Abstract
To detect the value of serum interleukin-17 (IL-17), tumour necrosis factor-α (TNF-α), and Dickkopf-1 (DKK-1) in rheumatoid arthritis (RA) at different disease stages. 141 RA patients were randomly obtained and diagnosed in a large tertiary first-class hospital in Jiangxi Province from November 2021 to January 2022. RA was divided into 38 low activity and remission phase (low remission patients), 72 moderate activity patients, 41 high activity patients, according to the disease activity score 28 (DAS28) of RA and 70 healthy controls. IL-17 and TNF-α in serum detected by flow cytometry; DKK-1by ELISA; rheumatoid factor (RF) and C-reactive protein (CRP) by rate scattering turbidimetry; erythrocyte sedimentation rate (ESR) by Widmanstat method; anti-cyclic citrullinated polypeptide antibody (Anti-CCP) by chemiluminescence. The changes among the groups were statistically analysed and evaluated their diagnostic value. ①Anti-CCP, CRP, and ESR levels in the moderate-to-high activity group were higher than controls, while IL-17, TNF-α, and DKK-1levels higher than low remission group, moderate activity group and controls (p < 0.05). ②IL-17, TNF-α and DKK-1 were positively correlated with RA disease activity, with the correlations of IL-17, TNF-α and DKK-1 all over 0.5 (p < 0.05). ③The ROC curve showed that among all indices the AUC of DKK-1 was the largest, 0. 922, and has the highest sensitivity and negative predictive value for RA, 0.965 and 0.953, respectively. The specificity and positive predictive value of TNF-α is highest, 0.918 and 0.921, respectively, combined them had the highest predictive value in moderate-to-high activity RA, with AUC of 0.968, and had the highest sensitivity of 0.965. The IL-17, TNF-α and DKK-1 levels were elevated in RA and positively correlated with disease activity, involved in the Wnt signalling pathway of inflammatory and joint destructive effects, combining them to monitor the RA disease process and biologically treat the cytokines in the pathogenesis of RA were valuable.
Introduction
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease characterised by synovial inflammation and destruction of bone and cartilage [Citation1,Citation2]. Multiple evidences indicate that Wnt signalling pathway involved in the pathogenesis of RA [Citation3]. Wnt protein is a glycoprotein that binds to Fz receptors on the cell surface, thereby performing a variety of important biological functions. In RA, the synovium expressed higher levels of Wnt and Fz genes than non-RA patients, the Wnt signalling pathway is involved in systemic and localised bone loss, plays a key role in maintaining the balance of the osteoblast-osteoclast axis [Citation4]. Wnt signalling inhibitor Dickkopf-1 (DKK-1) is higher in the serum of RA patients than in controls, and is associated with disease activity and more severe bone destruction in RA [Citation5,Citation6]. Wnt signalling pathway it can also be directly or indirectly down-regulated by pro-inflammatory factors, such as tumour necrosis factor-α (TNF-α), Interleukin-17 (IL-17), IL-1β, and IL-6 [Citation7,Citation8].
Dickkopf-1 (DKK-1) is a classic antagonist of Wnt/β-catenin signalling and is implicated in the pathogenesis of RA. It binded to the high-affinity low-density lipoprotein receptor-related protein 5/6 (LRP5/6) and a single-channel transmembrane co-receptor Kremen 1/2, then formed a ternary complex to endocytosed rapidly, leading Wnt/β-catenin signalling inhibited, resulting in bone flab formation in peripheral joints [Citation9,Citation10]. IL-17 is produced by T helper cell 17 (TH17) cells and has a facilitative role in inflammation. IL-17 regulates the proliferation, migration and apoptosis of vascular endothelial cells and smooth muscle cells through a variety of pathways and promotes the secretion of cytokines, leading to an immune response and the onset of cartilage and bone tissue destruction, with important effects on osteoclastogenesis and bone destruction. IL-17 also induces macrophage secreted TNF-α, which amplifies inflammation. TNF-α and IL-17 have synergistic effects in promoting IL-6, IL-8, and matrix metallo proteinase (MMP) production in fibroblast-like synoviocytes (FLS) [Citation11]. TNF-α released in active RA can increase DKK-1 level, systemic inhibition of TNF-α can reduce the level of DKK-1 in RA to almost normal [Citation12]. The correlation between IL-17, TNF-α and DKK-1 at different disease activity levels in RA is not well documented. The aim of this study was to assess the expression levels of IL-17, TNF-α and DKK-1 in RA at different activity levels, to explore the relevance of these three in the Wnt signalling pathway of RA, and to explore the impact of cytokines on this pathway and their possible clinical implications.
Data and methods
Study subjects
141 patients with RA diagnosed in a large tertiary hospital in Jiangxi Province from November 2021 to January 2022 were selected, and all RA patients met the diagnostic grading criteria of the relevant diseases, 38 cases in the low-remission group, 10 men and 28 women, with an average age of 48.90 ± 10.38 years, 72 cases in the moderate activity group, 16 men and 56 women, with an average age of 48.66 ± 11.94 years, and 41 cases in the high active group, 12 males and 29 females, with a mean age of 44.03 ± 11.10 years. 70 cases in the healthy control group, 18 males and 52 females, with a mean age of 47.73 ± 8.58 years, were obtained from the healthy population of the hospital physical examination centre. The study was approved by the ethics committee of a large tertiary first-class hospital in Jiangxi Province, and all participants signed a written informed consent.
Inclusion and exclusion criteria
Inclusion criteria: (1) Patients with RA met the diagnostic criteria for RA as revised by the European League Against Rheumatism (EULAR) at its annual meeting in 2021 [Citation13]. (2) Patients had a clear diagnosis of disease, complete imaging examinations such as X-ray and CT, pathological examinations and clinical data. (3) The study was approved by the medical ethics committee of a large tertiary first-class hospital in Jiangxi Province.
Exclusion criteria: (1) Those with extra-articular complications of RA or who have taken various anti-rheumatic drugs; (2) Those with other metabolic diseases or autoimmune diseases; (3) Those with underlying diseases such as diabetes mellitus, liver and kidney diseases, interstitial lung diseases, severe infections, and malignant neoplasms; (4) Pregnant or lactating women and growing children.
Apparatus, reagents and methods
General data such as gender, age, diagnosis, swollen joint count and tender joint count were collected from the included subjects through our Hospital Information System (His) system. All enrolled patients and control group had 3ml of fasting venous blood collected in EDTA tubes, and the serum was separated and collected after centrifugation at 1026 × g for 15 min at room temperature for natural clotting for 30 min, and stored frozen at -80 °C refrigerator. IL-17 and TNF-α were detected by FACS Calibur flow cytometry (BD, USA) and 12 cytokine test kits (Qingdao Ruiskell Biotechnology Co. Ltd.); DKK-1 was detected by commercial ELISA (R&D, USA), Anti-CCP was detected by chemiluminescence method (Shenzhen YOLO Biotechnology Co. Ltd) and complementary reagents. CRP and RF were detected by rate scattering turbidimetry method by automatic specific protein analysis system (Beckman Coulter IMMAGE 800). and complementary reagents. The ESR was measured by the Widmanstat method by automatic rapid erythrocyte sedimentation rate analyzer (Roller 20) and reagents (ALIFAX Company, Italy). The swollen joints count (0–28) and tender joint count (0–28) were recorded by two experienced rheumatic immunologists and the range of disease activity was assessed by the DAS28 score. The DAS28 score was calculated according to the formula; DAS28 score = 0.56*√(TJC28) + 0.28*√(SJC28) + 0.70*ln (ESR) + 0.014* (general health), the DAS28 score for low activity and remission (low remission group) was ≤3.2; for moderate activity group, 3.2 < DAS28 ≤ 5.1; for high activity group, DAS28 > 5.1 [Citation14]. All operations were performed strictly according to the requirements of the reagents, instruments instructions and the standard operation procedure (SOP) of a large tertiary first-class hospital in Jiangxi Province.
Data analysis
Data analysis was performed using SPSS 23.0 statistical software, with the number of cases for counting data and the χ2 test for comparison between groups; all data were analysed using the Kolmogorov-Smirnov test for normality, and the measurement data conforming to the normal distribution were statistically described by mean ± standard, with the t-test for comparison between two groups and the Analysis of Variance (ANOVA) for comparison between multiple groups. The non-normal distribution was statistically described by M (Q1, Q3) and the non-parametric test Kruskal Wallis for comparison between multiple groups and further two-by-two comparisons were made by Bonferroni; Spearman was used for correlation analysis; the receiver operating characteristic (ROC) curve was used to assess the diagnostic effectiveness of each group of indicators in RA further determined sensitivity, specificity, negative likelihood ratio (NLR), positive likelihood ratio (PLR), negative predictive value (NPV), positive predictive value (PPV) and Youden index by standard methods. The value of IL-17, TNF-α and DKK-1 in predicting moderate-to-high activity in RA also measured by ROC, and differences were considered statistically significant at p < 0.05.
Results
Comparison of general data
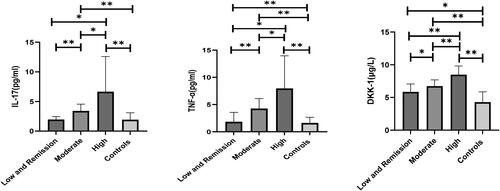
The differences in gender, age and course were not statistically significant in patients with RA of different activity levels or controls (p > 0.05); tender joint count (TJC), DAS28 score, RF, IL-17, TNF-α and DKK-1 were significantly higher in the high active group than in the low-remission group, moderate activity group and the controls; swollen joint count (SWJ), Anti-CCP, CRP and ESR were significantly higher in the high active group than in the low-remission group and the controls. The differences were statistically significant (p < 0.05).
Table 1. Basic information of each group.
Serum levels of IL-17, TNF-α and DKK-1 in the four groups
With disease activity increased, IL-17, TNF-α and DKK-1 expression levels increased significantly, and IL-17, TNF-α and DKK-1 expression levels in the high activity group were significantly higher than the low-remission group, moderate activity group and controls (p < 0.05, ).
Correlation analysis of serum IL-17, TNF-α and DKK-1 with clinical data
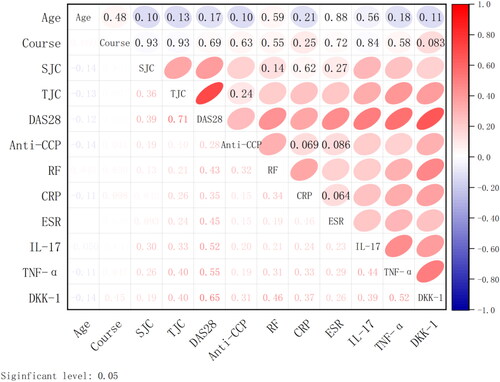
Spearman correlation analysis was used to analyse the correlation of IL-17, TNF-α and DKK-1 with age, course, SJC, TJC, DAS28, Anti-CCP, RF, CRP and ESR. The results showed that the correlations of IL-17, TNF-α and DKK-1 with DAS28, were all over 0.5, indicating that were correlated with the progression of RA disease, while IL-17, TNF-α and DKK-1 were not significantly correlated with age and course ().
Correlation of IL-17, TNF-α and DKK-1 expression levels with DAS28 scores in patients with RA
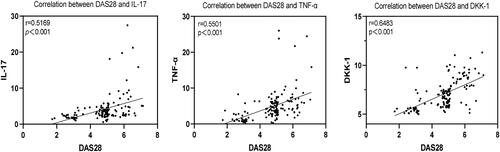
The results of Spearman correlation analysis showed that IL-17, TNF-α and DKK-1 were positively correlated with DAS28 scores (r = 0.5169, 0.5501, 0.6483, p < 0.001, ).
Evaluation of diagnostic value of each indices
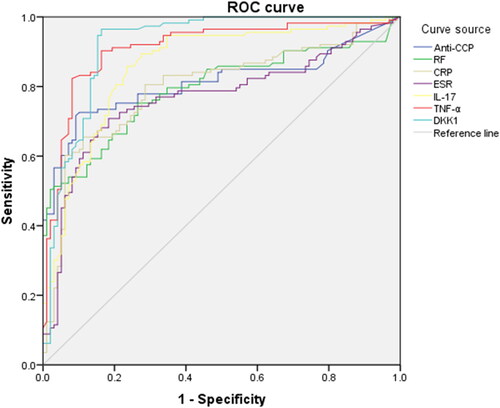
ROC curve results showed that the AUC of DKK-1 was the largest, 0.922, followed by TNF-α, IL-17, Anti-CCP, CRP, RF and ESR, with AUC areas of 0.911, 0.860, 0.811, 0.797 0.792 and 0.768, respectively. The evaluation of diagnostic value of each indices shows that DKK-1 has the highest sensitivity and negative predictive value for RA, 0.965 and 0.953, respectively. The specificity and positive predictive value of TNF-α is highest, 0.918 and 0.921, respectively ( and ).
Table 2. Clinical evaluation of research indices.
Diagnostic efficacy of IL-17, TNF-α and DKK-1 in RA
ROC curve analysis showed that combined IL-17, TNF-α and DKK-1 had the highest predictive value for the diagnosis of RA in the moderate to high activity group, with an AUC of 0.968, and had the highest sensitivity of 0.965. IL-17 had the highest specificity of 0.929 ( and ).
Figure 5. ROC Curves of IL-17, TNF-α and DKK-1 for the diagnosis of moderate to high activity RA.
Note: ROC, subject operating characteristic curve.
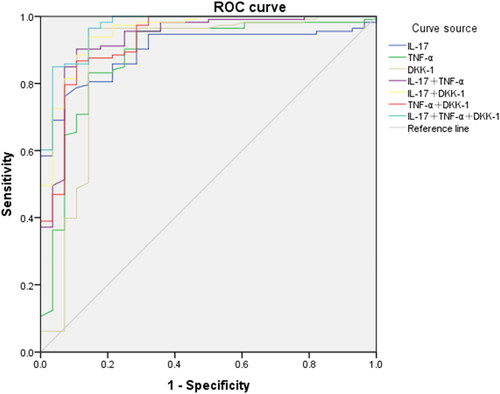
Table 3. Clinical evaluation of IL-17, TNF-α and DKK-1 in the diagnosis of moderate to high activity RA.
Discussion
RA is a chronic systemic autoimmune disease with an incidence of 0.5–1% of the world’s population [Citation15], with synovitis as the main lesion, accompanied by systemic inflammation and autoantibody production, and progressive RA can lead to bone and cartilage destruction and even joint deformity [Citation16]. Potential triggers for RA include autoantibodies and pro-inflammatory cytokines, with autoantibodies such as RF and Anti-CCP being the most well known of the specific autoantibodies, reflecting mature signs of joint inflammation [Citation17,Citation18]. However, these autoantibodies are also elevated in other non-RA patients, so conventional diagnostic markers are less specific in the diagnosis of RA and their changes as the disease progresses are unknown; cytokines regulate chronic inflammation and joint destruction and appear in the serum and arthritic synovial fluid of RA patients to further cause bone erosion and osteoclastogenesis [Citation19].
IL-17 is overexpressed in RA and is associated with joint inflammation and destruction, with biologically active IL-17 detected in the synovial fluid of RA patients; IL-17 induces the production of MMP-9 in human monocytes/macrophages and is associated with autocrine stimulation of TNF-α; IL-17 also induces TNF-α secretion by macrophages, and IL-17 acts synergistically with TNF-α to induce the release of IL-6, TNF-α and IL-1β to amplify the inflammatory process. In addition, by inducing receptor activator of nuclear factor κB ligand (RANKL), IL-17 is thought to have important effects on osteoclastogenesis and bone destruction, which may be the result of the imbalance in the production of pro-inflammatory cytokines and MMP-9 in diseased joint tissues through the interaction of IL-17 with macrophages in rheumatoid synovial membranes [Citation20,Citation21]. TNF-α plays a key role in the release of other cytokines and in the induction of chronic inflammation [Citation22], promotes inflammation, osteoclast formation, and subsequent joint tissue destruction and bone erosion within the peripheral joints, as well as the secretion of the Wnt inhibitor DKK-1 and blocks bone and cartilage formation [Citation23] .
The pro-inflammatory cytokines TNF-α and IL-17 directly or indirectly downregulate Wnt signalling and inhibit the Wnt/β-catenin signalling pathway [Citation7,Citation9], DKK-1 promotes synovial angiogenesis and is an inhibitor of Wnt signalling, which is a key process in the pathogenesis of RA [Citation24]. Like the pro-inflammatory cytokines TNF-α and IL-17 elevated in RA, CRP seems to rise, too. However, CRP is not only elevated in RA patients but also in the serum of infectious diseases, malignant tumours, and even normal people with low diagnostic specificity. Then this study not included it to discuss the association with disease activity of RA. The study by Diarra et al. [Citation12], also confirmed that cytokines such as TNF-α induce DKK-1 expression, and that DKK-1 and TNF-α are positively correlated with the disease process as bone destruction is gradually aggravated by irregular medication and treatment of patients. Systemic inhibition of TNF-α can reduce the level of DKK-1 in RA to almost normal; Fassio [Citation25] et al. found that after 2 months of treatment with the TNF-α inhibitor certolizumab, the serum levels of DKK-1 and sclerostin in RA patients decreased rapidly and were accompanied by significant changes in bone turnover, and Wang [Citation24] et al. also demonstrated that the use of the anti-TNF-α drug infliximab significantly reduced circulating levels of DKK-1, suggesting a close link between dysregulation of the Wnt signalling pathway in TNF-α-dependent inflammation and altered bone metabolism in RA. However, there is a lack of response to anti-TNF-α agents in patients with high baseline Th17(produce IL-17) cell levels, blocking IL-17A can inhibit the activity of synovial osteoclasts. IL-17 directly lead to the worsening of RA disease course, it is important factors that can be targeted to RA. There is hope that molecular therapy targeting IL-17 like Ixekizumab and secukinumab is necessary for autoimmune pathology [Citation26]. This article explores the clinical significance of IL-17, TNF-α and DKK-1 in the disease process and their role in arresting the disease process in RA by inhibiting signalling pathways and cytokines in the pathogenesis of the disease.
The results of the study showed that the levels of Anti-CCP, RF, CRP, ESR, were higher in RA patients in the moderate-to-high activity group than the controls, while IL-17, TNF-α and DKK-1 higher than the low-remission group and the controls (p < 0.05). As the degree of patients’ disease worsened, the results of Spearman correlation analysis showed that the expression levels of IL-17, TNF-α and DKK-1 were positively correlated with DAS28 (p < 0.05), the correlations of IL-17, TNF-α and DKK-1 with DAS28 scores, were all over 0.5, suggesting that high level of IL-17, TNF-α and DKK-1 can influence the severity of RA (p < 0.05). This is consistent with previous reports [Citation5,Citation27,Citation28]. The correlation between DKK-1 and DAS28 scores is highest, indicating that there is a strong correlation between serum DKK-1and disease activity of RA.
The ROC curve shows that the AUC of DKK-1 is the highest, followed by TNF-α, IL-17, Anti-CCP, CRP, RF and ESR, indicating that IL-17, TNF-αand DKK-1 diagnostic efficiency is more better. Evaluated each indices of diagnostic value shows that DKK-1 has the highest sensitivity and negative predictive value for RA, declared that DKK-1 could exclude false negative patients, improve the diagnosis rate of RA and delayed the progression of the disease. The specificity and positive predictive value of TNF-α is highest, declared that TNF-α specifically diagnosed RA and has an important predicted value.
According to the ROC curve showed that IL-17, TNF-α and DKK-1 had a predictive value for patients with moderate-to-high activity RA (p < 0.05), suggesting that combined diagnosis of IL-17, TNF-α and DKK-1 having the highest predictive value for patients with moderate-to-high RA, with an AUC of 0.968. The serum levels of IL-17, TNF-α and DKK-1 concentrations measured in this study were significantly higher in RA patients than patients in the low and remission and moderate RA group. The sensitivity of the combination of IL-17, TNF-α and DKK-1 is highest, 96.5%, which is a significant advantage in the combined diagnosis of RA. Thus, the combined detection of three bone and cartilage destruction-related indicators, IL-17, TNF-α and DKK-1, has diagnostic significance in moderate-to-high stage RA, and it is feasible to explore biological therapies such as IL-17 inhibitor therapy to modulate cytokines in disease pathogenesis for the diagnosis and treatment of RA, especially for patients with poor response to TNF-α indicators.
In conclusion, the concentration levels of all three indicators, IL-17, TNF-α and DKK-1, were elevated in the serum of RA patients in this study; the levels of IL-17, TNF-α and DKK-1 in the moderate and high stages were higher than low and remission activity RA and positively correlated with DAS28 scores, and the combination of the three could significantly improve the diagnosis rate of the disease and greatly improve the long-term quality of patients’ life.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the fundings of this study are availability from the corresponding author [QW], upon reasonable request.
Additional information
Funding
References
- Hulsmans H-M, Jacobs J-W, van der Heijde D-M, et al. The course of radiologic damage during the first six years of rheumatoid arthritis. Arthritis Rheum. 2000;43(9):1–7.
- Firestein G-S, McInnes I-B. Immunopathogenesis of rheumatoid arthritis. Immunity. 2017;46(2):183–196.
- Maruotti N, Corrado A, Neve A, et al. Systemic effects of wnt signaling. J Cell Physiol. 2013;228(7):1428–1432.
- Miao C-G, Yang Y-Y, He X, et al. Wnt signaling pathway in rheumatoid arthritis, with special emphasis on the different roles in synovial inflammation and bone remodeling. Cell Signal. 2013;25(10):2069–2078.
- Singh A, Gupta M-K, Mishra S-P. Study of correlation of level of expression of wnt signaling pathway inhibitors sclerostin and dickkopf-1 with disease activity and severity in rheumatoid arthritis patients. Drug Discov Ther. 2019;13(1):22–27.
- Seror R, Boudaoud S, Pavy S, et al. Increased dickkopf-1 in recent-onset rheumatoid arthritis is a new biomarker of structural severity. Data from the ESPOIR cohort. Sci Rep. 2016;6:18421.
- Munno OD, Ferro F. The effect of biologic agents on bone homeostasis in chronic inflammatory rheumatic diseases. Clin Exp Rheumatol. 2019;37(3):502–507.
- Cici D, Corrado A, Rotondo C, et al. Wnt signaling and biological therapy in rheumatoid arthritis and spondyloarthritis. Int J Mol Sci. 2019;20(22):5552.
- Xie W, Zhou L, Li S, et al. Wnt/β-catenin signaling plays a key role in the development of spondyloarthritis. Ann NY Acad Sci. 2016;1364(1):25–31.
- Ellwanger K, Saito H, Clement-Lacroix P, et al. Targeted disruption of the wnt regulator Kremen induces limb defects and high bone density. Mol Cell Biol. 2008;28(15):4875–4882.
- Fischer J-A, Hueber A-J, Wilson S, et al. Combined inhibition of tumor necrosis factor α and interleukin-17 as a therapeutic opportunity in rheumatoid arthritis: development and characterization of a novel bispecific antibody. Arthritis Rheumatol. 2015;67(1):51–62.
- Diarra D, Stolina M, Polzer K, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 2007;13(2):156–163.
- Fraenkel L, Bathon J-M, England B-R, et al. 2021 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2021;73(7):1108–1123.
- van Riel P-L, Renskers L. The disease activity score (DAS) and the disease activity score using 28 joint counts (DAS28) in the management of rheumatoid arthritis. Clin Exp Rheumatol. 2016;34(5 Suppl 101):S40–S44.
- Najm A, Masson F-M, Preuss P, et al. MicroRNA-17-5p reduces inflammation and bone erosions in mice with Collagen-Induced arthritis and directly targets the JAK/STAT pathway in rheumatoid arthritis fibroblast-like synoviocytes. Arthritis Rheumatol. 2020;72(12):2030–2039.
- Hu T, Liu Y, Tan L, et al. Value of serum collagen triple helix repeat containing-1(CTHRC1) and 14-3-3eta protein compared to anti-CCP antibodies and anti-MCV antibodies in the diagnosis of rheumatoid arthritis. Br J Biomed Sci. 2021;78(2):67–71.
- Volkov M, Karin-Anna S, Diane W. Autoantibodies and B cells: the ABC of rheumatoid arthritis pathophysiology. Immunol Rev. 2020;294(1):148–163.
- Tan L, Ouyang T, Li X, et al. Serum sirtuin-1 a potential marker in the diagnosis of rheumatoid arthritis. Autoimmunity. 2023;56(1):2181234.
- Wei S-T, Sun Y-H, Zong S-H, et al. Serum levels of IL-6 and TNF-alpha may correlate with activity and severity of rheumatoid arthritis. Med Sci Monit. 2015;21:4030–4038.
- Jovanovic D-V, Martel-Pelletier J, Di Battista J-A, et al. Stimulation of 92-kd gelatinase (matrix metalloproteinase 9) production by interleukin-17 in human monocyte/macrophages: a possible role in rheumatoid arthritis. Arthritis Rheumatism. 2000;43(5):1134–1144.
- Lee B, Jo Y, Kim G, et al. Specific inhibition of soluble gammac receptor attenuates Collagen-Induced arthritis by modulating the inflammatory T cell responses. Front Immunol. 2019;10:209.
- Lee S-J, Lee A, Hwang S-R, et al. TNF-alpha gene silencing using polymerized siRNA/thiolated glycol chitosan nanoparticles for rheumatoid arthritis. Mol Ther. 2014;22(2):397–408.
- Moelants E-A, Mortier A, Van Damme J, et al. Regulation of TNF-α with a focus on rheumatoid arthritis. Immunol Cell Biol. 2013;91(6):393–401.
- Wang S-Y, Liu Y-Y, Ye H, et al. Circulating dickkopf-1 is correlated with bone erosion and inflammation in rheumatoid arthritis. J Rheumatol. 2011;38(5):821–827.
- Fassio A, Adami G, Gatti D, et al. Inhibition of tumor necrosis factor-alpha (TNF-alpha) in patients with early rheumatoid arthritis results in acute changes of bone modulators. Int Immunopharmacol. 2019;67:487–489.
- Kuwabara T, Ishikawa F, Kondo M, et al. The role of IL-17 and related cytokines in inflammatory autoimmune diseases. Mediators Inflamm. 2017;2017:3908061.
- Ruaro B, Casabella A, Paolino S, et al. Dickkopf-1 (dkk-1) serum levels in systemic sclerosis and rheumatoid arthritis patients: correlation with the trabecular bone score (TBS). Clin Rheumatol. 2018;37(11):3057–3062.
- Zhang X, Yuan Y, Pan Z, et al. Elevated circulating IL-17 level is associated with inflammatory arthritis and disease activity: a meta-analysis. Clin Chim Acta. 2019;496:76–83.