Abstract
Lysosomal associated membrane protein 3 (LAMP3) has been reported to be a tumour promoter in multiple cancer types by modulating tumour cell autophagy. However, the potential mechanism of LAMP3 in radio-resistance of head and neck squamous cell carcinoma (HNSCC) remains unknown. Therefore, our current study aims to detect the impacts of LAMP3 on the resistance of HNSCC cells to radiotherapy and meanwhile explore its functional mechanism. Through RT-Qpcr examination, LAMP3 expression was identified to be expressed at a significantly high level in irradiation-resistant HNSCC cell lines compared with irradiation-sensitive HNSCC cell lines. Functional assays including CCK-8, colony formation and Transwell assays demonstrated that LAMP3 enhanced the radio-resistance through inducing autophagy to promote HNSCC cell growth. Furthermore, irradiation-resistant HNSCC cells could transfer exosomal LAMP3 to elevate LAMP3 expression in irradiation-sensitive HNSCC cells. Mechanistically, microRNA (miRNA) miR-526b-3p could inhibit LAMP3 expression so as to strengthen sensitivity of HNSCC cells to radiotherapy. In a word, exosomal LAMP3 expression promoted radioresistance of HNSCC cells via inducing autophagy, while this effect could be suppressed by miR-526b-3p in a targeted manner.
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer globally, which features high mortality rate [Citation1]. Surgery, anti-cancer drug therapy and radiotherapy remain to be three main therapeutic methods for HNSCC patients currently [Citation2]. Among them, radiotherapy was commonly applied for treating patients in multiple stages. However, resistance of patients to radiotherapy in late treatment stage largely impedes the therapeutic effect. Therefore, it is of great significance to explore the molecular mechanism affecting radioresistance.
Lysosomal associated membrane protein 3 (LAMP3), as a newly identified tumour-specific a protein, is revealed to be up-regulated in cancer tissues and association with cancer development [Citation3,Citation4]. As reported, LAMP3 can promote osteosarcoma cell growth by suppressing TP53 expression [Citation5]. Moreover, it can serve as the downstream target of HAGLR/miR-143-5p to facilitate metastasis in oesophageal cancer [Citation6]. It is known as an modulator in radioresistance of laryngeal squamous cell carcinoma [Citation7] and breast cancer [Citation8]. LAMP3 has also been illustrated to be relevant for prognosis in HNSCC [Citation9], but its detailed role in regulating radioresistance of HNSCC cells remains to be identified.
Autophagy is a self-protection mechanism widely existing in eukaryotic cells, which can realise cell metabolism and energy renewal and maintain cell homeostasis [Citation10]. Many researches have revealed the role of autophagy plays in regulating cancer development. Autophagy can suppress benign tumour growth, while it can also promote advanced cancer growth [Citation11,Citation12]. Recently, autophagy has been revealed to be involved in the development of radioresistance which poses a major cause of radiotherapy failure [Citation13–15]. LAMP3 is a member of the LAMP-family of proteins that are involved in the process of autophagy [Citation16,Citation17]. LAMP3 has been verified as a autophagy inducer for castration-resistant prostate cancer cells [Citation18]. However, whether it can regulate HNSCC cell autophagy and thus affect radioresistance is still unknown.
In summary, the preset study focused on the role of LAMP3-mediated autophagy and its effect on the radioresistance of HNSCC cells.
Materials and methods
Cell culture
HNSCC cells (SCC9, SCC15, FaDu, CAL-27, HN30) and human immortalised normal mucosal cell line (DOK) were bought from the Chinese Academy of Sciences Cell Bank (Shanghai, China). HNSCC cell lines were cultivated in DMEM (A4192101, Gibco, Grand Island, NY, USA) consisting of 10% FBS (16140071, Gibco), 0.08 mg ml−1 streptomycin and 80 U ml−1 penicillin, while DOK cells were grown in complete medium (RPMI-1640, A4192301, Gibco) supplemented with 10% FBS (16140071, Gibco), penicillin (100 U ml−1), streptomycin (100 mg ml−1), 2 mM glutamine and 25 mM 4-(2-hydroxyethyl)−1-piperazineethanesulphonic acid (HEPES). All cell lines were grown to monolayers in a 10 cm dish and kept at 37 °C in an incubator in a moist atmosphere with 5% CO2. Cells in exponential growth phase were utilised for subsequent experiments.
Establishment of the irradiation-resistant HNSCC cell lines
Briefly, 1 × 106 CAL-27 and FaDu cells were inoculated into a 25 cm culture flask and cultured for 2 days in RPMI-1640 medium (A4192301, Gibco) containing 10% FBS (16140071, Gibco) at 37 °C with 5% CO2. Then, cells were irradiated with 1 Gy of X-rays by a high-energy linear accelerator, at a dose rate of 100 cGy/min (X-RAD 225 high-energy biological X-ray irradiator, PXI, USA). The medium was changed immediately after irradiation, and the cells were further incubated until 90% confluence. Subsequently, the cells were collected after trypsinization and were sub-cultured into new flasks. Once these cells reached approximately 50% confluence, they were irradiated again with 100 cGy 3 times, 200 cGy 3 times, and 400 cGy 3 times. After treatment with a total dose of 2,100 cGy, we obtained the radiation-tolerant cell population, which were cultured for at least 1 month before using in experiments. Irradiation-resistant HNSCC cell lines were named CAL-27/IR and FaDu/IR for further investigations.
Clonogenic survival assay
The cells were seeded into 6-well plates after 48 h’ post-transfection followed by irradiation with x-ray treatment at different single radiation doses (0, 2, 4, 6, 8 Gy). After 2 weeks, the plates were fixed with 100% methanol (67-56-1, Bojing Chemical Co., Ltd., Shanghai, China) and then stained with 1% crystal violet (V5265, Sigma-Aldrich, St. Louis, MO, USA). Colonies were counted by a microscopic inspection, and the surviving fraction was calculated.
Total RNA extraction and quantitative real-time polymerase chain reaction (RT-qPCR)
Total RNA sample was extracted in HNSCC cells based on the instructions of TRIzol reagent (15596026, Invitrogen, Carlsbad, CA, USA). The extracted RNA inverse transcribed into complementary DNA (cDNA) using PrimeScript™ RT Master Mix kit (RR036Q, TaKaRa, Tokyo, Japan). After that, gene expression was tested utilising SYBR Green PCR Kit (4309155, Applied Biosystems, Foster city, CA, USA) on ABI 7500 fast PCR system (4351107, Applied Biosystems). The specific reaction conditions were as follows: pre-denaturation at 95 °C for 10 min, denaturation at 95 °C for 30 min. After renaturation for 30 s at 55 °C, it was extended for 2 min at 72 °C for a total of 35 cycles. The 2-ΔΔCt method was used for calculation of relative gene expression by taking GAPDH and U6 as reference genes.
Cell transfection
Short hairpin RNAs (shRNA) specifically targeting LAMP3 (named sh-LAMP3#1 and sh-LAMP3#2) was synthesised by GenePharma (Shanghai, China) for silencing of LAMP3 in irradiation-resistant HNSCC cells. In irradiation-sensitive HNSCC cells, LAMP3 whole sequence was sub-cloned into pcDNA3.1 vector to generate LAMP3 expression vector (named LAMP3-OE). A random sequence of shRNA (NC/shRNA) was used as negative control for sh-LAMP3 and the empty pcDNA3.1 vector was used as negative control for LAMP3 expression vector. In line with the supplier’s protocols, transfections were conducted with Lipofectamine 2000 (11668019, Invitrogen). Transfected cells were harvested for subsequent experiments after 48 h.
Cell counting kit-8 (CCK-8) assay
Cell viability was measured by CCK-8 kit (HY-K0301, MedChemExpress, Monmouth Junction, NJ, USA). In brief, transfected irradiation-resistant HNSCC cells and sensitive strains were planted into 96-well plates. After 10 μl of CCK-8 solution was added in cells at different time points (24 h, 48 h, 72 h). Finally, the absorbance values at a wavelength of 450 nm were measured using a spectrophotometer (840-210600, Thermo Fisher Scientific, Rockford, IL, USA).
Colony formation assay
After transfected for 48 h, cells were harvested and seeded into a 6-well plate at 600 cells per well and incubated for 14 d. The culture medium was then discarded and the cells were washed with PBS (SH30256.01B, Hyclone Logan, UT, USA). Cells were fixed with methanol (67-56-1, Bojing Chemical Co., Ltd.) for 15 min and stained by 1% crystal violet (V5265, Sigma-Aldrich) for 10 min at room temperature. Finally, colonies more than 50 cells were counted manually.
Transwell assays
HNSCC cells were planted on the top of 24-well transwell chambers (3428, Corning, NY, USA) coating Matrigel (356234, BD Biosciences, Franklin Lakes, NJ, USA) for invasion assay or without Matrigel for migration assay. The lower chambers were loaded with complete medium. Twenty-four hours later, cells in the upper layer were removed by a cotton swab and then fixed in methanol solution for 15 min. Next, the fixed cells were stained with 1% crystal violet (V5265, Sigma-Aldrich) for 10 min, and the invaded or migrated cells were observed and counted under a 10 × 10 optical microscope (DMI1, Leica, Wetzlar, Germany).
Western blot
Total protein extracted from irradiation-resistant cells and sensitive strains was isolated by RIPA buffer (R0278, Sigma-Aldrich). After being separated through 10-12% sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE), proteins were transferred to polyvinylidene fluoride (PVDF) membranes and blocked in 5% skim milk. The membranes were incubated with primary antibodies over night at 4 °C. After being washed for four times using 1 × TBST (T15207, Saint-Bio, Shanghai, China), the membranes were incubated for additional 1 h with the secondary antibody (1/2000, ab7063, Abcam, Cambridge, MA, USA) at room temperature. Finally, the bands were visualised by a ECL detection system (32134, Pierce Biotechnology, Rockford, IL, USA). The primary antibodies, including E-cadherin (ab40772, 1/1000), N-cadherin (ab76011, 1/5000), Vimentin (ab92547, 1/1000), Beclin1 (ab207612, 1/2000), p62 (ab207305, 1/1000), LC3I/II (ab128025, 1/1000), CD9 (ab236630, 1/1000), CD63 (ab193349, 1/1000), TSG101 (ab125011, 1/1000) and the internal control GAPDH (ab8245, 1/1000) were all obtained from Abcam.
Transferase-mediated dUTP nick-end labelling (TUNEL) assay
The prepared cells in 96-well plates were first fixed by 4% paraformaldehyde (E672002, Sangon Biotech, Shanghai, China) for 15 min, and then permeabilized with 0.1% Triton-X100 (R00285, Leagene, Beijing, China) for 20 min. TUNEL assays were conducted to measure cell apoptosis by using TUNEL Detection Kit (12156792910, Roche, Basel, Switzerland). Cell nucleus was visualised using DAPI solution (D9542, Sigma-Aldrich) to counterstain cells. The apoptotic cells were analysed using a fluorescence microscope (DMI8, Leica).
Flow cytometry analysis of cell apoptosis
Transfected cells were harvested for flow cytometry. Briefly, cells (1 × 105 cells/ml) were suspended in 1 × binding buffer. Cell suspension was mixed with 5 μl Annexin V-FITC kit (556547, BD Biosciences) in a dark room, and then incubated for 10 min at room temperature. After being rinsed with 1 × binding buffer, cells were centrifuged for 5 min at 1,000 × g. Next, the supernatant were removed and cells were re-suspended in 1 × binding buffer and stained with 10 μl PI (556463, BD Biosciences). Cell apoptosis was analysed using a flow cytometer (92821250S, Beckman Coulter, Kraemer Boulevard Brea, CA, USA).
Luciferase reporter assay
In order to verify the binding situation between LAMP3 and miR-526b-3p, Psi-CHECK2/-LAMP3 3′ UTR wild type (named LAMP3-WT) and Psi-CHECK2/-LAMP3 3′ UTR mutant type (named LAMP3-MUT) reporter plasmids were constructed in advanced. CAL-27/S and FaDu/S cells were co-transfected with 0.1 μg LAMP3-WT and LAMP3-MUT reporter plasmids along with miR-526b-3p inhibitor and control NC inhibitor using Lipofectamine 2000 (11668019, Invitrogen). To determine the effect of CAL-27/IR-exo and FaDu/IR-exo on the promoter activity of LAMP3, we constructed and transfected the pGL3-luciferase reporter vector containing the promoter of LAMP3 into CAL-27/S and FaDu/S cells and then treated with CAL-27/IR-exo and FaDu/IR-exo, respectively. After 48 h, the firefly and Renilla luciferase activities were detected with a Reporter Assay System (E1910, Promega, Madison, WI, USA) and recorded using GloMax 96 Microplate Luminometer (E6521, Promega).
Statistical analysis
Each experiment went through three independent repeats. Experimental data were illustrated as the means ± SD. The statistical analysis was made with SPSS 19.0 software (IBM, Armonk, NY, USA) by applying Student’s t-test to compare difference between two groups or one-way ANOVA to compare difference among multiple groups. The data were regarded as statistically significant in difference when p < 0.05.
Results
LAMP3 is up-regulated in irradiation-resistant HNSCC cells
First of all, we observed through GEPIA database (http://gepia.cancer-pku.cn/) that LAMP3 was up-regulated in HNSCC tissue samples in comparison with normal samples (). It was also verified through RT-qPCR data that LAMP3 presented higher expression in HNSCC cell lines (SCC9, SCC15, FaDu, CAL-27, HN30) than in human immortalised normal mucosal cell line DOK (). Among these HNSCC cell lines, FaDu and CAL-27 had the lowest LAMP3 expression. Therefore, we established irradiation-resistant HNSCC cell lines (FaDu/IR and CAL-27/IR) for further investigation on radioresistance. Since their parental cell lines were irradiation-sensitive HNSCC cell lines, we named them as FaDu/S and CAL-27/S. According to the results of CCK-8 assay, FaDu/IR and CAL-27/IR cells had significantly higher viability than FaDu/S and CAL-27/S cells (). The survival fraction of these four cell lines under irradiation treatment was also detected by colony formation assay. The results indicated that the survival fraction of FaDu/IR and CAL-27/IR cells were decreased slower than that of FaDu/S and CAL-27/S cells under increased dose of irradiation (), suggesting the radioresistance of FaDu/IR and CAL-27/IR cells. To detect the potential involvement of LAMP3 in radioresistance of HNSCC cells, we measured its expression level in four different cells. As illustrated in , LAMP3 expression was higher in FaDu/IR and CAL-27/IR cells than in FaDu/S and CAL-27/S cells. To conclude, LAMP3 is upregulated in irradiation-resistant HNSCC cells.
Figure 1. LAMP3 is up-regulated in irradiation-resistant HNSCC cells. (A) LAMP3 expression pattern in HNSC tissue samples and normal samples was obtained from GEPIA database. Red asterisk represents p value less than 0.01. (B) LAMP3 expression in five HNSCC cell lines (SCC9, SCC15, FaDu, CAL-27, HN30) in comparison with human immortalised normal mucosal cell line DOK was measured by RT-qPCR. (C) Cell Counting Kit-8 assay was applied to verify the viability of irradiation-resistant HNSCC cell lines (FaDu/IR and CAL-27/IR) and irradiation-sensitive HNSCC cell lines (FaDu/S and CAL-27/S). (D) The survival rate of cells were treated with different units of irradiation (0, 2, 4, 6, 8 Gy) was evaluated through colony formation assay. (E) LAMP3 expression in irradiation-resistant HNSCC cells and sensitive cells was measured by RT-qPCR. **P < 0.01.
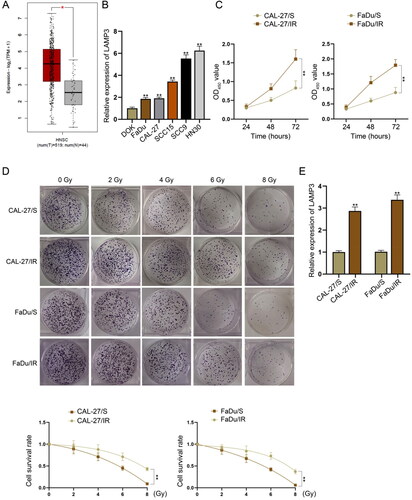
LAMP3 enhances radioresistance of HNSCC cells
We continued to explore whether LAMP3 could regulate the radioresistance of HNSCC cells through gain- or loss- of function assays. Before that, LAMP3 expression was overexpressed in CAL-27/S and FaDu/S cells while was silenced in CAL-27/IR FaDu/IR cells. The transfection efficiency of LAMP3-specific shRNAs and LAMP3 overexpression vector was confirmed by RT-qPCR ( and S1A). Through analysing the results of CCK-8 and colony formation assays, we confirmed that the viability and proliferation of irradiation-sensitive cells were strengthened after LAMP3 overexpression while those of irradiation-resistant cells were suppressed after LAMP3 knockdown ( and S1B–C). Moreover, we also detected the effect of LAPM3 silencing or overexpression on HNSCC cell invasion and migration using transwell assays. The results shown in and S1D–E revealed that overexpression of LAMP3 in sensitive strains promoted cell migration and invasion while the silencing of LAMP3 in resistant cells led to the opposite results. Epithelial mesenchymal transition (EMT) can contribute to cancer cell invasion and migration [Citation19,Citation20]. Here, we explored the effect of LAMP3 on EMT process by using western blot to measure the expression of EMT-related markers (E-cadherin, N-cadherin, Vimentin) in different groups. The increased level of the epithelial marker (E-cadherin) and the decreased level of mesenchymal markers (N-cadherin and Vimentin) in LAMP3-downregulated irradiation-sensitive cells indicated the reversal of EMT process, while the opposite changes in LAMP3-overexpressed irradiation-resistant cells suggested the progress of EMT process ( and S1F). Collectively, LAMP3 promotes radioresistance of HNSCC cells.
Figure 2. LAMP3 enhances radioresistance of HNSCC cells. (A) LAMP3 expression was overexpressed in CAL-27/S cells while was silenced in CAL-27/IR cells, as demonstrated by RT-qPCR. (B) The viability of CAL-27/S cells upon the ectopic expression of LAMP3 as well as that of CAL-27/IR cells with LAMP3 silence was analysed by cell counting kit-8 assay. (C) The proliferation abilities of LAMP3-overexpressed CAL-27/S cells and LAMP3-silenced CAL-27/IR cells were evaluated by colony formation assay. (D-E) Transwell assays were performed to verify the impact of LAMP3 overexpression (or knockdown) on the migration and invasion of CAL-27/S cells (or CAL-27/IR cells). (F) The protein levels of EMT-related markers (E-cadherin, N-cadherin, Vimentin) in CAL-27/S cells transfected with LAMP3 overexpression vector or CAL-27/IR cells transfected with LAMP3-specific shRNAs, as measured by western blot. **P < 0.01.
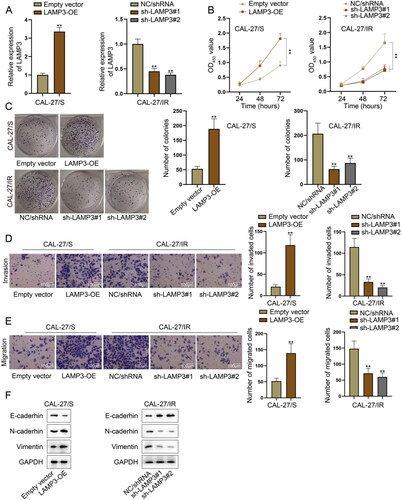
LAMP3 induces the autophagy of HNSCC cells
LAMP3 has been reported as an autophagy inducer [Citation21], and autophagy is closely associated with radioresistance of tumour cells [Citation22]. Therefore, we further investigated the role of LAMP3 in modulating autophagy. To obtain the initial evidence, we detected the expression of autophagy-related proteins (Beclin1, p62, LC3I/LC3II) in transfected irradiation-resistant and irradiation-sensitive cells. We then found that in LAMP3-overexpressed sensitive cell, the level of Beclin1 (the autophagy-promoting protein) was increased but the level of p62 (the autophagy-suppressing protein) was decreased in LAMP3-overexpressed irradiation-sensitive cells, meanwhile, the proportion of LC3II/LC3I was reduced. Conversely, the changes in the levels of abovementioned autophagy-related proteins were in an opposite tendency in LAMP3-silecned resistant cells ( and S2A), indicating the potential of LAMP3 in inducing autophagy of HNSCC cells. Furthermore, we applied immumofluorescence to measure the effect of LAMP3 on autophagy by measuring the fluorescence intensity of anti-LC3 labelled by red fluorescence (named RFP-LC3) or green fluorescence (named GFP-LC3). As shown in and S2B, the intensity of both RFP-LC3 and GFP-LC3 was enhanced in LAMP3-overexpressed sensitive cells while was weakened in LAMP3-silenced resistant cells. These results further demonstrated the autophagy-inducing role of LAMP3 in HNSCC. Autophagy can affect the apoptosis of tumour cells as an internal cause. Here, we further detected the apoptosis of HNSCC cells after silencing or overexpression of LAMP3 using flow cytometry and TUNEL assay. We observed through flow cytometry that LAMP3 up-regulation suppressed the apoptosis of sensitive cells while LAMP3 inhibition enhanced the apoptosis of resistant cells ( and S2C). Additionally, results of TUNEL assay shown in and S2D further indicated the suppressive effect of LAMP3 on apoptosis. Therefore, we could conclude that LAMP3 could induce autophagy of HNSCC cells.
Figure 3. LAMP3 induces the autophagy of HNSCC cells. (A) The expression of autophagy-related proteins (Beclin1, p62, LC3I/II) in CAL-27/S transfected with LAMP3 overexpression vector and CAL-27/IR cells LAMP3-specific shRNAs was analysed by western blot. (B) Immumofluorescence was utilised to measure the fluorescence intensity of anti-LC3 labelled by red fluorescence (named RFP-LC3) or green fluorescence (named GFP-LC3). (C) The apoptosis of CAL-27/S transfected with LAMP3 overexpression vector and CAL-27/IR cells LAMP3-specific shRNAs was assessed by flow cytometry. (D) TUNEL assay was taken to evaluate the apoptosis of CAL-27/S transfected with LAMP3 overexpression vector and CAL-27/IR cells LAMP3-specific shRNAs. **P < 0.01.
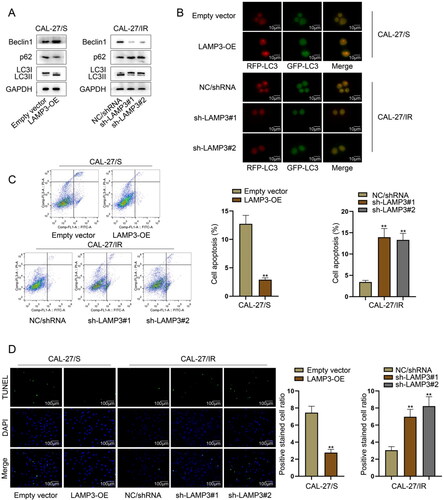
Irradiation-resistant HNSCC cells transfers exosomal LAMP3 into irradiation-sensitive HNSCC cells
Exosomes functioning as an important medium in intercellular communications has been elucidated to transmit signals from one cell to another cell so as to affect cell functions [Citation23,Citation24]. Previous researches have also reported that exosomes derived from cancer cells can affect radioresistance in cancer [Citation25,Citation26], so we hypothesised that exosomes might mediate the intercellular communication between irradiation-resistant HNSCC cells and irradiation-sensitive HNSCC cells. At first, we co-cultured irradiation-sensitive HNSCC cells with irradiation-resistant HNSCC cells to measure the viability and radioresistance of co-cultured irradiation-sensitive HNSCC cells (CAL-27/IR-CM and FaDu/IR-CM), and the normally cultured irradiation-sensitive HNSCC cells was taken as control. Through CCK-8 assay, we observed that the viability of CAL-27/IR-CM and FaDu/IR-CM was obviously higher than the control groups (). It was then manifested by colony formation assay that the survival rate of sensitive cells in CAL-27/IR-CM and FaDu/IR-CM groups was higher than those in control groups (). Considering the significant high expression of LAMP3 in resistant cells compared to sensitive cells, we supposed that LAMP3 might be transferred by resistant cells to sensitive cells and thus reverse the radiosensitivity of sensitive cells. To verify our hypothesis, we examined LAMP3 expression in sensitive cells co-cultured with resistant cells through RT-qPCR. It was found that the expression level of LAMP3 was significantly increased after co-culturing (). Subsequently, we extracted exosomes from two resistant cells for further analysis. With the application of electron microscope, we captured the exosomes in CAL-27/IR and FaDu/IR cells (named CAL-27/IR-exo and FaDu/IR-exo) (). Using the PKH67 Green Fluorescent Cell Linker Kit, we could see that PKH67-marked exosomes secreted by CAL-27/IR and FaDu/IR cells (). In addition, we found through western blot that the protein levels of exosomes markers (CD9, CD63, TSG101) in CAL-27/IR-exo and FaDu/IR-exo were higher than those in the cell lysate of CAL-27/IR and FaDu/IR cells (). To demonstrate the functions of exosomes secreted by CAL-27/IR and FaDu/IR cells in corresponding sensitive cells, we conducted CCK-8 assay and the result indicted that the viability of sensitive cells treated with CAL-27/IR-exo and FaDu/IR-exo had higher viability than untreated control cells (). Moreover, the survival rate of sensitive cells under irradiation treatment was higher in CAL-27/IR-exo and FaDu/IR-exo groups than that in control groups (). Finally, we detected LAMP3 expression using RT-qPCR in sensitive cells treated with or without exosomes. The results indicated that the expression level of LAMP3 was evidently increased after treated with CAL-27/IR-exo and FaDu/IR-exo (). However, is there exist transcriptional regulatory molecules delivered by radiotherapy-resistant cells, which promote the transcription of LAMP3 in sensitive cell lines, leading to its upregulation? To investigate this, we constructed a luciferase reporter gene vector containing the LAMP3 promoter and transfected it into sensitive cells. We then treated the cells with exosomes derived from radiotherapy-resistant cells and analysed changes in promoter activity. The results showed that exosome treatment did not alter the activity of the LAMP3 promoter, suggesting that exosomes do not regulate the transcription of LAMP3 (). These data indicated that irradiation-resistant HNSCC cells could transfer exosomes that carried LAMP3 into irradiation-sensitive cells.
Figure 4. Irradiation-resistant HNSCC cells transfers exosomal LAMP3 into irradiation-sensitive HNSCC cells. Irradiation-sensitive HNSCC cells co-cultured with irradiation-resistant HNSCC cells were named CAL-27/IR-CM and FaDu/IR-CM groups. The normally cultured irradiation-sensitive HNSCC cells were taken as control groups. (A) Cell counting kit-8 assay was taken to verify the viability of sensitive cells before or after co-culturing. (B) The survival rate of sensitive cells treated with different units of radiation (0, 2, 4, 6, 8 Gy) was analysed by colony formation assays before or after co-culturing. (C) LAMP3 expression was measured in CAL-27/IR-CM and FaDu/IR-CM and control sensitive cells by RT-qPCR. (D) The existence of exosomes in CAL-27/IR and FaDu/IR cells was detected using electron microscope. (E) PKH67 green fluorescence staining was used to label exosomes secreted by CAL-27/IR and FaDu/IR cells. (F) Exosome surface markers (CD9, CD63, TSG101) expression in CAL-27/IR-exo and FaDu/IR-exo and the cell lysate of CAL-27/IR and FaDu/IR cells, as detected by western blot. (G) Cell counting kit-8 assay was taken to measure the viability of irradiation-sensitive HNSCC cells under exosome treatment. (H) Colony formation assay was taken to detect the proliferation of irradiation-sensitive HNSCC cells under exosome treatment. (I) The expression level of LAMP3 in sensitive HNSCC cells was measured by RT-qPCR after treated with CAL-27/IR-exo or FaDu/IR-exo. (J) The effect of CAL-27/IR-exo and FaDu/IR-exo on the promoter activity of LAMP3 in CAL-27/S and FaDu/S cells was measured by luciferase reporter assay. **P < 0.01.
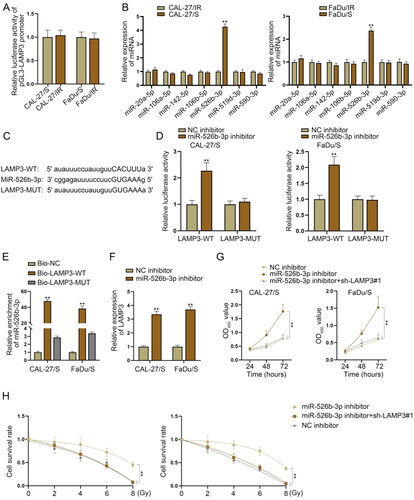
MiR-526b-3p suppresses LAMP3 expression to enhance radiosensitivity of HNSCC-sensitive cells
In this part, we wanted to explore whether there were certain genes that might inhibit LAMP3 expression to further reverse the radioresistance of sensitive HNSCC cells. To identify the upstream mechanism of LAMP3, we firstly performed luciferase reporter assay to measure the promoter activity of LAMP3 in resistant and sensitive cells. As shown in , the promoter activity of LAMP3 was not significantly different in resistant and sensitive cells, excluding the transcriptional regulation between these two cell lines. MicroRNAs (miRNAs) are essential upstream regulators for mRNAs, which can suppress the functions of their downstream mRNAs by directly targeting [Citation27,Citation28]. Herein, we applied starBase database to screen out potential miRNAs in the upstream of LAMP3. Under the condition of CLIP data: strict stringency (≥ 5), seven miRNA candidates were selected (miR-20a-5p, miR-106a-5p, miR-142-5p, miR-106b-5p, miR-526b-3p, miR-519d-3p, miR-590-3p). For further screening, we utilised RT-qPCR to measure their expression patterns and found that only miR-526b-3p was significantly up-regulated in irradiation-sensitive HNSCC cells in comparison with sensitive cells, while the expression of other miRNAs exerted no obvious variation in the two different groups of cells (). Therefore, miR-526b-3p was chosen for further investigations. The binding sites between miR-526b-3p and LAMP3 3’UTR as well as the mutant sequence of LAMP3 were shown in . Next, we demonstrated the interaction between miR-526b-3p and LAMP3 using luciferase reporter assay. The wild type sequence of LAMP3 3’UTR and the mutant one were separately inserted into pmirGLO reporter vector, and then co-transfected into sensitive HNSCC cells along with miR-526b-3p inhibitor and NC inhibitor. Next, we measured the firefly luciferase activity of each vector. It was observed that sensitive HNSCC cells exhibited higher luciferase activity in LAMP3-WT group after miR-526b-3p silencing, while the corresponding mutant group was barely affected (), indicating the interaction between miR-526b-3p and LAMP3. Furthermore, we conducted RNA pulldown assay to demonstrate the physic binding between miR-526b-3p and LAMP3. The biotin labelled probe contained the whole sequence of LAMP3 3’UTR that had the binding sites with miR-526b-3p was named Bio-LAMP3-WT, while that contained the sequence of LAMP3 3’UTR whose binding sites with miR-526b-3p were mutated was named Bio-LAMP3-MUT. The biotin labelled probe contained the random irrelevant sequence was named Bio-NC. The result showed that MiR-526b-3p was enriched in the pulldown products of Bio-LAMP3-WT group instead of the mutant group, which suggested the binding between miR-526b-3p and LAMP3 (). The expression of LAMP3 was elevated by miR-526b-3p silencing in CAL-27/S and FaDu/S cells (), manifesting the negative regulatory effect of miR-526b-3p on LAMP3 expression. Through rescue assays, we confirmed that the viability of CAL-27/S and FaDu/S cells was enhanced by miR-526b-3p knockdown, while this effect was reversed by inhibiting LAMP3 expression (). Moreover, after miR-526b-3p was silenced, the survival rate of sensitive cells under irradiation treatment was enhanced, while this tendency was abrogated by silencing of LAMP3 (). Therefore, we summarised that MiR-526b-3p suppresses LAMP3 expression to enhance radiosensitivity of HNSCC-sensitive cells.
Figure 5. MiR-526b-3p suppresses LAMP3 expression to enhance radiosensitivity of HNSCC-sensitive cells. (A) Luciferase reporter assay was applied to measure the promoter activity of LAMP3 in resistant and sensitive cells. (B) Expression of miRNAs potentially interacted with LAMP3 in irradiation-resistant FaDu/IR and CAL-27/IR cells and irradiation-sensitive FaDu/S and CAL-27/S cells was measured via RT-qPCR. (C) The binding sites between miR-526b-3p and LAMP3 3’UTR as well as the mutant sequence of LAMP3. (D) The wild type sequence of LAMP3 3’UTR and the mutant one were separately inserted into pmirGLO reporter vector, and then co-transfected into sensitive HNSCC cells along with miR-526b-3p inhibitor and NC inhibitor. The firefly luciferase activity of each vector was measured by a dual luciferase reporter assay system. (E) The enrichment of miR-526b-3p was detected in the pulldown products of Bio-LAMP3-WT, Bio-LAMP3-MUT and Bio-NC by RNA pulldown assay. (F) LAMP3 expression was measured in CAL-27/S and CAL-27/IR cells after miR-526b-3p silencing. (G) Cell counting kit-8 assay was taken to measure the viability of sensitive cells transfected with NC inhibitor, miR-526b-3p inhibitor or miR-526b-3p inhibitor + sh-LAMP3#1. (H) Colony formation assay was carried out in sensitive cells transfected with NC inhibitor, miR-526b-3p inhibitor or miR-526b-3p inhibitor + sh-LAMP3#1 to measure their survival rate under different dose of irradiation. **P < 0.01.
Discussion
Radiotherapy is a common therapeutic method for HNSCC patients. However, resistance of patients to radiotherapy in late treatment stage largely impedes the therapeutic effect. Therefore, it is necessary to find novel radiosensitizers. In the current study, we designed and conducted experiments in both irradiation-resistant HNSCC cells and their corresponding irradiation-sensitive cells.
LAMP3 belongs to the lysosome-associated membrane glycoprotein family and is associated with poor overall survival of cancer patients [Citation29]. Patients with high LAMP3 levels have a worse metastasis-free survival in HNSCC [Citation9]. However, its role in regulating radioresistance of HNSCC cells remain unknown. Our current study is the first one to reveal the effects of LAMP3 on the functions and radioresistance of HNSCC cells. In the current study, LAMP3 was identified to be upregulated in HNSCC tissues compared to normal tissues though using the bioinformatics tool GEPIA. Consistently, we confirmed the upregulation of LAMP3 in HNSCC cells compared with the normal cell. These data suggested the potential association between LAMP3 and HNSCC progression. Subsequently, we constructed irradiation-resistant cells and then demonstrated the high viability of survival rate of them compared to corresponding irradiation-sensitive cells. The high expression of LAMP3 in irradiation-resistant cells indicated the potential effect of LAMP3 on the radioresistance of HNSCC cells. Considering the difference of LAMP3 expression in irradiation-resistant cells and sensitive cells, we designed and carried out gain- and loss- of function assays, respectively. The experimental results demonstrated that LAMP3 overexpression promoted proliferation, invasion, migration and EMT process in irradiation-sensitive cells. In irradiation-resistant cells, LAMP3 knockdown led to the suppression on the proliferation, invasion, migration and EMT process. Therefore, we summarised that LAMP3 enhanced radioresistance of HNSCC cells.
Autophagy is a self-protection mechanism widely existing in eukaryotic cells, which can control cell apoptosis and renewal [Citation30]. According to previous studies, autophagy can be activated as a protective mechanism to mediate Multidrug resistance (MDR) during treatment [Citation31] and radioresistance [Citation13–15]. LAMP3 is closely correlated with autophagy, which has been verified as a autophagy inducer for castration-resistant prostate cancer cells [Citation18]. However, so far, whether it can regulate HNSCC cell autophagy and thus affect radioresistance has not been reported. Our current study firstly unveiled the role of LAMP3 in modulating HNSCC cell autophagy. By detecting the expression of autophagy-related proteins and the intensity of LC3 fluorescence, we found that LAMP3 could enhance the autophagy of HNSCC cells. Since autophagy can control cancer cell apoptosis, we further certified the effect of LAMP3 on HNSCC cell apoptosis. As expected, LAMP3 knockdown increased apoptosis rate of irradiation-resistant cells, while its overexpression led to the enhanced apoptosis rate of irradiation-sensitive cells. Hereto, we could conclude that LAMP3 induced autophagy so as to inhibit apoptosis and enhance the resistance of HNSCC cells to radiotherapy.
Exosomes are essential medium to transfer signalling from donor cells to recipient cells [Citation23,Citation24]. Previous researches have also reported that exosomes derived from cancer cells can affect radioresistance in cancer [Citation25,Citation26]. In this study, we explored whether exosomes mediated the intercellular communication between irradiation-resistant HNSCC cells and irradiation-sensitive HNSCC cells. After constructing the co-culture system, we confirmed that sensitive cells presented higher viability and resistance to irradiation after co-cultured with resistant cells. Moreover, the expression level of LAMP3 was significantly increased after co-culturing, indicating the potential delivery of LAMP3 from resistant cells to sensitive cells. Subsequently, we extracted exosomes from two resistant cells and confirmed that the high expression of LAMP3 in sensitive cells treated with exosomes secreted by resistant cells. The regulation of gene expression is influenced by multiple levels of control, with transcriptional regulation being one of the crucial factors. During our experimental process, we hypothesised whether the high expression of LAMP3 is regulated at the transcriptional level. To investigate this, we conducted luciferase reporter gene and the results confirmed that the upregulation of LAMP3 is not achieved through transcriptional regulation. This finding further elucidates that radioresistant cells exert their regulatory effects on radiosensitive cells through the direct delivery of LAMP3 mRNA via exosomes. Meanwhile, we confirmed that the viability and radioresistance of sensitive cells were strengthened by treating with exosomes secreted by resistant cells. These data indicated that irradiation-resistant HNSCC cells could transfer exosomes that carried LAMP3 into irradiation-sensitive cells.
In subsequence, we explored the upstream regulators of LMAP3 and thus seek for potential radiosensitizer for HNSCC cells. Through promoter luciferase reporter assay, the promoter activity of LAMP3 was not significantly different in resistant and sensitive cells, excluding the transcriptional regulation between these two cell lines. MiRNA-mRNA has been widely reported as a common post-transcriptional mechanism which affects cancer development [Citation32,Citation33]. Through bioinformatics selection, we chose miR-526b-3p for further analysis. Previous studies have suggested that miR-526b-3p can reverse lung cancer cisplatin-resistance [Citation34] and breast cancer stem cell property [Citation35]. Nevertheless, to our knowledge, it has not been reported in HNSCC. Our study firstly revealed that miR-526b-3p could suppress LAMP3 expression by directly targeting and thus reverse the radioresistance of HNSCC cells.
Conclusion
Our study demonstrated that high LAMP3 expression could aggravate the resistance of HNSCC cells to radiotherapy by inducing autophagy. Irradiation-resistant cells could transfer exosomal LAMP3 into sensitive cells. Mechanistically, miR-526b-3p could directly target LAMP3 to suppress LAMP3 expression. The current study unveiled a novel molecular pathway functioning in radioresistance of HNSCC cells, which might help to provide new insight on reversing radioresistance of HNSCC.
Supplemental Material
Download MS Word (795.8 KB)Acknowledgements
We are very grateful to all individuals and groups involved in this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Moskovitz J, Moy J, Ferris RL. Immunotherapy for head and neck squamous cell carcinoma. Curr Oncol Rep. 2018;20(2):1. PubMed PMID: 29502288; PubMed Central PMCID: PMCPMC5835060. eng.
- Kitamura N, Sento S, Yoshizawa Y, et al. Current trends and future prospects of molecular targeted therapy in head and neck squamous cell carcinoma. Int J Mol Sci. 2020;22(1):240. PubMed PMID: 33383632; PubMed Central PMCID: PMCPMC7795499. eng.
- Qiu X, You Y, Huang J, et al. LAMP3 and TP53 overexpression predicts poor outcome in laryngeal squamous cell carcinoma. Int J Clin Exp Pathol. 2015;8(5):5519–12. PubMed PMID: 26191259; PubMed Central PMCID: PMCPMC4503130. eng.
- Sun R, Wang X, Zhu H, et al. Prognostic value of LAMP3 and TP53 overexpression in benign and malignant gastrointestinal tissues. Oncotarget. 2014;5(23):12398–12409. PubMed PMID: 25362357; PubMed Central PMCID: PMCPMC4322976. eng.
- Liu S, Yue J, Du W, et al. LAMP3 plays an oncogenic role in osteosarcoma cells partially by inhibiting TP53. Cell Mol Biol Lett. 2018;23(1):33. PubMed PMID: 30008754; PubMed Central PMCID: PMCPmc6042264. eng.
- Yang C, Shen S, Zheng X, et al. Long noncoding RNA HAGLR acts as a microRNA-143-5p sponge to regulate epithelial-mesenchymal transition and metastatic potential in esophageal cancer by regulating LAMP3. Faseb J. 2019;33(9):10490–10504. PubMed PMID: 31311326; eng.
- Wu H, Li J, Chen J, et al. Efficacy of radiation exposure in laryngeal squamous cell carcinoma is mediated by the LAMP3/LAMC2/tenascin-C pathway. Exp Biol Med (Maywood). 2019;244(13):1070–1080.
- Nagelkerke A, Bussink J, van der Kogel AJ, et al. The PERK/ATF4/LAMP3-arm of the unfolded protein response affects radioresistance by interfering with the DNA damage response. Radiother Oncol. 2013;108(3):415–421. PubMed PMID: 23891100; eng.
- Nagelkerke A, Sweep FC, Stegeman H, et al. Hypoxic regulation of the PERK/ATF4/LAMP3-arm of the unfolded protein response in head and neck squamous cell carcinoma. Head Neck. 2015;37(6):896–905. PubMed PMID: 24634103; eng.
- Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17(9):528–542. PubMed PMID: 28751651; PubMed Central PMCID: PMCPMC5975367. eng.
- Onorati AV, Dyczynski M, Ojha R, et al. Targeting autophagy in cancer. Cancer. 2018;124(16):3307–3318. PubMed PMID: 29671878; PubMed Central PMCID: PMCPMC6108917. eng.
- Li X, He S, Ma B. Autophagy and autophagy-related proteins in cancer. Mol Cancer. 2020;19(1):12. PubMed PMID: 31969156; PubMed Central PMCID: PMCPMC6975070. eng.
- Kim J, Kang H, Son B, et al. NRBF2-mediated autophagy contributes to metabolite replenishment and radioresistance in glioblastoma. Exp Mol Med. 2022;54(11):1872–1885.
- Ikeh KE, Lamkin EN, Crompton A, et al. REV1 inhibition enhances radioresistance and autophagy. Cancers (Basel). 2021;13(21):5290.
- Sun Y, Chen K, Lin G, et al. Silencing c-Jun inhibits autophagy and abrogates radioresistance in nasopharyngeal carcinoma by activating the PI3K/AKT/mTOR pathway. Ann Transl Med. 2021;9(13):1085–1085. PubMed PMID: 34422997; PubMed Central PMCID: PMCPmc8339856. eng.
- Tanaka Y, Guhde G, Suter A, et al. Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature. 2000;406(6798):902–906. PubMed PMID: 10972293; eng.
- Eskelinen EL, Illert AL, Tanaka Y, et al. Role of LAMP-2 in lysosome biogenesis and autophagy. Mol Biol Cell. 2002;13(9):3355–3368. PubMed PMID: 12221139; PubMed Central PMCID: PMCPmc124165. eng.
- Pennati M, Lopergolo A, Profumo V, et al. miR-205 impairs the autophagic flux and enhances cisplatin cytotoxicity in castration-resistant prostate cancer cells. Biochem Pharmacol. 2014;87(4):579–597. PubMed PMID: 24370341; eng.
- Zhang Z, Lin M, Wang J, et al. Calycosin inhibits breast cancer cell migration and invasion by suppressing EMT via BATF/TGF-β1. Aging (Albany NY). 2021;13(12):16009–16023. PubMed PMID: 34096887; PubMed Central PMCID: PMCPmc8266341. eng.
- Zhang Y, Yuan Y, Zhang Y, et al. SNHG7 accelerates cell migration and invasion through regulating miR-34a-Snail-EMT axis in gastric cancer. Cell Cycle. 2020;19(1):142–152. PubMed PMID: 31814518; PubMed Central PMCID: PMCPMC6927713. eng.
- Nagelkerke A, Sieuwerts AM, Bussink J, et al. LAMP3 is involved in tamoxifen resistance in breast cancer cells through the modulation of autophagy. Endocr Relat Cancer. 2014;21(1):101–112. PubMed PMID: 24434718; eng.
- Digomann D, Linge A, Dubrovska A. SLC3A2/CD98hc, autophagy and tumor radioresistance: a link confirmed. Autophagy. 2019;15(10):1850–1851. PubMed PMID: 31276435; PubMed Central PMCID: PMCPMC6735542. eng.
- Naseri Z, Oskuee RK, Jaafari MR, et al. Exosome-mediated delivery of functionally active miRNA-142-3p inhibitor reduces tumorigenicity of breast cancer in vitro and in vivo. Int J Nanomedicine. 2018;13:7727–7747. PubMed PMID: 30538455; PubMed Central PMCID: PMCPMC6251455. eng.
- Jiang M, Zhang W, Zhang R, et al. Cancer exosome-derived miR-9 and miR-181a promote the development of early-stage MDSCs via interfering with SOCS3 and PIAS3 respectively in breast cancer. Oncogene. 2020;39(24):4681–4694. PubMed PMID: 32398867; eng.
- Chen X, Liu Y, Zhang Q, et al. Exosomal miR-590-3p derived from cancer-associated fibroblasts confers radioresistance in colorectal cancer. Mol Ther Nucleic Acids. 2021;24:113–126. PubMed PMID: 33738143; PubMed Central PMCID: PMCPMC7943971. eng.
- Chen X, Liu J, Zhang Q, et al. Exosome-mediated transfer of miR-93-5p from cancer-associated fibroblasts confer radioresistance in colorectal cancer cells by downregulating FOXA1 and upregulating TGFB3. J Exp Clin Cancer Res. 2020;39(1):65. PubMed PMID: 32293494; PubMed Central PMCID: PMCPMC7158087. eng.
- Jønson L, Christiansen J, Hansen TVO, et al. IMP3 RNP safe houses prevent miRNA-directed HMGA2 mRNA decay in cancer and development. Cell Rep. 2014;7(2):539–551. PubMed PMID: 24703842; eng.
- Bryan K, Terrile M, Bray IM, et al. Discovery and visualization of miRNA-mRNA functional modules within integrated data using bicluster analysis. Nucleic Acids Res. 2014;42(3):e17–e17. PubMed PMID: 24357407; PubMed Central PMCID: PMCPMC3919560. eng.
- Wang D, Cao X, Zhang Y, et al. LAMP3 expression correlated with poor clinical outcome in human ovarian cancer. Tumour Biol. 2017;39(3):1010428317695014. PubMed PMID: 28349821; eng.
- Maiuri MC, Zalckvar E, Kimchi A, et al. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8(9):741–752. PubMed PMID: 17717517; eng.
- Li YJ, Lei YH, Yao N, et al. Autophagy and multidrug resistance in cancer. Chin J Cancer. 2017;36(1):52. PubMed PMID: 28646911; PubMed Central PMCID: PMCPMC5482965. eng.
- Chen B, Sang Y, Song X, et al. Exosomal miR-500a-5p derived from cancer-associated fibroblasts promotes breast cancer cell proliferation and metastasis through targeting USP28. Theranostics. 2021;11(8):3932–3947. PubMed PMID: 33664871; PubMed Central PMCID: PMCPmc7914354. eng.
- Shi L, Wang Z, Geng X, et al. Exosomal miRNA-34 from cancer-associated fibroblasts inhibits growth and invasion of gastric cancer cells in vitro and in vivo. Aging (Albany NY). 2020;12(9):8549–8564. PubMed PMID: 32391804; PubMed Central PMCID: PMCPmc7244055. eng.
- Chen KB, Yang W, Xuan Y, et al. miR-526b-3p inhibits lung cancer cisplatin-resistance and metastasis by inhibiting STAT3-promoted PD-L1. Cell Death Dis. 2021;12(8):748. PubMed PMID: 34321456; PubMed Central PMCID: PMCPmc8319181. eng.
- Liu JH, Li WT, Yang Y, et al. MiR-526b-3p attenuates breast cancer stem cell properties and chemoresistance by targeting HIF-2α/notch signaling. Front Oncol. 2021;11:696269. PubMed PMID: 35004266; PubMed Central PMCID: PMCPmc8733566. eng.

