Citation21]. The Wnt/β-catenin signalling pathway regulates cell differentiation, proliferation, migration, and invasion [Citation22,Citation23]. Various studies have demonstrated that the Wnt/β-catenin signalling pathway is involved in the pathogenesis of RA [Citation24, Citation25].
The present study focussed on lncRNA OIP5-AS1. We elucidated the molecular mechanism of lncRNA OIP5-AS1 as a sponge that competitively binds to miR-410-3p, resulting in the upregulation of its target gene, Wnt7b, thereby regulating the downstream related factors of the Wnt/β-catenin signalling pathway and ultimately participating in the occurrence and development of RA.
Materials and methods
Establishment of the animal model
Male Sprague Dawley (SD) rats of specific pathogen-free (SPF) grade were selected for the experiment, and the adjuvant arthritis (AA) rat model was induced by Freund’s complete adjuvant (Sigma, USA). Normal control rats were injected with an equal volume of physiological saline under the same condition [Citation26]. Twenty days after inflammation, rats were anaesthetised with 3% pentobarbital sodium and sacrificed by removing blood from the abdominal aorta. This study was under the approval and guidance of the Medical Research Ethics Committee of Anhui University of Chinese Medicine.
Cell preparation and culture
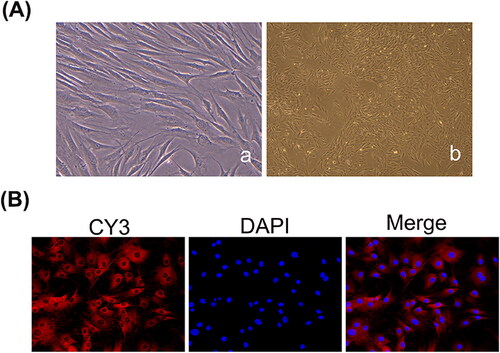
FLSs were cultured using the tissue block adherent method [Citation27]. Synovial tissue was removed from the knee joints of AA rats, and the tissue blocks were washed with PBS buffer (Gibco, USA). Synovial tissue was cut into pieces of approximately 1–2 mm3 and placed into DMEM (Gibco, USA) containing 20% foetal bovine serum (Lonsera, Uruguay) and 1% double antibiotics (100 U/mL penicillin and 100 g/mL streptomycin) (Beyotime, China), and the synovial tissue pieces were spread on one side of the cell culture bottle. The cell culture bottles were placed in a humidified incubator at 37 °C with 5% CO2 for 4–6 h with the synovial tissue piece side facing up and 3 mL of culture solution on the bottom of the bottle. After the cells adhered, 3–5 mL of complete medium (DMEM, 15% FBS, and 1% double antibiotics) was added to bottom of the cell culture bottle, and the cell culture bottle was placed in an incubator for additional culture. The culture medium was replaced every 2–3 d. An inverted fluorescence microscope was utilised to observe the migration of cells out of the tissue block, and the synovial tissue blocks were removed when the spindle cells detached from the tissue block. Fresh medium was added to continue the culture. When the FLSs reached 80% confluency on the bottom of the cell culture bottle, they were passaged. Primary FLSs from passages 3 to 5 were utilised for subsequent experiments. Synovial tissues from healthy SD rats were cultured using the same method as synovial cells in the normal control group. An inverted fluorescence microscope was used to observe the morphology of the cells. Immunofluorescence staining of vimentin was used to identify FLSs.
Cell transfection and siRNA
The NCBI database was searched to identify primers for lncRNA OIP5-AS1, and siRNA sequences were designed according to the full-length sequence information for rat OIP5-AS1 (NR_130141.1). The design and synthesis of lncRNA OIP5-AS1 siRNAs were completed by China Hefei Deir Spectrum Biotechnology Co., Ltd.
The lncRNA OIP5-AS1 siRNA sequences are shown in .
Table 1. LncRNA OIP5-AS1 siRNA sequences.
When FLSs reached 40% confluency, they were transfected with siRNAs using Lipo3000 reagent (Thermo Fisher, USA) according to the manufacturer’s protocol. After 48 h, quantitative real-time polymerase chain reaction (qRT-PCR) was conducted to examine gene expression levels.
Fluorescence in situ hybridisation (FISH)
Synoviocytes were seeded in 24-well plates containing climbing slices and allowed to reach 70% confluency. Cells were then fixed with 4% paraformaldehyde (Solarbio, China) for 20 min. After circles were drawn on the cell climbing slices using a gene pen, cells were digested with 10× proteinase K (at a concentration of 20 μg/mL; Solarbio, China) and washed with ultrapure water. A prehybridization solution (Sangon Biotech, China) was then added followed by incubation at 37 °C for 1 h. After discarding the prehybridization solution, the hybridisation solution was added dropwise followed by incubation at 4 °C overnight. The SSC eluate (Sangon Biotech, China) was washed away from the hybridisation solution, and DAPI staining solution (Solarbio, China) was added to the cell climbing slices followed by incubation for 8 min in the dark. Cells were then washed, and an anti-fluorescence quenching blocking agent (Beyotime, China) was added to the slices, which were observed and imaged using a fluorescence microscope (Nikon, Japan).
MTT essay
Synovial cells were seeded into 96-well plates, digested with trypsin, and incubated in a 5% CO2 incubator at 37 °C. After 24 h, the synoviocytes were treated according to the experimental groups. After 24, 48, and 72 h of treatment, 10 μL of MTT (Beyotime, China) and 100 μL of fresh medium were added to each well and incubated for 4 h in the dark. The medium was removed, and 100 μL of formazan solution was added to each well. When all the purple crystals were dissolved, the optical density at a wavelength of 570 nm was measured using an enzyme metre.
Enzyme-linked immunosorbent assay
FLS Cells were inoculated and cultured in 96-well plates and treated according to different groups. After 48h, the cell supernatant was taken and centrifuged. The concentration of inflammation-related indicators in serum were detected using the commercial enzyme-linked immunosorbent assay kit (ELISA,LAB, China) following the manufacturer’s protocols.
qRT-PCR
Synoviocytes were seeded in 6-well plates, and total cellular RNA was extracted using a total RNA extraction kit (Sangon Biotech, China) according to the manufacturer’s protocol. The extracted RNA was reverse transcribed into cDNA using a one-step reverse transcription kit (Invitrogen, Carlsbad, CA, USA), and the Poly (A) tailing method (Sangon Biotech, China) was used perform the tailing reaction of miR-410-3p and the synthesis of cDNA at the same time. TB green premix (Takara, Japan) was used for β-actin and U6 for internal standards. The expression levels of lncRNA OIP5-AS1 and miR-410-3p were detected using a LightCycler480 II System. Wnt7b and Wnt pathway-related components were evaluated according to the procedure used for lncRNA OIP5-AS1. The design and synthesis of primers were completed by China Hefei Deir Spectrum Biotechnology Co., Ltd. The primer sequences are shown in . The downstream primers and U6 primers of miR-410-3p were provided with the miRNA Poly (A) tailing kit.
Table 2. qRT-PCR primers.
Immunofluorescence assay
Cells were divided into four groups as follows: normal group, model group, lncRNA OIP5-AS1 siRNA group, and lncRNA OIP5-AS1 siRNA + NC group. After transfection, the medium was replaced with fresh medium, and cells were incubated at 37 °C with 5% CO2 for 24 h. Cells were then fixed with 4% paraformaldehyde (Solarbio, China) in the dark for 20 min, and cells were then washed with PBS and permeabilized with 0.5% Triton X-100 (Solarbio, China) for 20 min at room temperature. Cells were then blocked with goat serum for 30 min, and 1 mL of 5% BSA (Solarbio, China) was added to each well followed by incubation for 30 min. The blocking solution was removed by blotting with filter paper. Cells were incubated in a wet box overnight in the dark at 4 °C with the following diluted primary antibodies: Wnt7b (1:500), β-catenin (1:200), c-Myc (1:50), cyclin D1 (1:50), p-glycogen synthesis kinase 3β (p-GSK-3β) (Ser9) (1:400), and SFRP4 (1:500). The primary antibody solution was discarded, and cells were then incubated in a wet box at 37 °C for 1 h with diluted fluorescent-labelled goat anti-rabbit IgG (H&L, 1:1000; ZSGB Biotech, China). Nuclei were stained with 10 μg/mL DAPI (1:100; Solarbio, China) for 5 min, and cells were then sealed with an anti-fluorescence quenching agent (Beyotime, China). Cells were observed and imaged using a fluorescence microscope.
Western blot analysis
Total protein was extracted from FLSs using RIPA buffer (Beyotime, China). Protein concentration was measured using a BCA protein assay kit (Bio-Rad Laboratories, Inc.) with a standard protein concentration of 4 μg/μL. Proteins were then denatured and electrophoresed using SDS-PAGE (Sangon Giotech, China) to separate protein bands. The separated proteins were transferred onto PVDF membranes (Millipore, USA), and the membranes were blocked with 5% skim milk at room temperature for 2 h. The membranes were then incubated for 24 h with the following primary antibodies: β-actin (1:50000), Wnt7b (1:5000), β-catenin (1:6000), c-Myc (1:2000), cyclin D1 (1:1000), GSK-3β (1:1000), p-GSK-3β (Ser9) (1:1000), and SFRP4 (1:500). After washing three times, the membranes were incubated with an HRP-conjugated rabbit anti-human IgG (1:10000) for 1 h at room temperature. An ECL detection kit (Millipore, USA) was used to visualise the signals. ImageJ software was used for densitometry analysis.
Statistical analysis
All experiments were performed at least three times. SPSS 23.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis, and the measured data are expressed as mean ± standard deviation (SD) (±s). An independent sample t-test was used for comparison between the two groups. One-way analysis of variance (ANOVA) was used for the comparison among multiple groups. The difference was statistically significant when p < 0.05.
Results
Identification of FLSs
To verify that the cells used in the experiment are FLSs, the morphology and activity of the cells were observed using a fluorescence inverted microscope and vimentin immunofluorescence. Primary synovial cells were obtained by the tissue block adherence method, and most of the tissue blocks were adherent after 24 h as indicated by observation using an inverted phase contrast microscope. In approximately 2 to 3 days, a few spindle cells migrated along the edge of the tissue mass. After 5–6 days of continuous culture, the number of cells gradually increased and showed colony-like growth. After approximately 12 days, the cells grew rapidly and reached 80% confluency on the bottom of the culture bottle in 2–3 weeks. The morphology of synovial cells showed a short spindle shape at the initial stage and a long fusiform shape after three passages, and the nucleus was significant (). After passaging, the synoviocytes were vimentin-positive and were spindle-shaped. The nuclei were located centrally in the cells and were oval or oval in shape ().
LncRNA OIP5-AS1 knockdown inhibits the proliferation of FLSs
To select the best siRNA targeting sequence for lncRNA OIP5-AS1, we used qRT-PCR to evaluate the knockdown efficiency. Compared to the model group and NC group, the mRNA expression level of lncRNA OIP5-AS1 decreased to varying degrees after transfection with the three siRNA sequences. siRNA2 resulted in the most significant decrease in lncRNA OIP5-AS1 with a knockdown efficiency more than 50% (). Therefore, lncRNA OIP5-AS1 siRNA2 was utilised in subsequent experiments.
Figure 2. LncRNA OIP5-AS1 knockdown inhibits FLS proliferation. (A) Detection of transfection efficiency of lncRNA OIP5-AS1 siRNAs in FLSs from AA rats by qRT-PCR. (B) Inhibitory effect of lncRNA OIP5-AS1 siRNA on the proliferation of FLSs from AA rats at different times. ##P<0.01 compared to the normal group; *P<0.05 and **P<0.01 compared to the model group. Model: FLSs from AA rats; siRNAs 1, 2 and 3: different lncRNA OIP5-AS1 siRNA sequences; NC: negative control; and siRNA2 + NC: negative control of siRNA2.
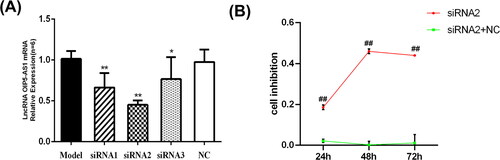
To test the effect of lncRNA OIP5-AS1 knockdown on the proliferation ability of FLSs isolated from AA rats, an MTT assay was performed at three different time points (24, 48, and 72 h). As shown in , lncRNA OIP5-AS1 knockdown had a weak inhibitory effect on FLS proliferation in AA rats after 24 h. After 48 h, the inhibition rate of FLS proliferation was the highest at 50%, and it decreased by 72 h. Therefore, 48 h was selected as the optimal time for the inhibitory effect of lncRNA OIP5-AS1 knockdown on cell proliferation.
LncRNA OIP5-AS1 is located in the cytoplasm
To determine the localisation of lncRNA OIP5-AS1 in FLSs from AA rats, FISH assays were performed. As shown in , CY3 labelled the cytoplasm of synoviocytes, whereas DAPI labelled the nuclei. The merged plot for the mRNA in situ hybridisation assay indicated positivity in the cytoplasm, which indicated that lncRNA OIP5-AS1 was mainly distributed in the cytoplasm.
LncRNA OIP5-AS1 knockdown inhibits the inflammation of RA
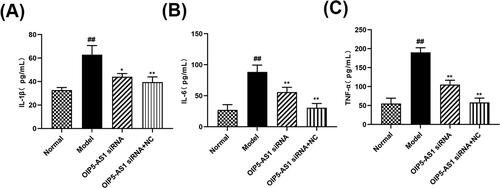
Inflammation runs through the whole pathogenesis of RA, and can cause joint redness, deformity and even disability in RA patients [Citation28]. ELISA assay was used to detect the effect of siRNA LncRNA OIP5-AS1 on inflammatory factors in the supernatant of RA-FLSs. As shown in , compared with the normal group, the expression of IL-6, IL-1β and TNF-α in the model group increased significantly, indicating that inflammation is involved in the pathogenesis of RA. Compared with the model group, the expression of IL-6, IL-1β and TNF-α in OIP5-AS1 siRNA group decreased significantly, The expression of IL-6, IL-1β and TNF-α was significantly reduced in the OIP5-AS1 siRNA group compared with the model group, indicating that the release of inflammatory factors in RA-FLSs was inhibited by the interfered OIP5-AS1.
Figure 4. LncRNA OIP5-AS1 siRNA inhibits the expression of IL-1β(A),IL-6(B) and TNF-α(C) in FLSs serum. ##P<0.01 compared to the normal group; *P<0.05 and **P<0.01 compared to the model group. Normal: normal synovial cells; Model: FLSs from AA rats; OIP5-AS1 siRNA: lncRNA OIP5-AS1 siRNA2; OIP5-AS1 siRNA + NC: Negative control for siRNA.
LncRNA OIP5-AS1 interacts with miR-410-3p
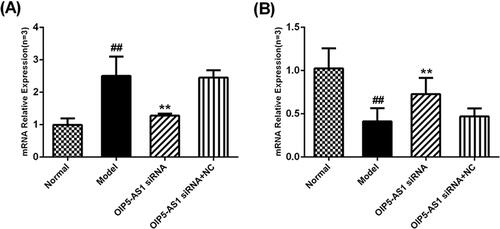
Both bioinformatics analysis and a literature review suggested that lncRNA OIP5-AS1 and miR-410-3p interact with each other, and this interaction has been confirmed in glioma [Citation19]. qRT-PCR was used to detect the effect of lncRNA OIP5-AS1 knockdown on the mRNA expression levels of lncRNA OIP5-AS1 and miR-410-3p. Compared to the model group, the expression level of lncRNA OIP5-AS1 was significantly decreased after lncRNA OIP5-AS1 knockdown (). Compared to the model group, the expression level of miR-410-3p was significantly increased after lncRNA OIP5-AS1 knockdown ().
Figure 5. Effects of lncRNA OIP5-AS1 knockdown on the expression of lncRNA OIP5-AS1 and miR-410-3p in FLSs from AA rats. (A) The effects of lncRNA OIP5-AS1 expression after siRNA. (B) The effects of miR-410-3p expression after siRNA. ##P<0.01 compared to the normal group; *P<0.05 and **P<0.01 compared to the model group. Normal: normal synovial cells; Model: FLSs from AA rats; OIP5-AS1 siRNA: lncRNA OIP5-AS1 siRNA2; OIP5-AS1 siRNA + NC: Negative control for siRNA.
LncRNA OIP5-AS1 knockdown inhibits Wnt/β-catenin signalling pathway activation
Wnt7b expression is inhibited by lncRNA OIP5-AS1 knockdown
Previous literature has shown that Wnt7b has a target relationship with miR-410-3p [Citation19]. Because Wnt7b overexpression is involved in the activation of the Wnt/β-catenin pathway, Wnt7b was selected as the pathway indicator. Immunofluorescence showed that Wnt7b was mainly positively expressed in the cytoplasm (). Compared to the normal group, the protein and mRNA expression levels of Wnt7b were significantly increased in the model group, and the expression of Wnt7b was significantly decreased by lncRNA OIP5-AS1 knockdown ().
Figure 6. Changes of Wnt7b protein and mRNA expression levels in FLSs from AA rats after lncRNA OIP5-AS1 siRNA. (A) The change of Wnt7b protein expression was observed by immunofluorescence (×200;a: normal group; b model group; c: lncRNA OIP5-AS1 siRNA group; and d lncRNA OIP5-AS1 siRNA + NC group). (B) The change of Wnt7b mRNA expression was evaluated by qRT-PCR. (C) The change of Wnt7b protein expression was evaluated by Western blot analysis. (D) Semiquantitative analysis of Wnt7b protein. ##P<0.01 compared to the normal group; *P<0.05 and **P<0.01 compared to the model group. Normal: normal synovial cells; Model: FLSs from AA rats; OIP5-AS1 siRNA: lncRNA OIP5-AS1 siRNA2; OIP5-AS1 siRNA + NC: negative control for siRNA.
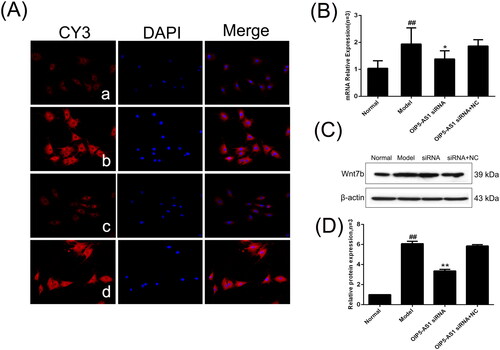
LncRNA OIP5-AS1 knockdown decreases β-catenin expression
β-catenin is a key component of the Wnt canonical pathway and a marker of Wnt pathway activation [Citation29]. The immunofluorescence assay showed positive expression of β-catenin in the nucleus and cytoplasm in the normal group, and the model group showed increased levels of β-catenin in the nucleus (). The protein and mRNA expression levels of β-catenin were significantly increased in the model group compared to the normal group, but LncRNAOIP5-AS1 knockdown significantly decreased the expression of β-catenin ().
Figure 7. The changes of β-catenin protein and mRNA expression levels in FLSs from AA rats after lncRNA OIP5-AS1 siRNA. (A) The change of β-catenin protein expression was observed by immunofluorescence (×200; a: normal group; b: model group; c: lncRNA OIP5-AS1 siRNA group; and d: lncRNA OIP5-AS1 siRNA + NC group). (B) The change of β-catenin mRNA expression was evaluated by qRT-PCR. (C) The change of β-catenin protein expression was evaluated by Western blot analysis. (D) Semiquantitative analysis of β-catenin protein. ##P<0.01 compared to the normal group; *P<0.05 and **P<0.01 compared to the model group. Normal: normal synovial cells; Model: FLSs from AA rats; OIP5-AS1 siRNA: lncRNA OIP5-AS1 siRNA2; OIP5-AS1 siRNA + NC: negative control for siRNA.
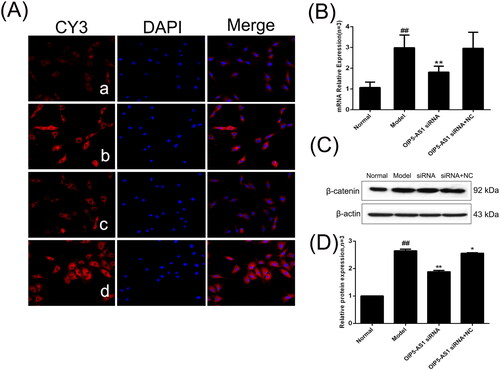
c-Myc expression is decreased after lncRNA OIP5-AS1 knockdown
c-Myc is a downstream effector of the Wnt signalling pathway [29]. The immunofluorescence assay showed that c-Myc was mainly positively expressed in the nucleus [Citation30] (). The protein and mRNA expression levels of c-Myc were significantly increased in the model group compared to the normal group, and lncRNA OIP5-AS1 knockdown significantly decreased the expression of c-Myc ().
Figure 8. The changes of c-Myc protein and mRNA expression in FLSs from AA rats after lncRNA OIP5-AS1 siRNA. (A) The change of c-Myc protein expression was observed by immunofluorescence (×200; a: normal group; b: model group; c: lncRNA OIP5-AS1 siRNA group; and d: lncRNA OIP5-AS1 siRNA + NC group). (B) The change of c-Myc mRNA expression was evaluated by qRT-PCR. (C) The change of c-Myc protein expression was evaluated by Western blot analysis. (D) Semiquantitative analysis of c-Myc protein. ##P<0.01 compared to the normal group; *P<0.05 and **P<0.01 compared to the model group. Normal: normal synovial cells; Model: FLSs from AA rats; OIP5-AS1 siRNA: lncRNA OIP5-AS1 siRNA2; OIP5-AS1 siRNA + NC: negative control for siRNA.
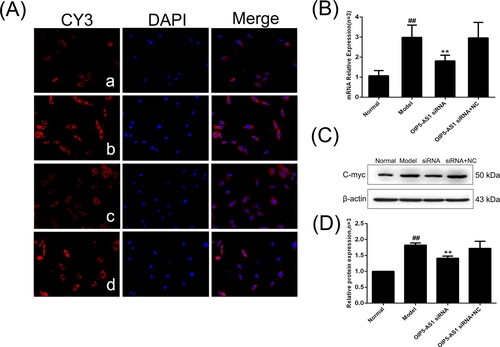
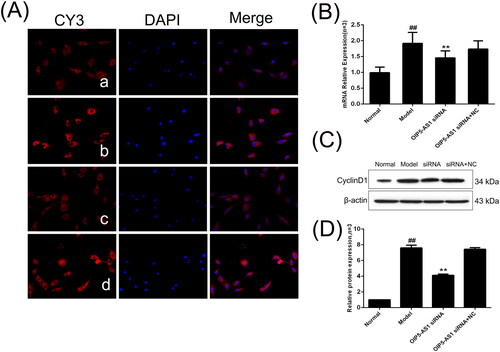
Cyclin D1 expression decreases after lncRNA OIP5-AS1 knockdown
Cyclin D1 and c-Myc are important downstream target genes of the Wnt/signalling pathway [Citation31]. Immunofluorescence showed that cyclin D1 was mainly expressed in the cytoplasm (). The protein and mRNA expression levels of cyclin D1 were significantly increased in the model group compared to the normal group, and lncRNA OIP5-AS1 knockdown significantly decreased the expression of cyclin D1 ().
Figure 9. The changes of cyclin D1 protein and mRNA expression in FLSs from AA rats after lncRNA OIP5-AS1 siRNA. (A) The change of cyclin D1 protein expression was observed by immunofluorescence (×200; a: normal group; b: model group; c: lncRNA OIP5-AS1 siRNA group; and d: lncRNA OIP5-AS1 siRNA + NC group). (B) The change of cyclin D1 mRNA expression was evaluated by qRT-PCR. (C) The change of cyclin D1 protein expression was analysed by Western Blot analysis. (D) Semiquantitative analysis of cyclin D1 protein. ##P<0.01 compared to the normal group; *P<0.05 and **P<0.01 compared to the model group. Normal: normal synovial cells; Model: FLSs from AA rats; OIP5-AS1 siRNA: lncRNA OIP5-AS1 siRNA2; OIP5-AS1 siRNA + NC: negative control for siRNA.
LncRNA OIP5-AS1 knockdown decreases GSK-3β and p-GSK-3β (Ser9) expression
GSK-3β is inhibited by phosphorylation and interacts with Axin to promote β-catenin phosphorylation [Citation32]. The immunofluorescence assay showed that p-GSK-3β was positively expressed in the cell membrane, nucleus, and cytoplasm (). As shown in , the mRNA expression levels of GSK-3β were not significantly different between the groups. However, the protein expression of p-GSK-3β/GSK-3β was significantly increased in the model group compared to the normal group, and lncRNA OIP5-AS1 knockdown significantly decreased the protein expression of p-GSK-3β/GSK-3β ().
Figure 10. The changes of GSK-3β and p-GSK-3β (Ser9) protein and mRNA expression in FLSs from AA rats after lncRNA OIP5-AS1 siRNA. (A) The change of p-GSK-3β (Ser9) protein expression was observed by immunofluorescence (×200; a: normal group; b: model group; c: lncRNA OIP5-AS1 siRNA group; and d: lncRNA OIP5-AS1 siRNA + NC group). (B) The change of GSK-3β mRNA expression was evaluated by qRT-PCR. (C) The change of p-GSK-3β (Ser9)/GSK-3β protein expression was evaluated by Western blot analysis. (D) Semiquantitative analysis of p-GSK-3β (Ser9)/GSK-3β protein. ##P<0.01 compared to the normal group; *P<0.05 and **P<0.01 compared to the model group. Normal: normal synovial cells; Model: FLSs from AA rats; OIP5-AS1 siRNA: lncRNA OIP5-AS1 siRNA2; OIP5-AS1 siRNA + NC: negative control for siRNA.
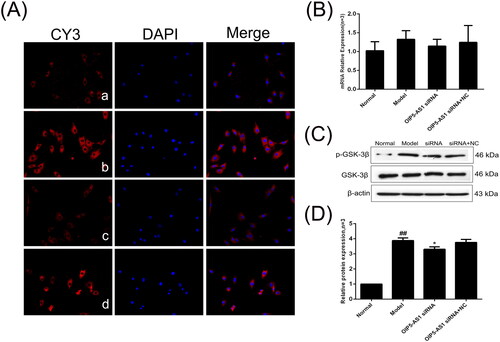
SFRP4 expression is increased after lncRNA OIP5-AS1 knockdown
SFRP4 is an antagonist of the Wnt pathway and negatively regulates the activation of the Wnt signalling pathway [Citation33]. The immunofluorescence assay showed that SFRP4 was mainly positively expressed in the cytoplasm (). The protein and mRNA expression levels of SFRP4 were significantly decreased in the model group compared to the normal group, and lncRNA OIP5-AS1 knockdown significantly increased the expression of SFRP4 ( and ).
Figure 11. The changes of SFRP4 protein and mRNA expression in FLSs from AA rats after lncRNA OIP5-AS1 siRNA. (A) The change of SFRP4 protein expression was observed by immunofluorescence (×200; a: normal group; b: model group; c: lncRNA OIP5-AS1 siRNA group; and d: lncRNA OIP5-AS1 siRNA + NC group). (B) The change of SFRP4 mRNA expression was evaluated by qRT-PCR. (C) The change of SFRP4 protein expression was evaluated by Western blot analysis. (D) Semiquantitative analysis of SFRP4 protein. ##P<0.01 compared to the normal group; *P<0.05 and **P<0.01 compared to the model group. Normal: normal synovial cells; Model: FLSs from AA rats; OIP5-AS1 siRNA: lncRNA OIP5-AS1 siRNA2; OIP5-AS1 siRNA + NC: negative control for siRNA.
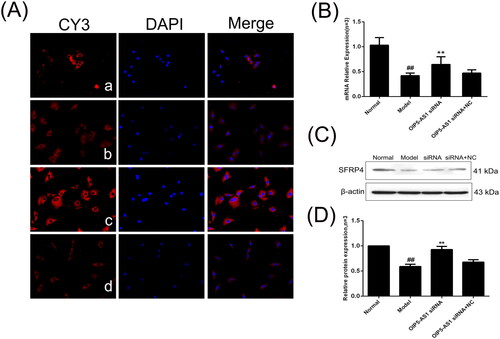
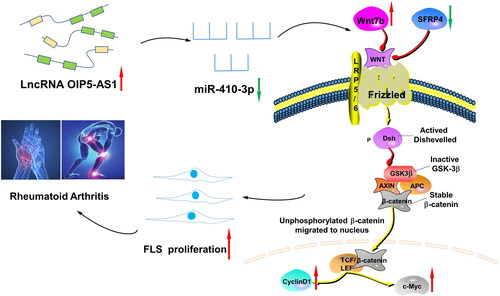
Figure 12. The lncRNA OIP5-AS1/miR-410-3p/Wnt7b axis promotes the proliferation of RA FLSs via regulating the Wnt/β-catenin pathway. When RA occurs, the expression of lncRNA OIP5-AS1 in FLSs increases, and the expression of miR-410-3p decreases after the competition. At the same time, the expression of Wnt7b, β-catenin, c-Myc, and cyclin D1 increases, which promotes the phosphorylation of GSK-3β, decreases the expression of SFRP4, activates the Wnt/β-catenin signalling pathway, and promotes FLS proliferation, thereby participating in the pathogenesis of RA.
Discussion
RA is an autoimmune disease of unknown aetiology with serious complications that predispose to joint damage. FLS activation is similar to tumour-like hyperplasia with strong invasion and migration ability [Citation4], and it is an important cause of cartilage injury and joint damage [Citation34]. In the present study, we showed that the expression of lncRNA OIP5-AS1 was upregulated in FLSs from AA rats, and the proliferation of FLSs in RA was suppressed after lncRNA OIP5-AS1 knockdown. In addition, we showed that lncRNA OIP5-AS1 promoted FLS proliferation by targeting the miR-410-3p/Wnt7b signalling axis, which may be related to the activation of the Wnt/β-catenin signalling pathway.
LncRNAs have been shown to be involved in regulating the development of a variety of diseases. LncRNA OIP5-AS1, a tumour suppressor lncRNA first identified in zebrafish, is involved in regulating the pathogenesis of many kinds of cancers [Citation35], and many studies on lncRNA OIP5-AS1 have been published. LncRNA OIP5-AS1, as a ceRNA in combination with miRNAs, negatively regulates its function to regulate the biological function of diseases, such as cancer. Wu et al. [Citation36] reported that lncRNA OIP5-AS1 inhibits breast cancer development by inducing the expression of GlO1 through competitively binding with miR-215a-5p. In gastric cancer (GC), miR-186 acts as a direct target of lncRNA OIP5-AS1, and the interaction regulates the process of aerobic glycolysis in GC cells [Citation37] LncRNA OIP5-AS1 has been reported to bind to miR-410-3p as a ceRNA in glioma, regulating glioma cell proliferation and apoptosis [19]. MiR-410-3p has also been found to play a role in inhibiting inflammation and proliferation of FLSs in RA pathogenesis [Citation19]. However, there are few studies on lncRNA OIP5-AS1 in RA. The targeting relationship of lncRNA OIP5-AS1 and miR-410-3p has been confirmed by dual-luciferase assays [Citation16,Citation17] In the present study, qRT-PCR showed that lncRNA OIP5-AS1 was upregulated and that miR-410-3p was downregulated in RA FLSs. Moreover, lncRNA OIP5-AS1 knockdown significantly decreased proliferation and correspondingly increased miR-410-3p expression. These results demonstrated that lncRNA OIP5-AS1 interacts with miR-410-3p in RA FLSs and has a regulatory effect on the proliferation of RA-FLSs. There have been various reports demonstrating the close relationship between RA and inflammation [Citation38,Citation39]. Knockdown of OIP5-AS1 also resulted in significant suppression of the inflammatory response in RA-FLSs, as confirmed by the results of assays for the pro-inflammatory cytokines IL-1β, IL-6, and TNF-α ().
Previous studies have demonstrated that miR-410-3p directly acts on Wnt7b and regulates the Wnt signalling pathway [Citation19] Wnt7b, as a Wnt non-transforming family member, is an important agonist of the Wnt/β-catenin signalling pathway and activates the Wnt signalling key effector, β-catenin, which in turn activates the Wnt/β-catenin signalling pathway [Citation40]. β-catenin is a key effector of the Wnt/β-catenin signalling pathway, which is involved in physiological processes, such as inflammation in several diseases, including RA, and Wnt/β-catenin signalling plays an important regulatory role in physiological processes, such as cell differentiation, proliferation, and apoptosis. When the Wnt signal is activated, the Wnt protein binds to Frizzle receptor proteins (Frz) and low-density lipoprotein receptor-related protein 5/6 (LRP5/6), thus activating dishevelled (Dvel, a FDZ domain-containing protein) and Axin (an axon protein) [Citation41]. Because the activity of GSK-3β is inhibited, β-catenin is not phosphorylated by GSK-3β, and the ubiquitin proteasome cannot recognise GSK-3β and degrade it. A large amount of β-catenin accumulates in the cell and enters the nucleus [Citation42] After interaction with transcriptional cofactor T cell factor (TCF)/lymphoid enhancer factor (LEF), it transactivates the related downstream factors, such as c-Myc and cyclin D1 [Citation43], thus participating in and regulating the occurrence and development of diseases in the body [Citation30,Citation44]. β-catenin translocation into the nucleus is considered to be an indicator of Wnt signalling pathway activation and plays a core role in the Wnt signal pathway [Citation6]. c-Myc and cyclin D1 are direct downstream targets of the Wnt/β-catenin signalling pathway [Citation45]. c-Myc is one of the major proto-oncogenes that regulates gene activation, transcription, and repression [Citation46]. Cyclin D1 is an important G1 phase regulator in the cell cycle, and overexpression of cyclin D1 shortens the cell cycle and promotes rapid cell proliferation [Citation47]. The present study showed that β-catenin, c-Myc, and cyclin D1 are highly expressed in FLSs and that their expression is significantly downregulated after OIP5-AS1 knockdown.
Glycogen synthesis kinase 3 (GSK-3) is a structurally active serine threonine kinase involved in the regulation of multiple signalling pathways and is currently known to comprise two isoforms, namely, GSK-3α and GSK-3β [Citation48]. GSK-3β is upstream of the Wnt/β-catenin signalling pathway, and the level of β-catenin is negatively regulated by GSK-3β-mediated phosphorylation or other degradation pathways [Citation49]. When phosphorylation occurs at Ser9, the activity of GSK-3β is inhibited, and the phosphorylation and degradation of β-catenin are blocked, thus positively regulating the Wnt/β-catenin signalling pathway [Citation50]. The present study showed that there was no significant difference in GSK-3β expression in FLSs between the groups; however, the expression of p-GSK-3β (Ser9)/GSK-3β was significantly increased in the model group compared to the normal group, and the expression level of p-GSK-3β (Ser9)/GSK-3β was significantly decreased after lncRNA OIP5-AS1 knockdown.
SFRP4 is considered a negative regulator of the Wnt/β-catenin pathway and plays an important role in the pathogenesis of RA [Citation51].Miao et al. [Citation52] demonstrated that DNMT1 activates the Wnt signal through the SFRP4 antagonist in FLSs from RA model rats. The present study showed that the expression of SFRP4 was low in FLSs, which was consistent with previous studies [Citation53]. Of note, the expression level of SFRP4 significantly increased after OIP5-AS1 siRNA treatment.
In summary, the present study demonstrated for the first time in RA FLSs that lncRNA OIP5-AS1 acts as a ceRNA to act on the miR-410-3p/Wnt7b signalling axis to regulate Wnt/β-catenin signalling pathway expression, which inhibits FLS cell proliferation, thereby alleviating the development and progression of RA (). Based on the results of the present study, we speculated that the lncRNA OIP5-AS1/miR-410-3p/Wnt7b signal axis is a potential target for the treatment of RA. Thus, the present study provides a scientific basis for the exploration of the pathogenesis of RA from the perspective of molecular biology.
Ethics approval and consent to participate
The experimental protocol was established according to the ethical guidelines of the Helsinki Declaration and was approved by the Animal Ethics Committee of Traditional Chinese Medicine of Anhui University.
Authors contributions
Yongzhong Wang contributed to the study concept and design; YuanSun wrote the manuscript and conducted the experiments; HuiJiang contributed to the analysis of experimental data and study design; Lingyu Pan and Yanquan Han contributed to the manuscript review; and YanChen and Yeke Jiang performed the animal model. All authors approved the results of this experiment and the final manuscript.
Acknowledgments
We appreciate the logistic support for the study design and encouragement from Mr. Fan Qiang and Mrs. Yao Lan.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Liu R, Zhao P, Tan W, et al. Cell therapies for refractory rheumatoid arthritis. Clin Exp Rheumatol. 2018;36:1–12.
- Guo Q, Wang Y, Xu D, et al. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018;6(1):15.
- Mo BY, Guo XH, Yang MR, et al. Long non-coding RNA GAPLINC promotes tumor-like biologic behaviors of fibroblast-like synoviocytes as MicroRNA sponging in rheumatoid arthritis patients. Front Immunol. 2018;9:702.
- Choi C, Jeong W, Ghang B, et al. Cyr61 synthesis is induced by interleukin-6 and promotes migration and invasion of fibroblast-like synoviocytes in rheumatoid arthritis. Arthritis Res Ther. 2020;22(1):275.
- Xing XW, Shi HY, Liu S, et al. miR-496/MMP10 is involved in the proliferation of IL-1beta-Induced Fibroblast-Like synoviocytes via mediating the NF-kappaB signaling pathway. Inflammation. 2021;44(4):1359–1369.
- Li LY, Yang JF, Rong F, et al. ZEB1 serves an oncogenic role in the tumourigenesis of HCC by promoting cell proliferation, migration, and inhibiting apoptosis via Wnt/beta-catenin signaling pathway. Acta Pharmacol Sin. 2021;42(10):1676–1689.
- Chen G, Wang Z, Wang D, et al. LncRNADisease: a database for long-non-coding RNA-associated diseases. Nucleic Acids Res. 2013;41(Database issue):D983–986.
- Yang X, Meng T. Long noncoding RNA in preeclampsia: transcriptional noise or innovative indicators? Biomed Res Int. 2019;2019:5437621.
- Arunkumar G, Anand S, Raksha P, et al. LncRNA OIP5-AS1 is overexpressed in undifferentiated oral tumors and integrated analysis identifies as a downstream effector of stemness-associated transcription factors. Sci Rep. 2018;8(1):7018.
- Wang Y, Wang H, Ruan J, et al. Long non-coding RNA OIP5-AS1 suppresses multiple myeloma progression by sponging miR-27a-3p to activate TSC1 expression. Cancer Cell Int. 2020;20:155.
- Tao Y, Wan X, Fan Q, et al. Long non-coding RNA OIP5-AS1 promotes the growth of gastric cancer through the miR-367-3p/HMGA2 axis. Dig Liver Dis. 2020;52(7):773–779.
- Zhi L, Zhao J, Zhao H, et al. Downregulation of LncRNA OIP5-AS1 Induced by IL-1beta Aggravates Osteoarthritis via Regulating miR-29b-3p/PGRN. Cartilage 2021;13(2_suppl):1345S–1355S.
- Qin GH, Yang WC, Yao JN, et al. LncRNA OIP5-AS1 affects the biological behaviors of chondrocytes of patients with osteoarthritis by regulating micro-30a-5p. Eur Rev Med Pharmacol Sci. 2021;25:1215–1224.
- Lai NS, Yu HC, Tung CH, et al. The role of aberrant expression of T cell miRNAs affected by TNF-alpha in the immunopathogenesis of rheumatoid arthritis. Arthritis Res Ther. 2017;19(1):261.
- Wang Y, Shang G, Wang W, et al. Magnoflorine inhibits the malignant phenotypes and increases cisplatin sensitivity of osteosarcoma cells via regulating miR-410-3p/HMGB1/NF-kappaB pathway. Life Sci. 2020;256:117967.
- Wang Y, Xu N, Zhao S, et al. miR-410-3p suppresses cytokine release from Fibroblast-Like synoviocytes by regulating NF-kappaB signaling in rheumatoid arthritis. Inflammation. 2019;42(1):331–341.
- Wang Y, Jiao T, Fu W, et al. miR-410-3p regulates proliferation and apoptosis of fibroblast-like synoviocytes by targeting YY1 in rheumatoid arthritis. Biomed Pharmacother. 2019;119:109426.
- Wang Y, Hou L, Yuan X, et al. LncRNA NEAT1 targets Fibroblast-Like synoviocytes in rheumatoid arthritis via the miR-410-3p/YY1 axis. Front Immunol. 2020;11:1975.
- Sun WL, Kang T, Wang YY, et al. Long noncoding RNA OIP5-AS1 targets Wnt-7b to affect glioma progression via modulation of miR-410. Biosci Rep. 2019;39(1):BSR20180395.
- Li B, Lee C, Cadete M, et al. Impaired Wnt/beta-catenin pathway leads to dysfunction of intestinal regeneration during necrotizing enterocolitis. Cell Death Dis. 2019;10(10):743.
- Lu Y, Deng X, Xiao G, et al. Circ_0001730 promotes proliferation and invasion via the miR-326/Wnt7B axis in glioma cells. Epigenomics. 2019;11(11):1335–1352.
- Qiao C, Qiao T, Yang S, et al. SNHG17/miR-384/ELF1 axis promotes cell growth by transcriptional regulation of CTNNB1 to activate Wnt/beta-catenin pathway in oral squamous cell carcinoma. Cancer Gene Ther. 2022, 29(1):122–132.
- Zhu H, Chen Z, Shen L, et al. Long noncoding RNA LINC-PINT suppresses cell proliferation, invasion, and EMT by blocking Wnt/beta-Catenin signaling in glioblastoma. Front Pharmacol. 2020;11:586653.
- Wang W, Guo P, Chen M, et al. FOXM1/LINC00152 feedback loop regulates proliferation and apoptosis in rheumatoid arthritis fibroblast-like synoviocytes via Wnt/beta-catenin signaling pathway. Biosci Rep. 2020;40(1):BSR20191900.
- Li Y, Yuan L, Jiang S, et al. Interleukin-35 stimulates tumor necrosis factor-alpha activated osteoblasts differentiation through Wnt/beta-catenin signaling pathway in rheumatoid arthritis. Int Immunopharmacol. 2019;75:105810.
- Lu J, Yang J, Zheng Y, et al. Resveratrol reduces store-operated Ca(2+) entry and enhances the apoptosis of fibroblast-like synoviocytes in adjuvant arthritis rats model via targeting ORAI1-STIM1 complex. Biol Res. 2019;52(1):45.
- Jia XY, Chang Y, Wei F, et al. CP-25 reverses prostaglandin E4 receptor desensitization-induced fibroblast-like synoviocyte dysfunction via the G protein-coupled receptor kinase 2 in autoimmune arthritis. Acta Pharmacol Sin. 2019;40(8):1029–1039.
- Weyand CM, Goronzy JJ. The immunology of rheumatoid arthritis. Nat Immunol. 2021;22(1):10–18.
- Luo J, Liu L, Shen J, et al. miR‑576‑5p promotes epithelial‑to‑mesenchymal transition in colorectal cancer by targeting the Wnt5a‑mediated Wnt/beta‑catenin signaling pathway. Mol Med Rep. 2021;23(2):94.
- Yu JE, Ju JA, Musacchio N, et al. Long noncoding RNA DANCR activates Wnt/beta-catenin signaling through MiR-216a inhibition in non-small cell lung cancer. Biomolecules. 2020;10(12):1646.
- Yang B, Zhao J, Huo T, et al. Effects of CircRNA-ITCH on proliferation and apoptosis of hepatocellular carcinoma cells through inhibiting Wnt/beta-catenin signaling pathway. J Buon. 2020;25:1368–1374.
- Lv Y, Li XJ, Wang HP, et al. TGF-beta1 enhanced myocardial differentiation through inhibition of the Wnt/beta-catenin pathway with rat BMSCs. Iran J Basic Med Sci. 2020;23:1012–1019.
- Bhuvanalakshmi G, Basappa KS, Rangappa A, et al. Breast cancer stem-like cells are inhibited by diosgenin, a steroidal saponin, by the attenuation of the Wnt beta-catenin signaling via the Wnt antagonist secreted frizzled related protein-4. Front Pharmacol. 2017;8:124.
- Zhang X, Zhang D, Wang Q, et al. Sprouty2 inhibits migration and invasion of Fibroblast-Like synoviocytes in rheumatoid arthritis by down-regulating ATF2 expression and phosphorylation. Inflammation. 2021;44(1):91–103.
- Kim J, Noh JH, Lee SK, et al. LncRNA OIP5-AS1/cyrano suppresses GAK expression to control mitosis. Oncotarget. 2017;8(30):49409–49420.
- Wu Z, Liu Y, Wei L, et al. LncRNA OIP5-AS1 promotes breast cancer progression by regulating miR-216a-5p/GLO1. J Surg Res. 2021;257:501–510.
- Huang J, Hou S, Xu J, et al. Long non-coding RNA OIP5-AS1 promotes cell proliferation and aerobic glycolysis in gastric cancer through sponging miR-186. Arch Med Sci. 2021;17:1742–1751.
- Yang Z, Lin SD, Zhan F, et al. LncRNA GAS5 alleviates rheumatoid arthritis through regulating miR-222-3p/Sirt1 signalling axis. Autoimmunity. 2021;54(1):13–22.
- Wu ZM, Luo J, Shi XD, et al. Icariin alleviates rheumatoid arthritis via regulating miR-223-3p/NLRP3 signalling axis. Autoimmunity. 2020;53(8):450–458.
- Liu Q, Wang Z, Zhou X, et al. miR-342-5p inhibits osteosarcoma cell growth, migration, invasion, and sensitivity to doxorubicin through targeting Wnt7b. Cell Cycle. 2019;18(23):3325–3336.
- Miao CG, Shi WJ, Xiong YY, et al. miR-375 regulates the canonical Wnt pathway through FZD8 silencing in arthritis synovial fibroblasts. Immunol Lett. 2015;164(1):1–10.
- Sun J, Yan P, Chen Y, et al. MicroRNA-26b inhibits cell proliferation and cytokine secretion in human RASF cells via the Wnt/GSK-3beta/beta-catenin pathway. Diagn Pathol. 2015;10(1):72.
- Liu F, van den Broek O, Destree O, et al. Distinct roles for xenopus tcf/lef genes in mediating specific responses to Wnt/beta-catenin signalling in mesoderm development. Development. 2005;132(24):5375–5385.
- Ashihara E, Takada T, Maekawa T. Targeting the canonical Wnt/beta-catenin pathway in hematological malignancies. Cancer Sci. 2015;106(6):665–671.
- Zhang QH, Hu QX, Xie D, et al. Ganoderma lucidum exerts an anticancer effect on human osteosarcoma cells via suppressing the Wnt/beta-Catenin signaling pathway. Integr Cancer Ther. 2019;18:153473541989091.
- Zhang Z, Liu M, Hu Q, et al. FGFBP1, a downstream target of the FBW7/c-Myc axis, promotes cell proliferation and migration in pancreatic cancer. Am J Cancer Res. 2019;9:2650–2664.
- Ma J, Yang D, Yang J, et al. Inhibition of Wnt/beta-catenin signal and NOX4 impairs repair of silica-induced lung epithelial cell injury.. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi Chin J Cell Mol Immunol. 2021;37:132–139.
- Faccidomo S, Holstein SE, Santanam TS, et al. Pharmacological inhibition of glycogen synthase kinase 3 increases operant alcohol self-administration in a manner associated with altered pGSK-3beta, protein interacting with C kinase and GluA2 protein expression in the reward pathway of male C57BL/6J mice. Behav Pharmacol. 2020;31:15–26.
- Reabroi S, Saeeng R, Boonmuen N, et al. The anti-cancer activity of an andrographolide analogue functions through a GSK-3beta-independent Wnt/beta-catenin signaling pathway in colorectal cancer cells. Sci Rep. 2018;8(1):7924.
- Lennox KA, Behlke MA. Cellular localization of long non-coding RNAs affects silencing by RNAi more than by antisense oligonucleotides. Nucleic Acids Res. 2016;44(2):863–877.
- Li W, Zhang Y, Lv J, et al. MicroRNA-137-mediated lysine demethylase 4A regulates the recovery of spinal cord injury via the SFRP4-Wnt/beta-Catenin axis. Int J Neurosci. 2023;133(1):37–50.
- Miao CG, Qin D, Du CL, et al. DNMT1 activates the canonical Wnt signaling in rheumatoid arthritis model rats via a crucial functional crosstalk between miR-152 and the DNMT1, MeCP2. Int Immunopharmacol. 2015;28(1):344–353.
- Jiang H, Liu J, Fan C, et al. lncRNAS56464.1 as a ceRNA promotes the proliferation of fibroblastlike synoviocytes in experimental arthritis via the Wnt signaling pathway and sponges miR1523p. Int J Mol Med. 2021;47(3):17.