Abstract
Infection by the Schistosoma japonicum can result in acute, chronic and late-stage manifestations. The latter often presents with severe organ failures and premature death. Importantly, infection can also produce autoimmune phenomena reflected by the development of autoantibodies. We wished to explore and profile the presence of autoantibodies in sera of patients with different stages of S. japonicum infection with the added aim of providing a reference assisting diagnosis. Blood samples from 55 patients with chronic and 20 patients with late-stage schistosomiasis japonica together, with a control group of 50 healthy people were randomly investigated against a microarray of 121 different autoantigens. In addition, the frequency of antibodies against Schistosoma egg antigen (SEA) was examined. In the sera from patients with chronic schistosomiasis japonica, 14 different highly expressed autoantibodies were detected, while patients with late-stage schistosomiasis were found to express as many as 43 autoantibody specificities together with a significantly higher frequency of antibodies against SEA compared to the control group. The findings presented suggest that autoantibody-based classification of schistosomiasis japonica represents a promising approach for the elucidation of subtypes of the disease. This approach may reflect differential disease mechanisms, which could ultimately lead to better treatment.
Introduction
Schistosomiasis, also known as bilharzia, is an infectious disease that affects more than 250 million people worldwide. According to the Institute for Health Metrics and Evaluation, Seattle (IHME), WA, USA was responsible for 11,500 deaths and 1.64 million disability-adjusted life years (DALYs) as of 2019 [Citation1]. The disease is caused by trematode parasitic worms of the genus Schistosoma that depends on a life cycle involving freshwater snails as intermediate hosts. Six schistosome species are adapted to humans, but the large majority of infections are caused by S. mansoni, S. haematobium and S. japonicum [Citation2]. The latter seriously jeopardises the health of residents in its major endemic areas (China and The Philippines), where it encumbers social and economic development [Citation3]. The clinical presentation of schistosomiasis japonica is reflected by the level of exposure to cercariae (the infectious stage released from the snail intermediate host) and for how long the infection has been active in the patient [Citation4]. The parasite is gender-differentiated and the disease is caused by immune reactions to eggs released from female schistosomes, while the worms themselves are of little pathological significance. S. japonicum infection can rapidly cause a severe clinical picture as each worm pair produces around 3,000 eggs per day, 10 times more than the average excreted by other schistosome species [Citation5]. Most eggs leave the host with faeces but a large proportion are stranded in the body and symptoms arise following immune responses to those carried to the liver by the blood circulation, eventually leading to periportal fibrosis, portal hypertension and hepatosplenomegaly. Pronounced obstruction of the venous blood flow in the portal system leads to late symptoms, such as ascites in the abdominal cavity and varicose veins in the oesophagus [Citation6]. A high level of schistosomiasis-induced morbidity was shown among subjects with signs and symptoms suggestive of schistosomiasis [Citation7]. Occasionally, S. japonicum eggs can also be found in other organs giving rise to other symptoms. Schistosomiasis generally presents as one of three clinical stages: (a) an acute stage characterised by dermatitis and fever (also called Katayama fever); (b) chronic manifestations that appear after many years with high worm burdens; and (c) a final late, advanced end stage with severe organ failures eventually resulting in premature death [Citation8].
In the chronic and late stages, some immune responses are down-regulated, resulting in a tempered immune response to eggs trapped in the body [Citation9]. Thus, the overall host immune response attempts to find a balance that limits tissue injuries, while still preventing new infections. However, this response pattern can “backfire” and trigger auto-immune reactions [Citation10]. Previous studies have shown that antinuclear autoantibodies (ANA) can exist in chronic and late schistosomiasis japonica patients suggesting that these patients are at particular risk of autoimmune disease [Citation11]. There is thus a need to find out which types of autoantibody are produced and whether or not they play a pathognomonic role.
Autoantibodies react with constituents of its own host (self-antigens) and come in two varieties, i.e. high-affinity IgG autoantibodies and less active IgM ones that only display a moderate affinity for self-antigens. The latter are often found in healthy individuals and do not seem to cause disease, while the former reflect an ongoing pathologic process [Citation12]. Autoantibodies are either of a general type or they are specific for certain diseases where they can offer diagnostic and therapeutic opportunities [Citation13]. The presence of specific autoantibodies in organ-specific autoimmune diseases strongly suggests that they are stimulated by long-standing inflammatory responses in target organs. Schistosomiasis may tender a similar prospect and previous findings [Citation14] have inspired the current search for additional, presumably specific autoantibodies.
Autoantibodies to intracellular antigens can be stimulated by antigens released from dying cells or appear in response to intrinsic abnormalities in lymphocytes of the B or T type [Citation15]. In schistosomiasis, the origin of autoantibodies is unclear and those found so far do not seem to be specific. It is therefore of interest to catalogue which autoantibodies are common in this disease. The aim of the present study was to screen for autoantibodies in a population in an area endemic for schistosomiasis to see if they can be used as markers for chronic and/or advanced schistosomiasis.
Materials and methods
Study site
We conducted a survey for biological indicators of autoimmune reactions in patients with S. japonicum infection in the provinces Hubei and Hunan, two major endemic areas for schistosomiasis japonica in China.
Study subjects
Serum samples were randomly collected during 2018 and 2019 from 125 people, including 55 patients with chronic schistosomiasis (average age 54.31 ± 3.91 years), 20 with late-stage disease (average age 68.30 ± 5.09 years) in the Affiliated Hospital of Yiyang Medical College in Hunan, China and 50 healthy adults (average age 48.71 ± 4.16 years) from non-endemic areas. All patients in this study were excluded from comorbidities such as hepatitis B virus infection and were non-autoimmune patients. The initial laboratory testing consisted of the demonstration of S. japonicum eggs in patient stools. All patients and healthy controls were tested for anti-SEA IgG; furthermore, a subset of patients were tested for autoantibodies. The distribution of the patients is shown in .
Table 1. Characteristics of schistosomiasis patients and controls.
General antibody assay
IgG antibodies against Schistosoma egg antigen (SEA) and their titres were measured in all patients with schistosome infection. Testing was carried out at the National Institute of Parasitic Diseases (NIPD), Chinese Centre for Disease Control and Prevention (China CDC) in Shanghai. The enzyme-linked immunosorbent assay (ELISA) was used to test for these antibodies using 96-well flat ELISA plates (Costar, NY, USA) coated with SEA. After blocking with 5% skimmed milk, the diluted serum samples were added to the plates that were then washed 3 times with phosphate-buffered saline (PBS) with 0.5% Tween-20 (PBST). The serum samples were diluted 1:100 with PBS and added to the plates. Horse radish peroxidase (HRP) conjugated mouse anti-human IgG (Sigma-Aldrich, St. Louis, MO, USA) was used as the secondary (detector) antibody. Absorbance was measured at 450 nm using a microtitre plate reader from Thermo Scientific (Bartlesville, OK, USA). Similarly, testing for autoantibodies was performed using the Human Anti-Bovine Amnion Type V Collagen IgG Antibody Assay Kit (Chondrex) and SSB IgG ELISA (NeoBioscience) and following manufacturer’s instructions.
Autoantibody profiling
We probed for reactivity against 121 purified or recombinant autoantigens and 4 control proteins) in serum samples from 15 chronic and 15 late-stage schistosomiasis japonica patients, who were randomly selected from 55 chronic schistosomiasis patients and 20 late-stage patients. The results were compared with that in sera from 30 and 7 age-matched healthy controls, respectively.
Autoantigen microarrays are high-throughput screening devices with the potential to identify autoantibody specificities against a wide spectrum of autoantigens and are therefore valuable for the evaluation of the correlation between autoantibodies and clinical manifestations [Citation15–17]. We tested the sera for IgG antibodies against a panel of 121 autoantigens in such an autoantigen microarray available from GeneCopoeia, Inc, Guangzhou, China. Briefly, samples were pre-treated with DNase I (an enzyme for digestion of DNA) from Qiagen, Germany and then diluted 1:50 in PBS with 0.5% Tween-20. The autoantigen array autoantigens and control proteins had been printed in duplicates onto Nitrocellulose film slides (Grace Bio-Labs, Oregon, USA). The diluted serum samples were incubated with the autoantigen arrays, with the autoantibodies revealed by adding cy3-labeled anti-human IgG (1:1000) from Jackson ImmunoResearch Laboratories, Baltimore, PA, USA, using Genepix 4400. A fluorescent microarray scanner (Molecular Devices, Sunnyvale, CA, USA) with laser wavelengths of 532 and 635 nm was used to image each microarray and read the median fluorescence intensity to quantify bindings. Significance analysis of microarrays was used to identify significant differences in IgG reactivity to the autoantigens between chronic schistosomiasis japonica patients and controls at p < 0.05 (fold change >2).
The resulting images were analysed using Genepix Pro 6.0 software (Molecular Devices). The median of the signal intensity for each spot was calculated and the local background around the spot subtracted. Data obtained from duplicate spots were averaged. The background subtracted signal intensity of each antigen was normalised to the average intensity of the total mouse IgG included on the array as internal controls. Finally, the net fluorescence intensity (NFI) for each antigen was calculated by subtracting the PBS control, which was included for each experiment as negative control. The signal-to-noise ratio (SNR) was used as a quantitative measurement of the true signal above the background. SNR values ≥3 were considered significantly higher than background and therefore accepted as true signals. The NFI of each autoantibody was used to generate heatmaps using Cluster and Treeview software (http://rana.bl.gov/EisenSoftware.htm) where each row represents an autoantibody and each column a sample.
Statistical analysis
All statistical analyses were done in R statistics (version 4.1.2) with the R Complex Heatmap package (version 2.10.0) used for plotting the results. Comparisons with reference values were done using the chi-square test. The experimental data conforming to normal distribution are expressed as mean ± standard deviation (SD). Independent sample t test was used to compare the levels of antibodies between groups. A significance level of 5% was used for statistical inference.
Results
Testing for anti-SEA IgG
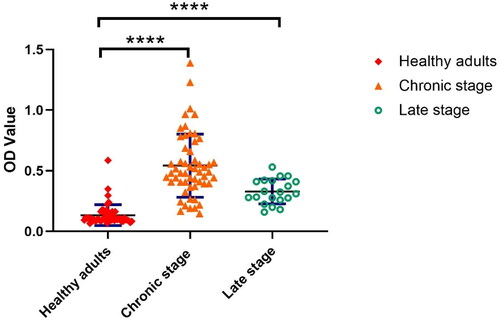
When sera from the study subjects, characterised as shown in , were tested for anti-SEA IgG, the titres were significantly higher in the sera from the patients with chronic and late-stage schistosomiasis compared to those in the healthy control group, though at a lower level in the late-stage group (). The mean OD value was 0.06 in 50 healthy individuals, 13.32 in 55 chronic patients and 0.56 in 20 late-stage patients with schistosomiasis japonica.
Figure 1. Quantification of anti-SEA IgG responses in healthy adults and in patients at different stages of schistosomiasis japonica. The error bars show the 95% CI optical density (OD) value at 450 nm. Healthy adults represent the control group. ****p < 0.001, the difference was highly significant.
Autoantibody testing
The results with reference to autoantibodies in chronic and late-stage schistosomiasis patients compared to control subjects are presented in supplementary figure 1 and in the form of digital images of representative antigen microarrays.
Figure 2. A hierarchically clustered heatmap of the 14 kinds of IgG autoantibodies with specificities against significant autoantigens detected in patients with chronic schistosomiasis japonica.
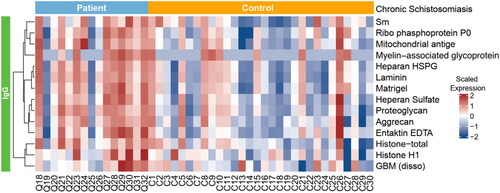
As shown in and , we identified 14 antoantigens demonstrating a significant increase in antibody-binding levels as revealed by the secondary antibody in the patients with chronic schistosomiasis japonica compared to the 30 age-matched healthy controls, including histone, Smith antigen (Sm), Ribophosphoprotein P0, Laminin, Glomerular basement membrane (GBM), Myelin − associated glycoprotein, et al. Similarly, there were also differences in the autoantibody responses between late-stage patients and the matched healthy controls. A total of 43 different autoantibodies with elevated levels were identified in late-stage schistosomiasis ( and ). Antibodies against autoantigens such as Sm, Mitochondrial antigen, GBM and Proteoglycan were expressed at higher levels in patients with late-stage disease, and they were also found to be highly expressed in chronic patients.
Figure 3. A hierarchically clustered heatmap of the 43 kinds of IgG autoantibodies with specificities detected in patients with late-stage schistosomiasis japonica.
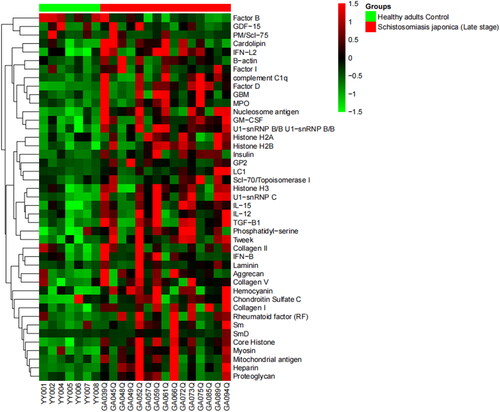
Table 2. Autoantigens in patients with schistosomiasis japonica.
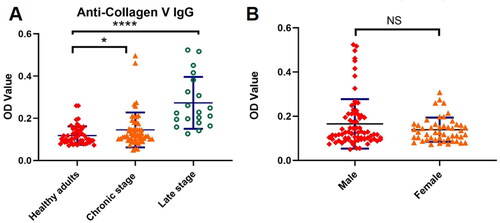
Based on the antigen microarray results, we selected several important types of antibodies as target antibodies such as anti-SSB antibody and anti-collagen V antibody and used enzyme linked immunosorbent assay (ELISA) to detect the expression of these antibodies in serum of patients with chronic schistosomiasis and patients with advanced schistosomiasis, respectively, and compared with healthy adults to verify the results of antigen microarray. The results showed that autoantibodies aginst collagen V seem to be very significant. The levels of anti-collagen V antibodies in the serum of schistosomiasis patients were significantly higher than those of healthy subjects (OD Value 0.19 ± 0.14 vs 0.12 ± 0.04, p < 0.001) and changed with the course of disease, with higher levels of anti-collagen V antibodies in the serum of patients with advanced schistosomiasis (OD Value 0.27 ± 0.12, p < 0.001), as shown in . In addition, there was no significant difference in the levels of anti-collagen V antibodies between male and female patients (OD Value 0.15 ± 0.06 vs 0.13 ± 0.03, p > 0.05), suggesting that the production of this type of autoantibody was not related to gender, as shown in .
Figure 4. (A) anti-Collagen V IgG antibody levels in schistosomiasis patients. (B) Anti-Collagen V IgG antibody levels in male and female schistosomiasis patients. *p < 0.05, the difference was significant; ****p < 0.001, the difference was highly significant; NS: the difference was not significant.
Discussion
In a previous paper, we reported on ANA incidence amounting to 6.7%, 23.3% and 70.0% in patients with acute, chronic and late stages of S. japonicum infection, respectively [Citation11]. These results mimic those in a report on S. mansoni infection in Egypt, where Abbas and Kader [Citation18] found circulating autoantibodies in sera from 54 schistosomal patients, 20 of whom with early infection, another 20 with post-schistosomal hepatic fibrosis and 14 described has having post-schistosomal glomerulonephritis. A few other papers on this issue have appeared over the years, the earliest from the 1960s [Citation19], but they have not resulted in much evidence of the role of the autoantibodies discovered. Neither have we, but the detailed enlargement of the antibody specificities found should improve the possibilities for comparing the spectrum of antibody specificities elicited by different parasite infections and how this relates to the development of autoimmune diseases.
Known autoimmune diseases are either systemic, e.g. systemic lupus erythematosus (SLE), which is a severe multi-organ manifestation often leading to kidney failure, or they are organ/tissue-specific, such as rheumatoid arthritis (RA), multiple sclerosis (MS), diabetes type-1, thyroiditis etc [Citation20]. The failure to distinguish self from nonself is often termed a breach of tolerance and is the basis for autoimmune disease. Infections can act as environmental triggers of specific diseases in genetically predisposed individuals. The association of parasitic infection and the development of autoimmune diseases could be due to microbial peptides similar to self-tissue that initiate autoimmunity through molecular mimicry [Citation21]. Specific infections associated with an increased autoantibody load may shed insight into the mechanisms. Berlin et al. [Citation22] tested sera from non-autoimmune patients with acute bacterial, viral, parasitic, and rickettsial infections and found that the most frequently detected autoantibodies were those against ANA and phospholipids, but there were also elevated titres of antibodies directed against annexin-V, prothrombin and laminin; differences depending on the type of infection were also noted. Interestingly, Rahima et al. [Citation23] reported that sera from SLE patients reacted with certain parasite extracts in a higher incidence (14.2%) compared to healthy subjects (3.7%) and also that ANA in the sera of acute schistosomiasis-infected mice could "trigger" SLE in subjects with the suitable immunogenetic background.
However, there are also studies finding autoimmunity to be a protective factor. Based on in vitro observations, experimental animal test results as well as human studies, Harboe [Citation24] noted a protective role for antibodies associated with RA in such widely different systems as trypanosome infection, malaria and schistosomiasis. Importantly, as shown by Amo et al. [Citation25], the parasite-induced protection in malaria may not be due to a systemic effect of infection on autoimmunity, but rather to specific alterations in immune cell infiltrates in kidneys and lymph nodes draining the kidneys. Infection of lupus-prone mice with the Plasmodium parasite did not reduce the levels or specificities of autoreactive antibodies, but reduced kidney-infiltrating CCL17-producing inflammatory dendritic cells that produced the desired effect.
Schistosomes are thought to manipulate host immune responses to enhance their survival and in so doing dampen the host immunopathology and reduce its susceptibility to allergic and auto-immune diseases [Citation26]. These autoantibodies may play a key role in the induction of pathological responses after parasite infections, which is crucial for parasite elimination. We found that anti-collagen V autoantibodies were significantly higher in schistosomiasis patients than in healthy people. Notably, anti-collagen antibodies have been reported to be associated with autoimmune disease. Autoantibodies aginst Collagen V are common in recipients developing Bronchiolitis obliterans syndrome (BOS) after lung transplant, and often precede development of fibrosis. Pre-existing Collagen V sensitisation may increase the risk of BOS [Citation27]. Mullazehi et al. [Citation28] analysed the anti-collagen II serology in patients with rheumatoid arthritis. RA patients with positive anti-collagen II antibodies had increased disease activity and more severe symptoms. Similarly, antibodies aginst collagen II was also positively correlated with serum IgG in SLE patients, and anti-collagen II antibodies may be a new indicator for monitoring SLE activity [Citation29]. Antibodies against collagen have been identified in schistosomiasis patients, suggesting an autoimmune response in schistosomiasis. This type of antibody was not associated with gender, but with the course of the disease. Patients with late-stage disease showed higher antibody levels than those with chronic disease. This indicates a correlation between anti-collagen V antibodies and the progression of schistosomiasis, suggesting that anti-collagen V antibodies may serve as a new serum marker for the transition to advanced stages of chronic schistosomiasis and requires further study.
A limitation of this study is that only chronic and late-stage patients were compared separately with healthy controls for differences in autoantibody expression by using autoantigen microarrays, but not between chronic and late-stage patients. Therefore, although some types of autoantibodies are highly expressed in both chronic and advanced patients, we could not clarify which disease stage they are more associated with. In conclusion, we identified several types of autoantibodies acting as serum markers that may give insight in the fate of immune mechanism to Schistosoma infection. These markers should be further investigated for their potential as biomarkers to guide individualised therapy. Our observations warrant further studies to validate them and their possible role in predicting clinical outcomes of schistosomiasis.
Ethics approval
Informed consent was obtained from each study participant, and the collection and use of serum samples for this study were approved by the Human Ethics Committee of the National Institute of Parasitic Diseases, Chinese Centre for Disease Control and Prevention (Chinese Centre for Tropical Diseases Research), Shanghai, China.
Author contributions
Zhiqiang Qin initiated and conceptualised the project. Xiaorong Zhou and Xi Wang performed most of the experiments and analysed the data and wrote the original draft. Jing Xu helped analysed the data. Robert Bergquist helped write the article. Xi Wang and Qi Tang helped Xiaorong Zhou with the ELISA experiments. Leming Shi helped with the Autoantibody detection experiments. All authors approved the final version.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
Not applicable.
Supplemental Material
Download MS Word (989.5 KB)Acknowledgments
We wish to thank all staff at the Gongan County Institute of Schistosomiasis Control and Prevention, Hubei Province and Yueyang County Institute of Schistosomiasis Control and Prevention, Hunan Province for their participation in sample collection and questionnaire administration. We thank Professor Quanzhen Li, Dr Jun Shang and Dr Wanwan Hou for their kind assistance and advice during the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- [cited 2023 Mar 25]. Available from: https://www.healthdata.org/results/gbd_summaries/2019/schistosomiasis-level-3-cause.
- McManus DP, Dunne DW, Sacko M, et al. Schistosomiasis. Nat Rev Dis Primers. 2018;4(1):1.
- Mutapi F, Maizels R, Fenwick A, et al. Human schistosomiasis in the post mass drug administration era. Lancet Infect Dis. 2017;17(2):e42–7.
- Li G, Huang S, Lian L, et al. Derivation and external validation of a model to predict 2-year mortality risk of patients with advanced schistosomiasis after discharge. EBioMedicine. 2019;47:309–318.
- Song L, Wu X, Ren J, et al. Assessment of the effect of treatment and assistance program on advanced patients with schistosomiasis japonica in China from 2009 to 2014. Parasitol Res. 2016;115(11):4267–4273.
- Burke ML, Jones MK, Gobert GN, et al. Immunopathogenesis of human schistosomiasis. Parasite Immunol. 2009;31(4):163–176.
- Olveda DU, Inobaya M, Olveda RM, et al. Diagnosing schistosomiasis-induced liver morbidity: implications for global control. Int J Infect Dis. 2017;54:138–144.
- Colley DG, Secor WE. Immunology of human schistosomiasis. Parasite Immunol. 2014;36(8):347–357.
- Fu CL, Odegaard JI, Herbert DR, et al. A novel mouse model of Schistosoma haematobium egg-induced immunopathology. PLoS Pathog. 2012;8(3):e1002605.
- Masi B, Perles-Barbacaru TA, Bernard M, et al. Clinical and preclinical imaging of hepatosplenic schistosomiasis. Trends Parasitol. 2020;36(2):206–226.
- Wang X, Fu Q, Song R, et al. Antinuclear antibodies and interleukin responses in patients with schistosoma japonicum infection. Parasite Immunol. 2018;40(10):e12577.
- Elkon K, Casali P. Nature and functions of autoantibodies. Nat Clin Pract Rheumatol. 2008;4(9):491–498.
- Prüss H. Autoantibodies in neurological disease. Nat Rev Immunol. 2021;21(12):798–813.
- Pereira LM, McFarlane BM, Massarolo P, et al. Specific liver autoreactivity in schistosomiasis mansoni. Trans R Soc Trop Med Hyg. 1997;91(3):310–314.
- Zhu H, Luo H, Yan M, et al. Autoantigen microarray for high-throughput autoantibody profiling in systemic lupus erythematosus. Genom Proteom Bioinform. 2015;13(4):210–218.
- Burberry A, Suzuki N, Wang JY, et al. Loss-of-function mutations in the C9ORF72 mouse ortholog cause fatal autoimmune disease. Sci Transl Med. 2016;8(347):347ra93.
- Chen X, Sun X, Yang W, et al. An autoimmune disease variant of IgG1 modulates B cell activation and differentiation. Science. 2018;362(6415):700–705.
- Abbas MM, Kader SA. A study of autoimmunity in schistosomiasis. J Egypt Soc Parasitol. 1993;23(1):289–296.
- Kurata M. Schistosomiasis japonica and "autoimmunity" experimental studies. Kurume Med J. 1965;12(1):7–9.
- Ngo ST, Steyn FJ, McCombe PA. Gender differences in autoimmune disease. Front Neuroendocrinol. 2014;35(3):347–369.
- Wang L, Wang FS, Gershwin ME. Human autoimmune diseases: a comprehensive update. J Intern Med. 2015;278(4):369–395.
- Berlin T, Zandman-Goddard G, Blank M, et al. Autoantibodies in nonautoimmune individuals during infections. Ann NY Acad Sci. 2007;1108:584–593.
- Rahima D, Tarrab-Hazdai R, Blank M, et al. Anti-nuclear antibodies associated with schistosomiasis and anti-schistosomal antibodies associated with SLE. Autoimmunity. 1994;17(2):127–141.
- Harboe M. Rheumatoid factors in leprosy and parasitic diseases. Scand J Rheumatol Suppl. 1988;75:309–313.
- Amo L, Kole HK, Scott B, et al. CCL17-producing cDC2s are essential in end-stage lupus nephritis and averted by a parasitic infection. J Clin Invest. 2021;131(11):e148000.
- Zhang Y, Xiong DH, Li Y, et al. Schistosoma japonicum infection in Treg-Specific USP21 knockout mice. J Immunol Res. 2021;2021:6613162.
- Foster MH. Basement membranes and autoimmune diseases. Matrix Biol. 2017;57–58:149–168.
- Mullazehi M, Mathsson L, Lampa J, et al. High anti-collagen type-II antibody levels and induction of proinflammatory cytokines by anti-collagen antibody-containing immune complexes in vitro characterise a distinct rheumatoid arthritis phenotype associated with acute inflammation at the time of disease onset. Ann Rheum Dis. 2007;66(4):537–541.
- He C, Mao T, Feng Y, et al. Anti-CII antibody as a novel indicator to assess disease activity in systemic lupus erythematosus. Lupus. 2015;24(13):1370–1376.

