Abstract
Long-chain noncoding small nucleolar RNA host gene 14 (LncRNA SNHG14) is highly expressed in various diseases and promotes diseases progression, but the role and mechanism of LncRNA SNHG14 on targeting miR-137 in promoting osteoarthritis (OA) chondrocyte injury remains unclear. To measure the expression of the LncRNAs SNHG14 and miR-137, cell survival, inflammatory response, chondrocyte apoptosis, and extracellular matrix (ECM) levels, we subjected human chondrocytes to a variety of lipopolysaccharide (LPS) concentrations. To measure the luciferase activity of SNHG14-WT and SNHG14-MUT transfected with miR-137 mimic or miR-NC mimic, luciferase reporter genes were utilized. The results showed that chondrocyte viability was significantly inhibited with LPS treatment and chondrocyte inflammatory response, apoptosis and extracellular matrix degradation were significantly increased. However, the above results were significantly reversed after LncRNA SNHG14 inhibition. The luciferase activity bound to miR-137 was decreased in SNHG14-WT group, but there was no change in SNHG14-mut group, which indicated that LncRNA SNHG14 inhibited miR-137 expression as a miRNA sponge. In conclusion, inhibition of LncRNA SNHG14 attenuates chondrocyte inflammatory response, apoptosis and extracellular matrix degradation by targeting miR-137 in LPS induced chondrocytes.
1. Introduction
Osteoarthritis is a prevalent degenerative condition linked to age, obesity, gender, weight, and trauma [Citation1]. Synovial hyperplasia, osteophyte development, subchondral bone sclerosis, and progressive articular cartilage loss are its distinguishing features. The mismatch between the synthesis and catabolism of the extracellular matrix led to cartilage degradation [Citation2]. Around 250 million people worldwide suffer with OA, which has an annual economic cost of more than 89.1 billion dollars. At present, the treatment strategy for early OA is to use drugs to relieve joint pain, and the classic drugs are non-steroidal anti-inflammatory drugs (NSAIDs) [Citation3]. In addition, patients who do not respond to NSAIDs may benefit from intra-articular (IA) injections of hyaluronic acid (HA), corticosteroid, and platelet-rich plasma (PRP) [Citation4, Citation5]. But the sharp arthroplasty is the sole option for OA patients at KLIII-KLIV stage [Citation6]. Although some symptom relief and life quality improvements were possible with these medications, OA development could not be halted.
In articular cartilage, only chondrocytes are permanently present. According to some studies, extracellular matrix breakdown, apoptosis, and chondrocyte inflammation all played a role in the etiology of OA [Citation7]. According to Robert Goggs et al. chondrocyte apoptosis and the loss of chondrocyte survival signals lead to the deterioration of articular cartilage and further aggravate the course of the disease [Citation8]. TNF-alpha, IL-Iβ, and IL-6 are abnormally highly produced in OA chondrocytes, which hastens chondrocyte apoptosis and inhibits the formation of extracellular matrix [Citation9]. Additionally, increasing pro-inflammatory cytokine expression in OA chondrocytes may trigger the c-Jun/p38/NF-κB signaling pathway, which in turn will enhance production of IL-6 and IL-1β [Citation10].
MicroRNAs (miRNAs), as endogenous non-coding RNAs containing about 20 nucleotides, regulate gene expression through binding to the 3′-untranslated region (3′-UTR) of target mRNAs, leading to translational repression [Citation11]. Many evidence indicated miRNAs play vital roles in cell proliferation, differentiation, inflammatory response and apoptosis [Citation12]. Xu W. et al. found MiR-27a could promotes the apoptosis of IL-1β treated-articular chondrocytes in OA through PI3K/AKT/mTOR signaling pathway [Citation13]. In rat OA model, intra-articular injection of miRNA-140 alleviates OA by modulating ECM homeostasis [Citation14]. According to a recent study, miR-137 drastically reduced the number of chondrocytes in OA patients, restricted their ability to proliferate, and increased their likelihood of dying [Citation15].
LncRNAs, a group of ncRNAs that have greater than approximately 200 (nucleotides) lengths, play essential role in many biological processes [Citation16]. Some studies review the association between OA and lncRNAs [Citation17, Citation18]. LncRNAs are implicated in catabolic processes involved in the onset and progression of OA, such as cartilage destruction, inflammatory, autophagy and apoptosis [Citation19, Citation20]. Further, some studies also revealed lncRNAs may have values in the diagnosis and therapy of OA [Citation21]. Recently, studies reported LncRNA SNHG14 is highly expressed in various diseases and promotes diseases progression. Zhang Guanlin. et al. reported LncRNA SNHG14 promotes ischemic brain injury via regulating miR-199b/AQP4 axis [Citation22]. In colorectal cancer, LncRNA SNHG14 promotes cell proliferation and invasion through modulating miR-519b-3p/DDX5 axis [Citation23]. Zhu J. et al. found down-regulation of SNHG14 attenuates lung injury and inflammation in LPS-stimulated acute lung injury via miR-34c-3p/WISP1 signal pathway [Citation24]. Whether LncRNA SNHG14 was involved in OA chondrocyte inflammatory response, apoptosis and extracellular matrix degradation by regulating miR-137 have not been elucidated. Therefore, our study aimed to investigate the role and association of LncRNA SNHG14 and miR-137 on chondrocyte inflammatory, apoptosis and extracellular matrix degradation in OA.
2. Materials and methods
2.1. Materials
LPS and PCR kits were purchased from Sigma Corporation in the United States. Human chondrocytes (CHON-001) were purchased from ATCC Corporation. Dulbecco’s modified Eagle F/12 medium (DMEM F/12), fetal bovine serum, G-418 and Liposome 3000 were purchased from Invitrogen. ELISA test kit and the CCK-8 kit were purchased from Biyuntian Biological Company. Luciferase test kits were purchased from Shanghai Yisheng Biological Company.
2.2. Methods
2.2.1. Chondrocytes culture and LPS treatment
Human chondrocytes were cultured with DMEM F/12 medium containing 100 U/mL penicillin, 10% fetal bovine serum, 50 µg/mL ascorbic acid (AA, Sigma) and 100 μg/mL streptomycin. Based on prior studies, we divided chondrocytes at 90% cell confluence and performed our investigations using passages 2 to 5 [Citation25]. Chondrocytes were treated with different concentrations of LPS (0, 1, 5, 10 μg/mL) according to previous studies [Citation26–28] for 12 hrs to induce injury in vitro.
2.2.2. Chondrocyte transfection
LncRNA SNHG14 low expression sequence (si-SNHG14), miR-137 inhibitor mimics, and the corresponding controls were obtained from GenePharma (Shanghai, China). Chondrocytes were transfected with si-SNHG14, si-NC, si-SNHG14 + miR-137 inhibitor, si-SNHG14 + NC inhibitor and then treated with LPS (10 μg/mL) for 12 hrs according to the Liposome® 3000 transfection reagent operation instructions (Invitrogen; ThermoFisher Scientific, Inc.), respectively.
2.2.3. CCK8 assay
The CCK-8 was used to detect chondrocyte viability in our experiment. A total of 5 × 103 cells were seeded in 96-well cell culture plates. After incubation, chondrocytes were washed twice with PBS and CCK-8 solution (10 µL) was pipetted into each well at different times (0, 24, 48, 72 hrs) respectively. Then, the plates were incubated at 37 °C for 2 hrs without light, and the absorbance at 450 nm was recorded with the microplate reader (Elx808™ Bio-Tek Instruments, Winooski, VT).
2.2.4. Enzyme-linked immunosorbent assay
Chondrocytes were treated under the certain conditions (transfected with interfere sequence (si-NC/si-SNHG14) and miR mimics (miR-137/miR-NC inhibitor) and further treated with LPS), then centrifuged at 4◦C, 1000 rpm for 10 min. The TNF-alpha (Beyotime Biotechnology, Catalog No: PT512) and IL-6 (Beyotime Biotechnology, Catalog No: PI326) levels were determined by ELISA Kits. Briefly, the cell-free supernatant and standard protein were added to the coated ELISA plates, then incubated at room temperature for 1 hrs, washed by PBS five times. The horseradish peroxidase-labeled secondary antibody was added before incubation at room temperature without light for 20 min, then washed five times. Then, chromogenic substrate and the stop solution was added. The OD450 value was recorded using microplate reader (Elx808™ Bio-Tek Instruments, Winooski, VT).
2.2.5. Flow cytometry (FCM)
Chondrocytes were transfected with interfere sequence (si-NC/si-SNHG14) and miR mimics (miR-137/miR-NC inhibitor) and then treated with LPS. The supernatant was discarded and washed with PBS 2 times. Chondrocyte apoptosis was detected using the apoptosis detection kit (Invitrogen, Waltham, MA, USA, Catalog No: V13242) according to instructions. Briefly, chondrocytes were centrifuged at 1000 rpm for 3 min, then 190 µL Annexin V-FITC and 10 µL PI binding solution were used to resuspend the cells and Chondrocytes apoptosis was detected by the flow cytometer (BD Biosciences, San Jose, CA, USA). The percentage in Q2 and Q4 gates was considered for apoptosis of cells.
2.2.6. Q-PCR experiments
Total RNA was extracted using the TRIZOL kit (Invitrogen, Waltham, MA, USA, Catalog No.15-596-018) and quantified by microplate reader (Elx808™ Bio-Tek Instruments, Winooski, VT). Then, 1 μg/μL RNA was used to synthesize cDNA according to the reverse transcription kit (Applied Biosystems, Foster City, CA, USA) according to the instructions. Quantitative PCR was performed on the ABI7500qPCR system. Taking cDNA as the template, denaturing at 95 °C, annealing at 57 °C, and extending at 72 °C, 35 cycle amplifications were performed to record CT values. GAPDH and U6 were selected as the internal reference. The 2−△△Ct method was used to determine the relative quantification of mRNA. All Q-PCR reactions were repeated three times. The specific primer sequences were designed by Sheng gong Biotech. Co., Ltd. (Shanghai, China) and listed in .
Table 1. Lists of PCR specific primer sequences.
2.2.7. Western blot analysis
Chondrocytes were collected and washed 2 times with PBS, then protein samples were extracted using 200 μL RIPA buffer solution. Afterward, protein concentration was determined by Bradford method. Then protein samples were isolated with 10% SDS-PAGE and transferred to a PVDF membrane (Millipore, Bedford, MA, USA). Later, the membrane was blocked in 5% skim milk for 2 hrs and incubated at 4 °C for primary antibodies overnight. Next, the membranes were washed with TBST solution for 3 times and incubated with the sheep anti-rabbit secondary antibody (1:2000) for 1 hrs, further treated with ECL luminescent reagent. Human β-actin was used as internal control for protein loading and protein expressions were quantified by Quantity One software (Bio-Rad, Inc., USA) based on greyscale values [Citation25]. The information of antibodies is listed in .
Table 2. Lists of antibodies.
2.2.8. Dual-luciferase reporter assay
The target fragments of wild-type SNHG14 and mutant SNHG14 were constructed and integrated into pGL3 vector (Promega, Madison, WI, USA) to construct pGL3-SNHG14-wild type (SNHG14-WT) and pGL3-SNHG14-mutant (SNHG14-MUT) reporter vector. NC-mimic + SNHG14 WT, miR-317 mimic + SNHG14 WT, NC-mimic + SNHG14 MUT, miR-317 mimic + SNHG14 MUT were co-transfected to chondrocytes for 48 hrs with Lipofectamine®3000. The dual-luciferase reporter assay was operated according to the luciferase activity assay kit instructions. Briefly, chondrocytes were cleaned three times with PBS and treated with 100 µL Lysis Buffer per well, 20 µL cell lysate solution, 100 µL of LARII working solution and Stop & Glo Reagent, respectively. Then, chondrocytes were detected by luminescence detector.
2.2.9. Statistical analyses
Statistical analyses were performed using Graph Pad Prism 8.0 (GraphPad Software, San Diego, CA, US). All data are presented as the mean ± SD of three independent experiments. The student’s t-test were used for two groups and one-way ANOVA was used for multi-group comparisons. The difference in p < 0.05 was considered statistically significant.
3. Results
3.1. LncRNA SNHG14 was high expressed in LPS treated chondrocytes
Human articular chondrocytes were treated with different concentrations LPS (0, 1, 5, 10 μg/mL) for 12 hrs to establish OA cellular model. The chondrocyte viability was detected by CCK-8. The results showed that cell viability was decreased in a dose-dependent manner. In particular, when chondrocytes were treated with 10 μg/mL LPS, cell viability was decreased about 50% (p < 0.001, ). Therefore, 10 μg/mL LPS was used in subsequent studies. In addition, Q-PCR results showed that the SNHG14 expression was gradually increased along with LPS treatment (p < 0.001, ). The above results showed that SNHG14 is highly expressed in LPS induced OA chondrocytes.
Figure 1. The expression of SNHG14 was increased in the LPS induced chondrocytes. (A)The cell viability was detected by CCK-8 assay in different concentrations of LPS (0, 1, 5, 10 μg/mL). (B)The mRNA levels of SNHG14 in different concentrations of LPS (0, 1, 5, 10 μg/mL). data were compared by unpaired t test, ***p < 0.001: n = 3.
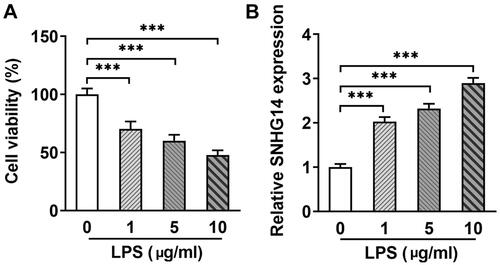
3.2. LncRNA SNHG14 knockdown inhibited chondrocyte inflammatory and apoptosis and increased cell viability
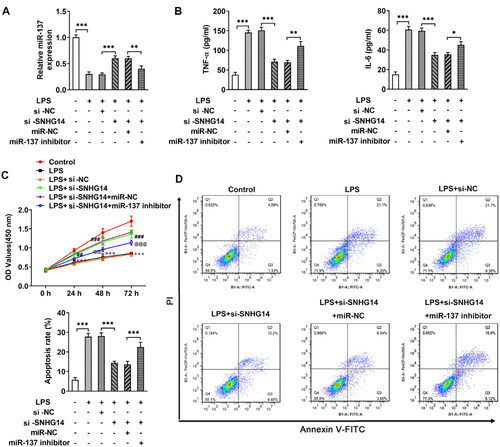
To explore the role of SNHG14 in OA, we transfected interfere sequence to downregulate LncRNA SNHG14 expression (). Then, chondrocyte inflammatory, apoptosis and cell viability were determined by Q-PCR, Flow cytometry and CCK-8 assay, respectively. The results indicated that LncRNA SNHG14 knockdown reduced TNF-alpha and IL-6 concentrations (p < 0.01, ). The CCK-8 results showed that cell viability was elevated after LncRNA SNHG14 knockdown (compared with the LPS + si-NC group) (p < 0.001, ). Furthermore, Flow cytometry results showed that chondrocyte apoptosis rate was increased in LPS group and reduced in the LPS + si-SNHG14 group (p < 0.001, ). Taken together, these data further indicated that knocking down SNHG14 in LPS induced chondrocytes inhibited inflammatory response, apoptosis and enhanced cell viability.
Figure 2. si-SNHG14 inhibited inflammation response and apoptosis in LPS-induced chondrocytes. (A)the mRNA levels of SNHG14 was measured by Q-PCR. (B) the TNF-alpha and IL-6 protein expression were detected by ELISA. (C) Cell viability was detected by CCK-8 assay. (D) Chondrocytes apoptosis were detected by FCM. (E) Quantitative analysis of chondrocyte apoptosis in different groups. The results are presented as the means ± SD of three independent experiments and statistical significance was determined by one-way ANOVA. **p < 0.01, ***p < 0.001: n = 3.
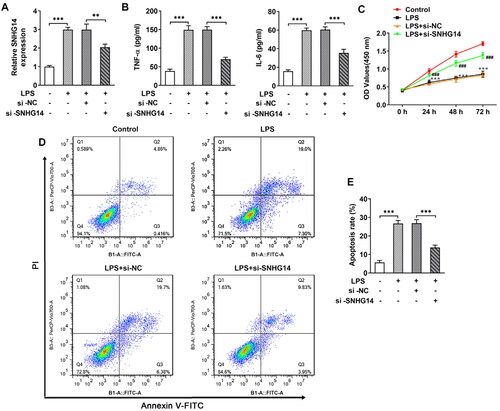
3.3. Knocking down SNHG14 inhibits extracellular matrix degradation
Extracellular matrix degradation accelerates OA development. LncRNA SNHG14 knockdown sequences were transfected into chondrocytes. The ADAMTS-5, MMP13, Collagen II and Aggrecan were detected by Western blot. The results showed that the ADAMTS-5 and MMP13 expression in the LPS groups were markedly increased (p < 0.001, ), while the Collagen II and Aggrecan were significantly decreased (p < 0.001, ). Besides, when SNHG14 was knockdown, the expression of ADAMTS-5, MMP13, Collagen II and Aggrecan were reversed compared with LPS + si-NC group (p < 0.001, ). The above results showed that knocking down SNHG14 could prevent the chondrocyte extracellular matrix degradation.
Figure 3. The effect of SNHG14 knockdown on the protein levels of ADAMTS-5, MMP13, Collagen II and Aggrecan. (A) The level of ADAMTS-5, MMP13, Collagen II and Aggrecan in chondrocytes were measured by western blotting. (B-E) Quantitative analysis for the expression of ADAMTS-5, MMP13, Collagen II and Aggrecan in different groups. The results are presented as the means ± SD of three independent experiments and statistical significance was determined by one-way ANOVA. **p < 0.01, ***p < 0.001: n = 3.
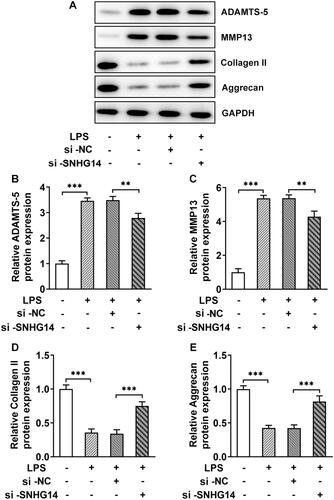
3.4. Lnc RNA SNHG14 targets miR-137
We analyzed the targeted relationship between LncRNA SNHG14 and miR-137 through the Star-Base database (http://starbase.sysu.edu.cn/). The results showed they have binding sites with each other. The Q-PCR results showed that the miR-137 expression gradually decreased with LPS treatment (p < 0.001, ). In addition, and the miR-137 expression was increased after LncRNA SNHG14 inhibition (p < 0.001, ). For further to determine the relationship between LncRNA SNHG14 and miR-137 expression, SNHG14-WT vector and SNHG14-MUT vector were transfected with miR-137 mimic or miR-NC mimic. The luciferase reporter gene results showed that the luciferase activity in the SNHG14-WT group was reduced, while no significantly changed in the SNHG14-MUT group (p < 0.001, ). The above results indicated that SNHG14 targeted miR-137 and inhibited the miR-137 expression.
Figure 4. SNHG14 targeted inhibition of miR-137 expression. (A) the mRNA levels of miR-137 were measured by RT-qPCR in different concentrations of LPS (0,1,5,10 μg/mL) treatment. (B) the mRNA levels of miR-137 in control, LPS group, LPS + si-NC group and LPS + si-SNHG14 group. (C) Luciferase reporter gene was used to detect the Regulatory relationship between miR-137 and SNHG14. The results are presented as the means ± SD of three independent experiments and statistical significance was determined by one-way ANOVA. **p < 0.01, ***p < 0.001: n = 3.
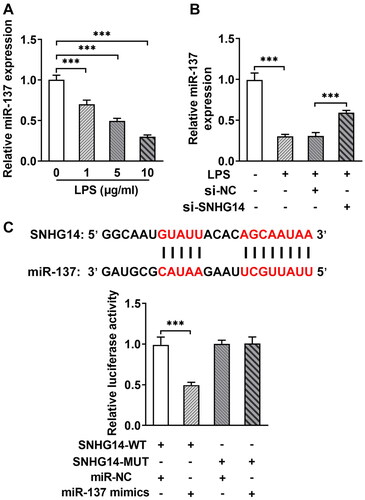
3.5. SNHG14 regulated chondrocyte proliferation, apoptosis and inflammation via inhibiting miR-137
Next, in order to explore whether SNHG14 regulates inflammatory response, apoptosis, and cell viability by targeting the miR-137. We transfected miR-137 mimics and/or si-SNHG14 into chondrocytes. The Q-PCR results showed that the miR-137 expression was increased in the LPS + si-SNHG14 group compared with LPS + si-NC group. While, the expression of miR-137 was reduced in LPS + si-SNHG14 + miR-137 inhibitor group compared with LPS + si-SNHG14 + miR-NC group (p < 0.05, ). Besides, the TNF-alpha and IL-6 concentrations in the LPS + si-SNHG14 + miR-137 inhibitor group were increased (p < 0.05, ). Moreover, the cell viability of the LPS + si-SNHG14 + miR-137 inhibitor group was reduced compared with the LPS + si-SNHG14 + miR-NC group (p < 0.05, ). Flow cytometry results showed that chondrocytes apoptosis in the LPS + si-SNHG14 + miR-137 inhibitor group was increased compared with the LPS + si-SNHG14 + miR-NC group (p < 0.05, ). The above results indicated that inhibition of miR-137 expression in LPS induced chondrocytes could reverse the effect of knockdown SNHG14 on chondrocyte proliferation, apoptosis and inflammation.
Figure 5. si-SNHG14 regulates inflammatory response, apoptosis and cell viability by targeting miR-137 in LPS-induced chondrocytes. (A) the expression of SNHG14 was measured by Q-PCR. (B) the levels of TNF-alpha and IL-6 was detected ELISA. (C) Cell viability was detected by cck-8 assay. (D) Chondrocytes apoptosis was detected by FCM. The results are presented as the means ± SD of three independent experiments and statistical significance was determined by one-way ANOVA. *p < 0.05, **p < 0.01, ***p < 0.001: n = 3.
3.6. SNHG14 regulate extracellular matrix degradation by target miR-137
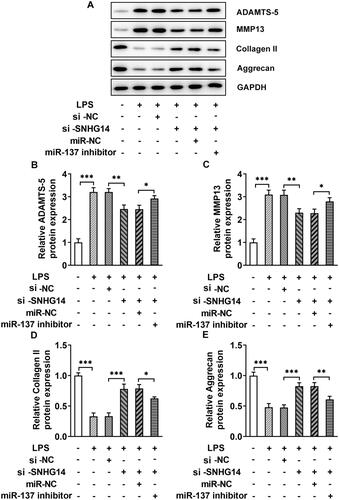
The western blot results showed that the ADAMTS-5 and MMP13 expression in the LPS + si-SNHG14 + miR-137 inhibitor group were increased (p < 0.05) and collagen II and Aggrecan were decreased compared with the LPS + si-SNHG14 + miR-NC group (p < 0.05, ). Then, LncRNA SNHG14 may inhibit chondrocytes extracellular matrix degradation by regulating miR-137 expression.
Figure 6. The effect of miR-137 knockdown on the protein levels of ADAMTS-5, MMP13, Collagen II and Aggrecan. (A) The level of ADAMTS-5, MMP13, Collagen II and Aggrecan were measured by western blotting. (B-E) Quantitative analysis for the expression of ADAMTS-5, MMP13, Collagen II and Aggrecan in different groups. The results are presented as the means ± SD of three independent experiments and statistical significance was determined by one-way ANOVA. *p < 0.05, **p < 0.01, ***p < 0.001: n = 3.
4. Discussion
In this study, we found that SNHG14 was markedly overexpressed in LPS induced chondrocytes, while miR-137 was downregulated. Furthermore, inhibition of SNHG14 showed potent anti-inflammatory, apoptosis and extracellular matrix degradation effects on chondrocytes. Then, the synthesis of biological agents that inhibition of SNHG14 may be effective way to treat OA.
LncRNAs are known to function as competitive endogenous RNA (ceRNAs) in isolating miRNAs from target mRNAs [Citation29, Citation30]. In recent years, numerous studies have shown that LncRNAs were involved in regulating cell proliferation, apoptosis, inflammatory response, metabolic balance of the extracellular matrix during the OA process [Citation31–34]. For example, Cao L. et al. found that knocking down LncRNA FOXD2-AS1 promotes osteoarthritis chondrocyte proliferation and inhibits apoptosis by targeting the miR-206 [Citation35]. Besides, knocking down LncRNA PVT1 inhibits chondrocytes apoptosis in OA by targeting miR-488-3p [Citation31]. LncRNA CIR enhances OA articular cartilage degeneration by inducing autophagy [Citation36]. At the same time, Shen H. et al. reported LncRNA SNHG5 promotes chondrocytes proliferation through the miR-26a/SOX2 signaling axis [Citation37]. Some studies have reported that LncRNA SNHG14 was participated in arthritis progress. Zhang Jihui et al. found LncRNA SNHG14 induced proinflammatory cytokine production in arthritis via the regulation of the miR-17-5p/MINK1-JNK pathway [Citation38]. Moreover, down-regulation of LncRNA SNHG14 has been shown to inhibit FSTL1-mediated activation of the NLRP3 and TLR4/NF-κB signaling pathways by targeting miR-124-3p, thereby reducing the inflammatory response in OA [Citation39]. Consistent with previously findings, our study found that LncRNA SNHG14 in LPS induced chondrocytes were overexpressed and knocking down LncRNA SNHG14 could inhibit the inflammatory response, apoptosis and extracellular matrix degradation.
MiR-137, as an important miRNA, plays an increasingly important role in OA chondrocytes. Overexpression of miR-137 targets the inhibition of TCF4 to reverse the OA progression through the AMPK/NF-kB signaling pathway [Citation15]. Besides, Zhang Y. et al. founded miR-137 inhibits cell growth and extracellular matrix degradation through regulating ADAMTS-5, thus playing a protective role in OA [Citation40]. Furthermore, in rheumatoid arthritis fibroblast-like synoviocytes, miR-137 decreases proliferation, migration and invasion [Citation41]. More importantly, recent studies have shown that LncRNA GAS5 induced chondrocytes apoptosis by down-regulating miR-137 [Citation42]. However, our study found that the miR-137 expression was reduced in LPS induced chondrocytes, and knockdown LncRNA SNHG14 reversed the miR-137 expression. Meanwhile, bioinformatics analysis found that LncRNA SNHG14 contains a binding site of miR-137. Dual-Luciferase Reporter assay verified the association between LncRNA SNHG14 and miR-137. Knocking down LncRNA SNHG14 in LPS induced chondrocytes, the proliferative capacity of chondrocytes was increased. While chondrocytes apoptosis, inflammatory response and extracellular matrix degradation were decreased. These results suggested knocking down LncRNA SNHG14 inhibited chondrocytes inflammatory response, apoptosis and extracellular matrix degradation by competitive binding miR-137.
Nonetheless, there are some major limitations in this study that could be addressed in future research. First, adult human chondrocytes often require years or even decades, and the development of OA and chondrocyte injuries may accumulate gradually during the progression of OA. In our experiment, we performed LPS to induce OA cell model, which may not be completely the same as the normal pathological process of OA. In addition, there is a possibility of initiation of pathways leading to other associated problem because of knocking down lncRNA SNHG14 leading to rheumatoid arthritis, heart disease, diabetes etc. Thus, animal experiments should be needed to further validate our results in future.
In summary, LncRNA SNHG14 expression was elevated and miR-137 expression was decreased in LPS induced chondrocytes. Inhibiting the expression of LncRNA SNHG14 could increase cell viability and decrease chondrocyte apoptosis, inflammatory response and extracellular matrix degradation by regulating miR-137 expression. This study explored the role of SNHG14 in targeting miR-137 to promote chondrocyte injury and LncRNA SNHG14 may be a new biological target for the treatment of OA.
Authors’ contributions
ZD and XC contributed to the conception and design of the study. CT and LSW carried out the principal experiments. WLL and YKY contributed to the analysis and interpretation of the data. XC contributed to the drafting of the article. JCG contributed to the critical revision of important intellectual content. All authors read and approved the final manuscript.
Consent for publication
Not applicable.
| Abbreviations | ||
| LncRNA SNHG14 | = | long-chain noncoding small nucleolar RNA host gene 14 |
| OA | = | osteoarthritis |
| LPS | = | lipopolysaccharide |
| ECM | = | extracellular matrix |
| NSAIDs | = | non-steroidal anti-inflammatory drugs |
| IA | = | intra-articular |
| HA | = | hyaluronic acid |
| PRP | = | platelet-rich plasma |
| miRNAs | = | microRNAs |
| 3′-UTR | = | 3′-untranslated region |
| DMEM F/12 | = | Dulbecco’s modified Eagle F/12 medium |
| AA | = | ascorbic acid |
| FCM | = | flow cytometry |
| ceRNAs | = | competitive endogenous RNA |
| ADAMTS-5 | = | a disintegrin and metalloproteinase with thrombospondin motif -5 |
| MMP13 | = | matrix metalloproteinase 13 |
Acknowledgments
We want to thanks JSJ for giving suggestions.
Conflicts of interest
The authors declare that they are free from conflict of interest in presenting it.
Data availability
The datasets used in the present study are available from the corresponding authors on reasonable request.
Additional information
Funding
References
- Wang Y, Fan X, Xing L, et al. Wnt signaling: a promising target for osteoarthritis therapy. Cell Commun Signal. 2019;17(1):1.
- Chen D, Shen J, Zhao W, et al. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res. 2017;5(1):16044.
- Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019;27(11):1578–10.
- Everts P, Onishi K, Jayaram P, et al. Platelet-Rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci. 2020;21(20):7794.
- Ma L, Zheng X, Lin R, et al. Knee osteoarthritis therapy: recent advances in intra-articular drug delivery systems. Drug Des Devel Ther. 2022;16:1311–1347.
- Quicke JG, Conaghan PG, Corp N, et al. Osteoarthritis year in review 2021: epidemiology and therapy. Osteoarthr. Cartilage. 2022;30(2):196–206.
- Woodell-May JE, Sommerfeld SD. Role of inflammation and the immune system in the progression of osteoarthritis. J Orthop Res. 2020;38(2):253–257.
- Goggs R, Carter SD, Schulze-Tanzil G, et al. Apoptosis and the loss of chondrocyte survival signals contribute to articular cartilage degradation in osteoarthritis. Vet J. 2003;166(2):140–158.
- Wang T, He C. Pro-inflammatory cytokines: the link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018;44:38–50.
- Mengshol JA, Vincenti MP, Coon CI, et al. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor kappaB: differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum. 2000;43(4):801–811.
- Si HB, Zeng Y, Liu SY, et al. Intra-articular injection of microRNA-140 (miRNA-140) alleviates osteoarthritis (OA) progression by modulating extracellular matrix (ECM) homeostasis in rats. Osteoarthr. Cartilage. 2017;25(10):1698–1707.
- Xia M, Lu J, Wu Y, et al. MicroRNA-4287 alleviates inflammatory response via targeting RIPK1 in osteoarthritis. Autoimmunity. 2022;55(5):301–309.
- Xu W, Hang M, Yuan CY, et al. MicroRNA-139-5p inhibits cell proliferation and invasion by targeting insulin-like growth factor 1 receptor in human non-small cell lung cancer. Int J Clin Exp Pathol. 2015;8:3864–3870.
- Cai C, Min S, Yan B, et al. MiR-27a promotes the autophagy and apoptosis of IL-1β treated-articular chondrocytes in osteoarthritis through PI3K/AKT/mTOR signaling. Aging. 2019;11(16):6371–6384.
- Wang J, Fang L, Ye L, et al. miR-137 targets the inhibition of TCF4 to reverse the progression of osteoarthritis through the AMPK/NF-κB signaling pathway. Biosci Rep. 2020;40(6).
- Maass PG, Luft FC, Bähring S. Long non-coding RNA in health and disease. J Mol Med (Berl). 2014;92(4):337–346.
- Nie T, Zhang C, Zhang G, et al. LncRNA CALML3-AS1 regulates chondrocyte apoptosis by acting as a sponge for miR-146a. Autoimmunity. 2021;54(6):336–342.
- Zou H, Lu C, Qiu J. Long non-coding RNA LINC00265 promotes proliferation, apoptosis, and inflammation of chondrocytes in osteoarthritis by sponging miR-101-3p. Autoimmunity. 2021;54(8):526–538.
- Jiang S, Liu Y, Xu B, et al. Noncoding RNAs: new regulatory code in chondrocyte apoptosis and autophagy. Wiley Interdiscip Rev RNA. 2020;11:e1584.
- Zhu J, Yu W, Wang Y, et al. lncRNAs: function and mechanism in cartilage development, degeneration, and regeneration. Stem Cell Res Ther. 2019;10(1):344.
- Budd E, Nalesso G, Mobasheri A. Extracellular genomic biomarkers of osteoarthritis. Expert Rev Mol Diagn. 2018;18(1):55–74.
- Zhang G, Li T, Chang X, et al. Long noncoding RNA SNHG14 promotes ischemic brain injury via regulating miR-199b/AQP4 axis. Neurochem Res. 2021;46(5):1280–1290.
- Wang X, Yang P, Zhang D, et al. LncRNA SNHG14 promotes cell proliferation and invasion in colorectal cancer through modulating miR-519b-3p/DDX5 axis. J Cancer. 2021;12(16):4958–4970.
- Zhu J, Bai J, Wang S, et al. Down-regulation of long non-coding RNA SNHG14 protects against acute lung injury induced by lipopolysaccharide through microRNA-34c-3p-dependent inhibition of WISP1. Respir Res. 2019;20(1):233.
- Xu C, Ni S, Zhuang C, et al. Polysaccharide from angelica sinensis attenuates SNP-induced apoptosis in osteoarthritis chondrocytes by inducing autophagy via the ERK1/2 pathway. Arthritis Res Ther. 2021;23(1):47.
- Chen G, Liu T, Yu B, et al. CircRNA-UBE2G1 regulates LPS-induced osteoarthritis through miR-373/HIF-1a axis. Cell Cycle. 2020;19(13):1696–1705.
- Ding Y, Wang L, Zhao Q, et al. MicroRNA‑93 inhibits chondrocyte apoptosis and inflammation in osteoarthritis by targeting the TLR4/NF‑κB signaling pathway. Int J Mol Med. 2019;43(2):779–790.
- Zhang Q, Bai X, Wang R, et al. 4-octyl itaconate inhibits lipopolysaccharide (LPS)-induced osteoarthritis via activating Nrf2 signalling pathway. J Cell Mol Med. 2022;26(5):1515–1529.
- Zhou Y, Li X, Duan Y, et al. LncRNA MALAT-1 regulates the growth of interleukin-22-stimulated keratinocytes via the miR-330-5p/S100A7 axis. Autoimmunity. 2022;55(1):32–42.
- Sun Y, Jiang H, Pan L, et al. LncRNA OIP5-AS1/miR-410-3p/Wnt7b axis promotes the proliferation of rheumatoid arthritis fibroblast-like synoviocytes via regulating the wnt/β-catenin pathway. Autoimmunity. 2023;56(1):2189136.
- Li Y, Li S, Luo Y, et al. LncRNA PVT1 regulates chondrocyte apoptosis in osteoarthritis by acting as a sponge for miR-488-3p. DNA Cell Biol. 2017;36(7):571–580.
- Huang H, Yan J, Lan X, et al. LncRNA WDR11-AS1 promotes extracellular matrix synthesis in osteoarthritis by directly interacting with RNA-Binding protein PABPC1 to stabilize SOX9 expression. Int J Mol Sci. 2023;24(1):817.
- Rocha FAC, Ali SA. Soluble biomarkers in osteoarthritis in 2022: year in review. Osteoarthr. Cartilage. 2023;31(2):167–176.
- Wang P, Wang Y, Ma B. Long noncoding RNA NAV2-AS5 relieves chondrocyte inflammation by targeting miR-8082/TNIP2 in osteoarthritis. Cell Cycle. 2023;22(7):796–807.
- Cao L, Wang Y, Wang Q, et al. LncRNA FOXD2-AS1 regulates chondrocyte proliferation in osteoarthritis by acting as a sponge of miR-206 to modulate CCND1 expression. Biomed Pharmacother. 2018;106:1220–1226.
- Wang CL, Peng JP, Chen XD. LncRNA-CIR promotes articular cartilage degeneration in osteoarthritis by regulating autophagy. Biochem Biophys Res Commun. 2018;505(3):692–698.
- Shen H, Wang Y, Shi W, et al. LncRNA SNHG5/miR-26a/SOX2 signal axis enhances proliferation of chondrocyte in osteoarthritis. Acta Biochim Biophys Sin (Shanghai). 2018;50(2):191–198.
- Zhang J, Lei H, Li X. LncRNA SNHG14 contributes to proinflammatory cytokine production in rheumatoid arthritis via the regulation of the miR-17-5p/MINK1-JNK pathway. Environ Toxicol. 2021;36(12):2484–2492.
- Wang B, Li J, Tian F. Downregulation of lncRNA SNHG14 attenuates osteoarthritis by inhibiting FSTL-1 mediated NLRP3 and TLR4/NF-κB pathway through miR-124-3p. Life Sci. 2021;270:119143.
- Zhang Y, Wang G, Ma L, et al. miR-137 suppresses cell growth and extracellular matrixdegradation through regulating ADAMTS-5 in chondrocytes. Am J Transl Res. 2019;11:7027–7034.
- Du J, Zhang F, Guo J. miR‑137 decreases proliferation, migration and invasion in rheumatoid arthritis fibroblast‑like synoviocytes. Mol Med Rep. 2018;17:3312–3317.
- Gao ST, Yu YM, Wan LP, et al. LncRNA GAS5 induces chondrocyte apoptosis by down-regulating miR-137. Eur Rev Med Pharmacol Sci. 2020;24:10984–10991.

