Abstract
Glioblastoma is the most common glioma with high mortality and poor prognosis. Radiation resistance is one of the large challenges in the treatment of glioma. The study aimed to identify whether DNA polymerase ζ affects glioma cell radiosensitivity. The mRNA and protein levels of REV3L and REV7 were examined using quantitative real-time PCR and western blot. After REV3L and REV7 knockdown in a GBM cell line (A172), we assessed cell viability, colonies, apoptosis, and immune escape. The underlying mechanisms were evaluated using western blot and were confirmed using rescue experiments. The results showed that REV3L and REV7 levels were increased in glioma and related to poor survival. γ-ray treatment inhibited cell viability, survival fraction, and immune escape, and induced apoptosis of glioma cells from a GBM cell line, whereas knockdown of REV3L or REV7 enhanced these effects. Mechanically, silencing of REV3L or REV7 inactivated the PI3K/AKT/mTOR pathway. IGF-1 treatment abrogated the effects on cell viability, colonies, and apoptosis induced by REV3L or REV7 knocking down. Taken together, silencing of REV3L and REV7 inhibited radiation resistance via inactivating the PI3K/AKT/mTOR pathway, suggesting that targeting DNA polymerase ζ may be a new strategy to reduce the radiotherapy resistance of glioma.
Introduction
Glioma is a central nervous system (CNS) tumor in adults with significant incidence and mortality. It can be classified as adult-type diffuse gliomas, pediatric-type diffuse low-grade gliomas, pediatric-type diffuse high-grade gliomas, and circumscribed astrocytic gliomas, accounting for 1/4 of all primary CNS tumors [Citation1]. The prognosis of glioblastoma (GBM) is poor, with a median survival rate of 14 months [Citation2]. Treatment options for malignant glioma have major limitations. Like most cancers, the standard treatments of glioma include surgery and chemoradiotherapy [Citation3]. However, radiation resistance is a major obstacle to the success of radiotherapy [Citation4]. Therefore, exploring appropriate methods to enhance the sensitivity of radiotherapy may be helpful to improve the clinical outcomes of glioma.
Immunotherapy is becoming a promising treatment strategy for glioma due to its ability to penetrate the blood-brain barrier [Citation5]. Radiotherapy can enhance antitumor immune activation, and immunotherapy in turn sensitizes tumors to radiotherapy [Citation6]. Although the use of immune checkpoint inhibitors has significantly improved the therapeutic outcomes of cancers; unfortunately, they have little success in glioma treatment [Citation7]. Tumor immune escape is common; however, the mechanisms of glioma escape in immunotherapy are still unclear [Citation7]. Therefore, clarifying the immune escape of glioma may provide a theoretical reference for improving the effect of immunotherapy and radiotherapy resistance.
DNA polymerase ζ (pol ζ), consisting of REV3L and REV7 catalytic subunits, maintains genomic stability during normal DNA replication by hindering DNA replication forks [Citation8,Citation9]. pol ζ can effectively extend DNA past mismatched bases and bypass DNA lesions [Citation10]. Inactivation of pol ζ leads to chromosome breakage and disrupts the viability of cells and embryos, leading to death [Citation11]. The decreased expression and/or dysfunction of pol ζ increase the risk of cancers, such as skin, lung, colorectal, and breast cancers [Citation12]. REV3L and REV7 have been identified to contribute to chemotherapy resistance in glioma [Citation13,Citation14]. Additionally, overexpression of pol ζ inhibits the radiosensitivity of cancer cells [Citation15]. However, whether pol ζ affects radiotherapy resistance in glioma remains not understood.
In the current study, we aimed to clarify the effects of pol ζ on the radiosensitivity of glioma cells. We speculated that interference with REV3L and REV7 inhibited the proliferation, and immune escape, and facilitated apoptosis of glioma cells from a GBM cell line A172 by modulating the PI3K/AKT/mTOR pathway. The findings suggest that pol ζ may be a target to enhance the radiation sensitivity of glioma cells.
Materials and methods
Tissue specimens
The operations protocol involving tissue extraction is approved by the Ethics Committee of The First Affiliated Hospital of Jinan University (Approval No. 20221124). Informed contents were obtained from all subjects. Glioma tissues (n = 40) and paracancerous normal tissues (n = 40) from patients with glioma were obtained in The First Affiliated Hospital of Jinan University. All tissues were collected during surgery and stored in liquid nitrogen. These glioma types were all astrocytomas.
Cell culture
GBM cell lines (A172, LN229, and U251) and normal human astrocytes (NHAs) were grown in Dulbecco’s modified eagle medium (Gibco) supplemented with 10% fetal bovine serum (Gibco) at 37 °C in 5% CO2.
Cell transfection
A172 cells were seeded into 6-well plates and incubated until 80% cell confluence. The cells were transfected with small interfering RNA (si)-REV3L 1#, si-REV3L 2#, si-REV7 1#, si-REV7 2#, and si-negative control (NC) (Genepharma) using Lipofectamin™ 2000 (Invitrogen) for 48 h.
Cell treatment
Transfected A172 cells were exposed to 0.8 Gy/min of γ-ray with 60Co. The unirradiated cells were the control cells.
Cell counting kit-8 (CCK-8) assay
γ-ray treated cells were seeded into 96-well plates and incubated at 37 °C for 24 h. Subsequently, 10 μL CCK-8 reagent from a CCK-8 kit (Dojindo) was incubated with the cells for 4 h. The optical density (OD) value of each well was measured using the microplate reader at 450 nm.
Colony formation assay
After irradiation, A172 cells were seeded into 6-well plates and incubated at 37 °C for 14 days. Cell colonies were stained with 0.5% crystal violet and counted under a microscope. Survival fractions were quantified.
Flow cytometry
To assess A172 cell apoptosis, the cells after irradiation were washed with PBS and resuspended using the 1× binding buffer. The cells were incubated with 5 μL Annexin V/PE and 10 μL 7-AAD from an Annexin V-PE/7-AAD apoptosis detection kit (Yeasen) for 15 min in the dark. Apoptosis was measured using a flow cytometer (Beckman Coulter).
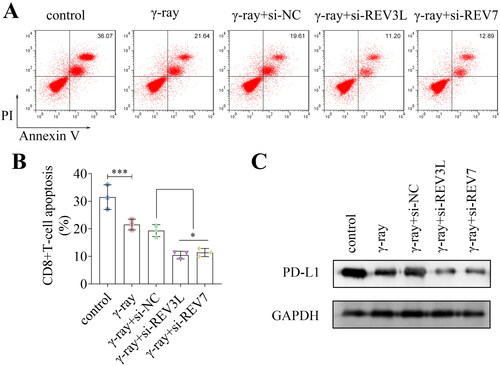
To assess CD8+ T cell apoptosis, peripheral blood mononuclear cells (PBMCs) were obtained and purified from the blood of patients with glioma using the Ficoll-Paque assay. Human CD8+ T cells were purified using the EasySep human CD8+ T cell Enrichment kit (StemCell Technologies). Then, CD8+ T cells were co-cultured with A172 cells at 37 °C for 24 h. Apoptosis of CD8+ T cells was detected using an annexin V-FITC/PI kit (Yeasen) before the flow cytometry was performed. The apoptosis was analyzed using the Flowjo 7.6 software.
Quantitative real-time PCR (qRT-PCR)
Total RNA was isolated from tissues and A172 cells using the MolPure Cell RNA Kit (Yeasen). After measuring RNA concentration, total RNA (1 μg) was reverse transcribed to complementary DNA using a miScript II RT kit (Qiagen). qPCR was assessed using the miScript SYBR Green PCR kit (Qiagen) using an ABI 7500 real-time PCR system (Applied Biosystems). Relative gene expression was calculated using the 2−ΔΔCt method. GAPDH was the housekeeping control. The primer sequences are listed below: REV3L forward: 5′-GCTCCAGTATGTGTACCATCTTGT-3′ and reverse: 5′-ATGGATATCTCGAAGTAACACGTC-3′; REV7 forward: 5′-TCTTAATCAAGCTTTCCGTCT-3′ and reverse: 5′-CTCAGATAAGCCGTTACCTCC-3′; GAPDH forward: 5′-ACTCCTCCACCTTTGACGC-3′ and reverse: 5′-GCTGTAGCCAAATTCGTTGTC-3′.
Western blot
Total protein was extracted from A172 cells using the RIPA lysis buffer. About 25 μg protein was separated using 10% SDS-PAGE. The separated proteins were electro-transferred to PVDF membranes (Millipore). The membrane was blocked in 5% non-fat milk, incubated with primary antibodies overnight at 4 °C, and incubated with HRP-conjugated secondary antibodies for 2 h at 25 °C. Protein bands were visualized using the ECL reagent under a Gel Doc EZ Gel imaging system (Bio-Rad). The protein levels were quantified using the ImageJ software.
Statistical analysis
Data were acquired from three repeated experiments and analyzed using the GraphPad Prism 7.0 software. Comparisons between the two groups were carried out using the student’s t-test, and comparisons among multiple groups were performed using one-way ANOVA. Data were expressed as mean ± standard deviation. The prognosis of patients with glioma was evaluated using the Kaplan-Meier analysis. p < 0.05 was identified as statistical significance.
Results
Pol ζ is highly expressed in glioma and correlated to poor prognosis
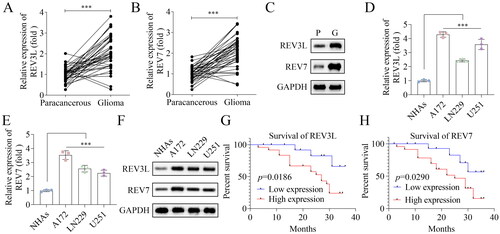
First, the mRNA expression of pol ζ including REV3L and REV7 was measured in tissues. The results showed that both REV3L and REV7 were highly expressed in glioma tissues, compared with normal brain tissues (). Similarly, the protein levels of REV3L and REV7 were both elevated in tumor tissues (). In addition, REV3L and REV7 were increased in glioma cells at mRNA and protein levels, including A172, LN229, and U251 cells, especially in A172 cells, compared with that in NHAs (). Additionally, the survival rate was detected. High mRNA expression of REV3L and REV7 were correlated to poor prognosis of patients with glioma (). The results show that high expressed pol ζ is associated with low survival of patients with glioma.
Figure 1. Pol ζ is highly expressed in glioma and correlated to poor prognosis. The mRNA expression of (A) REV3L and (B) REV7 in 40 paired glioma tissues and paracancerous tissues. n = 40. (C) The protein levels of REV3L and REV7 in tumor and adjacent normal tissues. n = 3. The mRNA expression of (D) REV3L and (E) REV7 in GBM cells (A172, LN229, and U251) and normal cells (NHAs). n = 3. (F) The protein levels of REV3L and REV7 in A172, LN229, U251, and NHAs. n = 3. The prognosis of patients with glioma has high or low (G) REV3L and (H) REV7 expression. n = 40. The expression of REV3L and Rev7 in tissues was assessed using student’s t-test. The prognosis of patients with glioma was evaluated using the Kaplan-Meier analysis. Other comparisons were analyzed using one-way ANOVA. ***p < 0.001.
Effects of pol ζ on the radiosensitivity of GBM cells
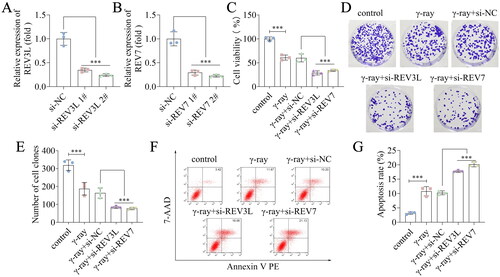
Subsequently, we explored the role of REV3L and REV7 knockdown in A172 cells. REV3L and REV7 mRNA expression was downregulated following transfection with siRNAs (). The si-REV3L 2# and si-REV7 2# transfected cells were used in the subsequent study. We used γ-ray to treat these transfected cells to analyze the effects of REV3L and REV7 on radiosensitivity. We found that γ-ray suppressed A172 cell viability, and silencing of REV3L and REV7 further suppressed the viability of γ-ray treated cells (). The survival fraction was reduced by γ-ray and further decreased following REV3L and REV7 knockdown (). Besides, γ-ray promoted cell apoptosis, and knockdown of REV3L and REV7 enhanced apoptosis induced by γ-ray (). In addition, we evaluated the effects of REV3L and REV7 on these biological behaviors. The results showed that interference with REV3L and REV7 inhibited A172 cell viability and colonies, and facilitated apoptosis (). Taken together, the results indicate that silencing of pol ζ enhances radiosensitivity, inhibits proliferation, and induces apoptosis of A172 cells.
Figure 2. Effects of pol ζ on the radiosensitivity of GBM cells. (A) The efficiency following transfection with (A) si-REV3L 1#, si-REV3L 2#, and si-NC. (B) The efficiency following transfection with si-REV7 1#, si-REV7 2#, and si-NC. (C) Cell viability of transfected cells treated with γ-ray. (D,E) Cell survival was measured after A172 cell transfection and irradiation. (F,G) Cell apoptosis of transfected cells after irradiation. n = 3 in all experiments. The comparisons were analyzed using one-way ANOVA. ***p < 0.001.
Effects of pol ζ on the immune escape of GBM cells
To analyze whether REV3L and REV7 affected immune escape, the apoptosis of CD8+ T cells was detected. Knockdown of REV3L and REV7 inhibited CD8+ T cell apoptosis when co-cultured with A172 cells under γ-ray treatment (). Additionally, γ-ray downregulated the protein levels of PD-L1, and the depletion of REV3L and REV7 further reduced the PD-L1 levels (). Taken together, the silencing of pol ζ inhibits the immune escape of A172 glioma cells.
Effects of pol ζ on the PI3K/AKT/mTOR pathway
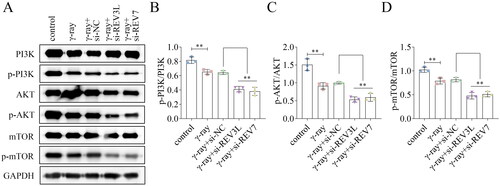
To assess the underlying mechanism, the protein levels of related factors of the PI3K/AKT/mTOR pathway were detected. The protein levels of PI3K, AKT, and mTOR were not affected by REV3L and REV7. Additionally, γ-ray inhibited the phosphorylation levels of PI3K, AKT, and mTOR, while depletion of REV3L and REV7 decreased the levels of p-PI3K, p-AKT, and p-mTOR in γ-ray-treated cells (). The ratios of p-PI3K/PI3K, p-AKT/AKT, and p-mTOR/mTOR were reduced by γ-ray and further reduced by REV3L and REV7 knockdown (). The results show that silencing of pol ζ inactivates the PI3K/AKT/mTOR pathway.
Figure 4. Effects of pol ζ on the PI3K/AKT/mTOR pathway. (A) The protein levels of PI3K, p-PI3K, AKT, p-AKT, mTOR, and p-mTOR in A172 cells treated with γ-ray and transfected with si-REV3L and si-REV7. The ratios of (B) p-PI3K/PI3K, (C) p-AKT/AKT, and (D) p-mTOR/mTOR were quantified. n = 3 in all experiments. The comparisons were analyzed using one-way ANOVA. **p < 0.01.
Pol ζ affects the radiosensitivity via the PI3K/AKT/mTOR pathway
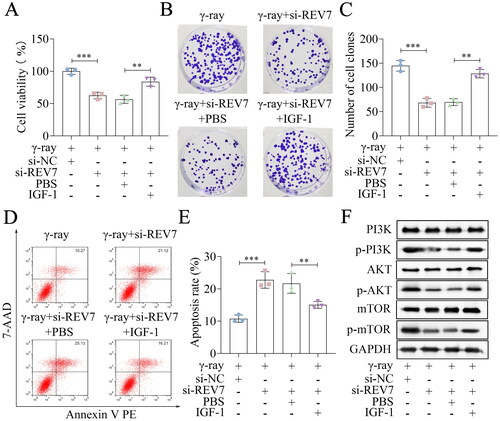
Then, the rescue experiments were performed. IGF-1 was used to activate this pathway in A172 cells, and cellular processes were evaluated. Cell viability was inhibited by knockdown of REV3L or REV7 in the presence of γ-ray or not, which was partly abolished by IGF-1 treatment (; Supplementary Figure 2(A)). Depletion of REV3L or REV7 inhibited the survival fraction, and IGF-1 reversed the inhibition of survival fraction (; ). Cell apoptosis was induced by REV3L or REV7 knockdown, whereas IGF-1 abrogated the effects on apoptosis in the conditions of γ-ray treatment or no treatment (; ). The protein levels of p-PI3K, p-AKT, and p-mTOR were downregulated by REV3L or REV7 knockdown, which was counteracted by IGF-1 treatment (). To sum up, the knockdown of Pol ζ enhances the radiosensitivity of A172 cells by inactivation of the PI3K/AKT/mTOR pathway.
Figure 5. REV3L affects the radiosensitivity via the PI3K/AKT/mTOR pathway. (A) Cell viability of si-REV3L transfected cells treated with IGF-1. (B,C) Cell survival was measured after transfection and IGF-1 treatment. (D,E) Cell apoptosis of transfected cells after IGF-1 treatment. (F) the protein levels of PI3K, p-PI3K, AKT, p-AKT, mTOR, and p-mTOR. n = 3 in all experiments. The comparisons were analyzed using one-way ANOVA. ***p < 0.001. **p < 0.01.
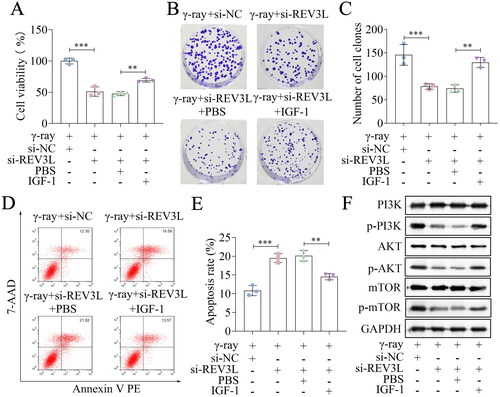
Figure 6. REV7 affects the radiosensitivity via the PI3K/AKT/mTOR pathway. (A) Cell viability of si-REV7 transfected cells treated with IGF-1. (B,C) Cell survival was measured after transfection and IGF-1 treatment. (D,E) Cell apoptosis of transfected cells after IGF-1 treatment. (F) the protein levels of PI3K, p-PI3K, AKT, p-AKT, mTOR, and p-mTOR. n = 3 in all experiments. The comparisons were analyzed using one-way ANOVA. ***p < 0.001. **p < 0.01.
Discussion
The overall survival of GBM is poor, and one of the reasons is the development of resistance to radiotherapy or chemotherapy, which significantly reduces their therapeutic efficiency. Thus, it is necessary to understand the mechanisms of GBM radiotherapy resistance. DNA interstrand crosslinks interfere with DNA replication and RNA transcription, inducing cell death. Pol ζ and another DNA polymerase pol θ are essential in DNA interstrand crosslinks repair that bypass DNA lesions [Citation16,Citation17]. Pol ζ is involved in DNA damage repair in tumor cells during anti-cancer treatment, especially in chemoradiotherapy. Accumulating evidence has revealed that Pol ζ is the potential target to enhance the chemoradiotherapy sensitivity of cancer cells [Citation18,Citation19]. For example, overexpressing of Pol ζ, including REV7 and REV3L, inhibits radiosensitivity, apoptosis, and oxidative stress of lung cancer cells [Citation15,Citation20]. Downregulation of REV3 suppresses cisplatin resistance and cisplatin-induced mutagenicity in colon cancer cells [Citation21]. Additionally, Pol ζ has the potential to become a chemoradiation resistance and biomarker of cervical cancer [Citation22]. In glioma, REV3L contributes to enhancing the resistance to temozolomide and fotemustine, and REV7 increases the cisplatin resistance [Citation13,Citation14]. Chemotherapy and radiotherapy are commonly used for cancer therapy after surgical resection. They are effective in killing remaining tumors, cause a variety of side effects, and lead to tolerance [Citation23]. The regulatory mechanisms of Pol ζ to chemotherapy resistance is mainly to regulate biological behaviors of tumor cells, such as apoptosis [Citation15]. However, the effects of Pol ζ on radiotherapy resistance in glioma remain unknown. Inhibition of the pol ζ REV3 catalytic subunit impairs UV damage-induced DNA mutagenesis, suggesting pol ζ may be involved in the effects of radiation on DNA damage [Citation10]. The results of our study showed that REV3L and REV7 mRNA and protein levels were upregulated in glioma tissues and cells, consistent with the previous study. Furthermore, the knockdown of REV3L and REV7 suppressed radiation resistance by inhibiting viability, immune escape, and inducing apoptosis of A172 cells treated with γ-ray. The results suggest that Pol ζ is an oncogene in GBM, which depletion increased the radiotherapy sensitivity of GBM cells.
The PI3K/AKT/mTOR pathway is important in tumorigenesis and cancer development that has been widely studied in a variety of cancers [Citation24]. This pathway is aberrantly activated in cancers and acts as an oncogenic driver to regulate biological functions, including glioma [Citation25]. It is related to cell survival, growth, metabolism, metastasis, angiogenesis, and drug resistance [Citation26,Citation27]. Previous studies have shown that the PI3K/AKT/mTOR pathway promotes radiation resistance. PDK1-drive resistance to radiotherapy is linked to the activation of the PI3K pathway in liver cancer [Citation28]. Additionally, the PI3K/AKT/mTOR pathway is commonly activated and leads to radioresistance in GBM cells [Citation29]. In the current study, the results indicated that the knockdown of REV3L and REV7 suppressed the activation of the PI3K/AKT/mTOR pathway. Activation of this pathway using the IGF-1 abrogated the effects on the radiosensitivity of GBM cells induced by REV3L or REV7 knockdown. Taken together, silencing of Pol ζ sensitizes GBM cells to radiation resistance by inactivating the PI3K/AKT/mTOR pathway.
One of the limitations of this study was that we only used one cell line to study the effects of Pol ζ. Besides, the role of Pol ζ in vivo remains unknown. We will further study these issues in our future work.
In conclusion, REV3L and REV7 levels were upregulated in glioma. Depletion of REV3L and REV7 enhanced γ-ray-induced radiosensitivity by suppressing the activation of the PI3K/AKT/mTOR pathway. The data suggested that Pol ζ might be a novel target during glioma radiotherapy.
Ethical approval
This work was approved by the Ethics Committee of The First Affiliated Hospital of Jinan University (Approval No. 20221124). Informed contents were obtained from all subjects.
Authors’ contributions
All authors participated in the design, interpretation of the studies and analysis of the data, and review of the manuscript. JD drafted the work and revised it critically for important intellectual content; ZC, WD, and YX were responsible for the acquisition, analysis, or interpretation of data for the work; JY made substantial contributions to the conception or design of the work.
Supplemental Material
Download MS Word (679.5 KB)Acknowledgments
Not applicable.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1–8. doi:10.1093/neuonc/noab106
- Lin J, Bytnar JA, Theeler BJ, et al. Survival among patients with glioma in the US military health system: a comparison with patients in the surveillance, epidemiology, and end results program. Cancer. 2020;126(13):3053–3060. doi:10.1002/cncr.32884
- Wang TJC, Mehta MP. Low-grade glioma radiotherapy treatment and trials. Neurosurg Clin N Am. 2019;30(1):111–118. doi:10.1016/j.nec.2018.08.008
- Mudassar F, Shen H, O'Neill G, et al. Targeting tumor hypoxia and mitochondrial metabolism with anti-parasitic drugs to improve radiation response in high-grade gliomas. J Exp Clin Cancer Res. 2020;39(1):208. doi:10.1186/s13046-020-01724-6
- Xu S, Tang L, Li X, et al. Immunotherapy for glioma: current management and future application. Cancer Lett. 2020;476:1–12. doi:10.1016/j.canlet.2020.02.002
- Wang Y, Liu ZG, Yuan H, et al. The reciprocity between radiotherapy and cancer immunotherapy. Clin Cancer Res. 2019;25(6):1709–1717. doi:10.1158/1078-0432.CCR-18-2581
- Daubon T, Hemadou A, Romero Garmendia I, et al. Glioblastoma immune landscape and the potential of new immunotherapies. Front Immunol. 2020;11:585616. doi:10.3389/fimmu.2020.585616
- Martin SK, Wood RD. DNA polymerase ζ in DNA replication and repair. Nucleic Acids Res. 2019;47(16):8348–8361. doi:10.1093/nar/gkz705
- Ben Yamin B, Ahmed-Seghir S, Tomida J, et al. DNA polymerase zeta contributes to heterochromatin replication to prevent genome instability. EMBO J. 2021;40(21):e104543. doi:10.15252/embj.2020104543
- Zan H, Komori A, Li Z, et al. The translesion DNA polymerase zeta plays a major role in Ig and bcl-6 somatic hypermutation. Immunity. 2001;14(5):643–653. doi:10.1016/S1074-7613(01)00142-X
- Lange SS, Tomida J, Boulware KS, et al. The polymerase activity of mammalian DNA pol ζ is specifically required for cell and embryonic viability. PLoS Genet. 2016;12(1):e1005759. doi:10.1371/journal.pgen.1005759
- Shilkin ES, Boldinova EO, Stolyarenko AD, et al. Translesion DNA synthesis and carcinogenesis. Biochemistry. 2020;85(4):425–435. doi:10.1134/S0006297920040033
- Roos WP, Tsaalbi-Shtylik A, Tsaryk R, et al. The translesion polymerase Rev3L in the tolerance of alkylating anticancer drugs. Mol Pharmacol. 2009;76(4):927–934. doi:10.1124/mol.109.058131
- Yang J, Ding W, Wang X, et al. Knockdown of DNA polymerase ζ relieved the chemoresistance of glioma via inhibiting the PI3K/AKT signaling pathway. Bioengineered. 2021;12(1):3924–3933. doi:10.1080/21655979.2021.1944027
- Chen X, Ji R, Liu J, et al. Roles of DNA polymerase ζ in the radiotherapy sensitivity and oxidative stress of lung cancer cells. Cancer Chemother Pharmacol. 2022;89(3):313–321. doi:10.1007/s00280-021-04360-9
- Sharma S, Canman CE. REV1 and DNA polymerase zeta in DNA interstrand crosslink repair. Environ Mol Mutagen. 2012;53(9):725–740. doi:10.1002/em.21736
- Zan H, Shima N, Xu Z, et al. The translesion DNA polymerase theta plays a dominant role in immunoglobulin gene somatic hypermutation. EMBO J. 2005;24(21):3757–3769. doi:10.1038/sj.emboj.7600833
- Nicolay NH, Helleday T, Sharma RA. Biological relevance of DNA polymerase β and translesion synthesis polymerases to cancer and its treatment. Curr Mol Pharmacol. 2012;5(1):54–67. doi:10.2174/1874467211205010054
- Mohni KN, Thompson PS, Luzwick JW, et al. A synthetic lethal screen identifies DNA repair pathways that sensitize cancer cells to combined ATR inhibition and cisplatin treatments. PLOS One. 2015;10(5):e0125482. doi:10.1371/journal.pone.0125482
- Wang W, Sheng W, Yu C, et al. REV3L modulates cisplatin sensitivity of non-small cell lung cancer H1299 cells. Oncol Rep. 2015;34(3):1460–1468. doi:10.3892/or.2015.4121
- Lin X, Trang J, Okuda T, et al. DNA polymerase zeta accounts for the reduced cytotoxicity and enhanced mutagenicity of cisplatin in human colon carcinoma cells that have lost DNA mismatch repair. Clin Cancer Res. 2006;12(2):563–568. doi:10.1158/1078-0432.CCR-05-1380
- Shi TY, Yang L, Yang G, et al. DNA polymerase ζ as a potential biomarker of chemoradiation resistance and poor prognosis for cervical cancer. Med Oncol. 2013;30(2):500. doi:10.1007/s12032-013-0500-4
- Cao W, Gu Y, Meineck M, et al. The combination of chemotherapy and radiotherapy towards more efficient drug delivery. Chem Asian J. 2014;9(1):48–57. doi:10.1002/asia.201301294
- Polivka JJr., Janku F. Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol Ther. 2014;142(2):164–175. doi:10.1016/j.pharmthera.2013.12.004
- Shahcheraghi SH, Tchokonte-Nana V, Lotfi M, et al. Wnt/beta-catenin and PI3K/Akt/mTOR signaling pathways in glioblastoma: two main targets for drug design: a review. Curr Pharm Des. 2020;26(15):1729–1741. doi:10.2174/1381612826666200131100630
- Alzahrani AS. PI3K/akt/mTOR inhibitors in cancer: at the bench and bedside. Semin Cancer Biol. 2019;59:125–132. doi:10.1016/j.semcancer.2019.07.009
- Akbarzadeh M, Mihanfar A, Akbarzadeh S, et al. Crosstalk between miRNA and PI3K/AKT/mTOR signaling pathway in cancer. Life Sci. 2021;285:119984. doi:10.1016/j.lfs.2021.119984
- Bamodu OA, Chang HL, Ong JR, et al. Elevated PDK1 expression drives PI3K/AKT/mTOR signaling promotes radiation-resistant and dedifferentiated phenotype of hepatocellular carcinoma. Cells. 2020;9(3):746. doi:10.3390/cells9030746
- Liu Y, Shen Y, Sun T, et al. Mechanisms regulating radiosensitivity of glioma stem cells. Neoplasma. 2017;64(5):655–665. doi:10.4149/neo_2017_502