Abstract
Increase of regulatory T cells (Tregs) in the tumour microenvironment predicts worse survival of patients with various types of cancer. Recently, B cells play a significant role in the maintenance of Treg cells. However, the relevance of regulatory B cells (Bregs) to tumour immunity in humans remains elusive. Flow cytometry analysis was used to detect the Bregs and Tregs. Double staining results illustrated that the proportion of Bregs and Tregs were prominently higher in cervical cancer than normal tissues. Increase of Bregs and Tregs in cervical cancer microenvironment was associated with poor survival. Furthermore, Bregs cocultured with cervical cancer cell lines increased and induced Tregs. To sum up, the increased expression of Bregs contributes to the differentiation of CD4+ T cells into Tregs in the cervical cancer.
Introduction
The cellular immunity and humoral immunity are involved in tumorigenesis and development. T cell-mediated cellular immunity is the main type of anti-tumour immunity. T cells have a variety of biological functions, such as direct killing of target cells, assisting, or inhibiting B cells to produce antibodies, responding to specific antigens and mitogens, and producing cytokines [Citation1–3]. The regulatory T cells (Tregs) derived from peripheral T cells mainly maintain peripheral immune tolerance and immune response homeostasis. The induction of Tregs mainly plays an inhibitory role in the anti-tumour immune response and then promotes the occurrence and development of tumours [Citation4–6].
Studies have shown that the proportion of Tregs in peripheral blood of cervical cancer patients is higher than that of healthy people, and it is closely related to the clinical stage of HR-HPV infection and the degree of lesions [Citation7]. The level of Treg-specific expression factor Foxp3 increased significantly with the increase of cervical lesion grade [Citation8]. This indicates that the increase in the proportion of Treg cells promotes cervical cancer. However, the precise mechanism of Tregs activation in cervial cancer microenvironment remains elusive.
B cells secrete specific antibodies and present antigens to promote immune response [Citation9,Citation10]. Recent studies have found that regulatory B cells (Bregs), a subset of B cells, plays a negative role in immune function regulation [Citation11–13]. In recent years, the role of Bregs in promoting immune escape and tumour growth has attracted increasing attention. It secretes interleukin 10 (IL-10), interleukin 35 (IL-35), and transforming growth factor-β (TGF-β), and inhibits anti-tumour immune effector cells to promote tumour cell proliferation and metastasis [Citation14–17].
Although studies have demonstrated a correlation between Bregs and immune escape, but the distribution and function of Bregs in human cervical cancer have not been elucidated. We hypothesised that the Bregs promote Tregs production in cervical cancer. In the present study, we aimed to illustrate the clinical relevance between Bregs and Tregs isolated from peripheral blood of patients with cervical cancer. In addition, in vitro experiments were performed to mimic the generation of Bregs in tumour microenvironment and elucidate the possible mechanism.
Methods and materials
Clinical research objects
This study has been approved by the Research Ethics Committee of Third Affiliated Hospital of Guangzhou Medical University [2022(128)], and all recruited subjects were fully informed of the information collected in this study and the use of clinical samples prior to signing the informed consent form. Peripheral blood (10 mL) was obtained from 53 patients with cervical cancer (none of them received chemotherapy or radiotherapy prior to surgical treatment) and 53 healthy volunteers. All patients underwent no chemotherapy or radiotherapy before surgical treatment. Tumor node metastasis (TNM) staging was determined according to the 2002 International Union against Cancer (UICC) TNM classification of malignant tumour. The mean follow-up was 49.5 months (range 2–80 months). Clinical information of the samples is summarised in .
Table 1. Clinicopathologic characteristics of study subjects.
Cell culture
Human cervical cancer cell lines, including SiHa, HeLa, CaSki, and SW756 were obtained from ATCC. The cervical epithelial immortalised cell H8 was used as the normal control. Cells were cultured in minimum essential medium (MEM)(Procell Life Science&Technology, Wuhan, Hubei, China) containing 10% foetal bovine serum (FBS) (Thermo Fisher Scientific, Rockford, IL, USA) and 1% penicillin-streptomycin (Hyclone, Logan, Utah, USA) with a 37 °C and 5% CO2 incubator.
Isolation and culture of Bregs
Fasting blood samples were collected from the patients, and peripheral blood monocytes were isolated by density gradient centrifugation. CD19 Bregs were isolated by a Mitenyi Biotech kit (Cologne, Bergesch Gladbach, Germany) by indirectly magnetically labelled antibodies against CD2, CD14, CD16, CD36, CD43, CD235a to the isolation of highly pure CD19+ B cells by depletion of magnetically labelled cells [Citation18]. After the purity reached 98% by flow cytometry analysis, cells (1 × 106 cell/mL) were divided into 11 groups: untreated control cells, cocultured with H8, SiHa, HeLa, CaSki, C33A, and SW756 cell lines at a ratio of 100:1, two positive control stimulated with lipopolysaccharide (LPS, 5 μg/mL; Sigma-Aldrich, Shanghai, China), CD40L (1 μg/mL, ab281905, Abcam, Shanghai, China), one negative control treated with PBS for 72 h. Anti-CD40L antibody (Santa Cruz Biotechnology, Dallas, Texas, USA) was added at a concentration of 20 μL/1 × 106 cells in the CD40L block groups.
Isolation and culture of Tregs
High-purity CD4+ T cells were isolated by removing magnetically labelled cells by negative selection using the Treg Cell Isolation Kit II (Miltenyi Biotech, Cologne, Bergesch Gladbach, Germany), followed by bead-conjugated anti-CD25 antibody for CD25+ cell depletion [Citation19]. An aliquot of CD4+CD25- cells (1 × 106 cell/mL) was stained with anti-human CD4-APC-CY7, CD25-PE, CD45RA-FITC antibody for 30 min and followed by flow cytometry analysis.
Flow cytometry analysis
The relative amounts of Bregs and Tregs in peripheral blood were measured by flow cytometry. Bregs were stained with anti-human CD19 FITC (ab1167, Abcam, Shanghai, China), CD27 PE-CY7 antibody (4 µL/106 cell, ab233581, Abcam, Shanghai, China) for 30 min. Then, cells were stained with anti-human IL-10 APC antibody (1/5000, ab221277, Abcam, Shanghai, China) followed by flow cytometry analysis. Tregs were stained with anti-human CD4-APC-CY7 (4 µL, ab233298, Abcam, Shanghai, China), CD25-PE (1/1000, ab283576, Abcam, Shanghai, China), CD45RA-FITC (ab18240, Abcam, Shanghai, China) antibody for 30 min and followed by flow cytometry analysis. Cells were obtained and analysed by CellQuest Pro software (BD Bioscience, Franklin Lakes, NJ, USA).
Coculture of Bregs and Tregs
Finally, the Bregs were cocultured with the CD4+CD25− Tregs in CD3 antibody-coated plate for 48 h. The cocultured cells were stained with anti-human CD4 APC-CY7 antibody for 30 min, then anti-human FOXP3-Alexa 488 (3 µg/mL, ab187598, Abcam, Shanghai, China) were used, followed by flow cytometry analysis. The anti-IL-10 antibody (1/200, ab133575, Abcam, Shanghai, China) was added to the IL-10 block group. Supernatant of the cocultured cells was analysed for IL-10, TGF-β, IFN-γ, and IL17 by ELISA (Dakewei, Shenzhen, Guangdong, China).
Statistical analysis
SPSS 25.0 was used to analyse the statistical data expressed as mean ± (SD). The χ2 tests were used to analyse the relationship between Tregs/Bregs expression and clinic pathologic characteristics. The prognosis of the patients was analysed by combining the survival of the included patients with the relative number of Bregs or Tregs. Survival curves were plotted by the Kaplan–Meier method and compared using the log-rank test. Spearman rank correlation test was used to analyse the relationship between Tregs and Bregs. Differences between the two groups were evaluated using Student’s t-test and one-way ANOVA for multiple groups. p < 0.05 was considered as significant. All experiments were repeated at least three times.
Results
The percentage of Bregs and Tregs are positively correlated in cervical cancer
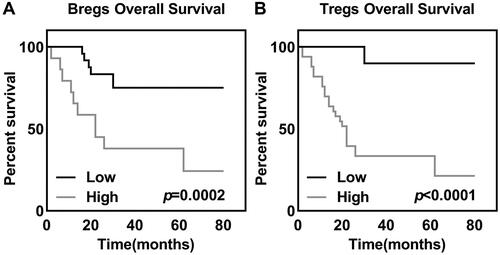
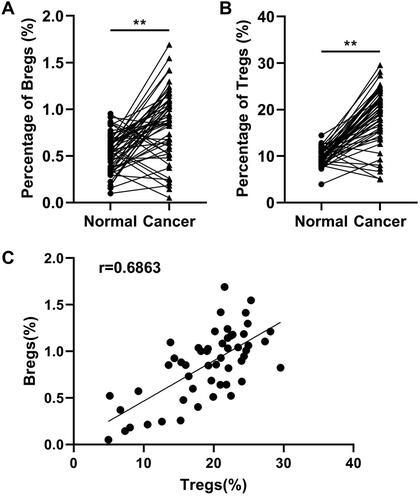
Firstly, data from flow cytometry illustrated that the proportion of Bregs () and Tregs () were both prominently higher than that in normal group. Result of Spearman rank correlation test illustrated that the distribution of Bregs and Tregs was correlative (r = 0.6863, confidence interval: 0.5108 to 0.8069). High expression of Bregs suggested the high expression of Tregs. The distribution of Bregs and Tregs in cervical cancer was inversely associated with the overall survival of cervical cancer patient, as revealed by the results of Kaplan–Meier analysis and log-rank test. The overall survival of Bregs (, p = 0.0002) and Tregs (, p = 0.0003) low expression group were higher than that of the corresponding high expression groups in cervical cancer. Furthermore, analysis of the clinicopathologic characteristics of patients obtained revealed that high Bregs and Tregs may associated with higher clinical stages of cervical cancer ().
Figure 1. Percentage of (A) Bregs and (B) Tregs in cervical cancer. (C) Positive association between Bregs and Tregs (r = 0.6863, confidence interval: 0.5108–0.8069). The t-test was used to analyse data between normal and cancer groups, and spearman rank correlation test was used to analyse the relationship between Tregs and Bregs. **p < 0.01.
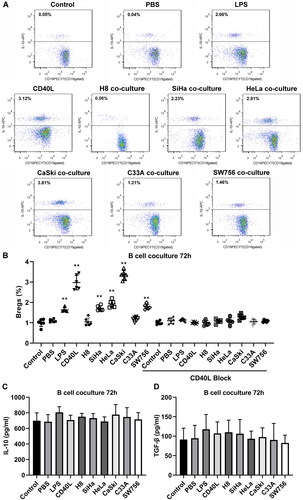
Cervical cancer cells promote the generation of IL-10+CD19+ B cells
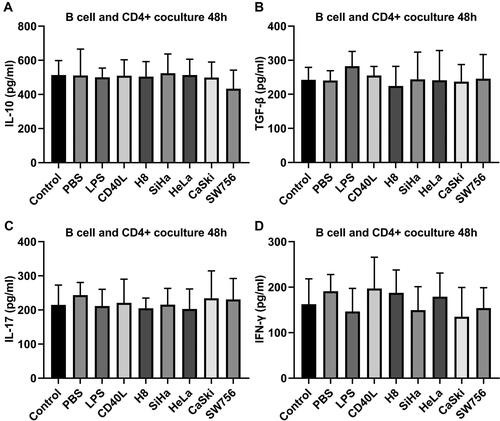
IL-10+CD19+ Bregs were separated by flow cytometry (), and phenotypes of the cells were subsequently identified. The results showed that the separated Bregs were enriched in CD27+ (). Then, the sorted CD19+ Bregs were cocultured with different cervical cancer cell lines and normal cervical epithelial cells. 72 h later, percentage of IL-10+CD19+ Bregs in LPS, CD40L, SiHa, HeLa, CaSki, and SW756 groups was prominently higher than the PBS control group. However, the addition of anti-CD40L antibody reduced the induction effect of Bregs ( and ). Furthermore, secretion of IL-10 () and TGF-β () of IL-10+CD19+ Bregs were detected in the supernatant of each group mentioned above, and the concentration of two factors was not significantly changed.
Figure 3. B Cells were stained for CD19 (A) before and after the sorting, and (B) the relative number and phenotype of Bregs in the sorted B cells were determined before cocultivation.
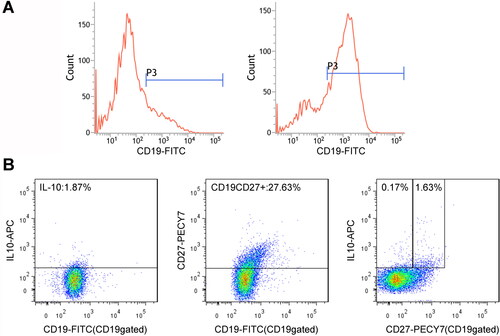
Figure 4. (A) Flow cytometry of sorted CD19+Bregs after 72 h coculturing with cervical cancer cells, and the surfaces of the cells were stained for CD19 expression and cytoplasmic IL-10. (B) The scatter diagram shows the Breg frequencies from 10 subjects. (C) IL-10 and (D) TGF-β production in the supernatant of each group after coculture for 72 h. Differences between multiple groups were evaluated using one-way ANOVA analysis. **p < 0.01.
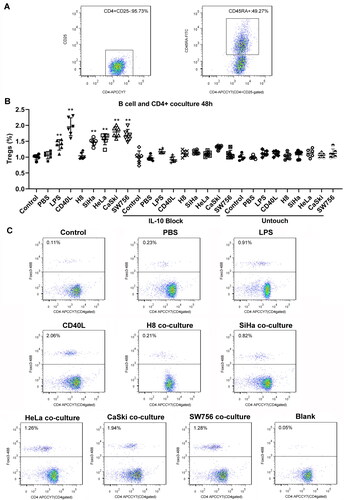
Bregs induce the generation of CD4+Foxp3+ Tregs
Phenotypic staining of sorted T cell was performed before cocultivation, and naive T cells CD4+CD25-CD45+ was about 49.27% (). Then nine groups of Bregs were cocultured with CD4+CD25− T cells in the presence of plated-CD3 antibody for 48 h; the results of flow cytometry analysis showed the percentage of CD4+Foxp3+ Tregs of SiHa, HeLa, CaSki, and SW756 stimulated Breg coculture group. LPS and CD40L-stimulated Breg groups were higher than control Breg group. Coculture with normal cells did not increase Breg. However, the addition of anti-IL10 antibody or untouched coculture did not largely affect the Tregs induction ( and ). ELISA assay demonstrated that there was no significant differences between IL-10 (), TGF-β (), IL-17 (), and IFN-γ () expression in the supernatant of each group.
Figure 5. Foxp3 expression in sorted CD4+ T cells after coculture with stimulated B cells 48 h. (A) Relative number of CD4+CD25-CD45+ T cells in sorted CD4+CD25-T cells before coculture. (B-C) Sorted CD4+CD25− T cells were cocultured with B cells, then stained for CD4 and Foxp3 expression. Differences between multiple groups were evaluated using one-way ANOVA analysis. **p < 0.01.
Discussion
Currently, little is known about the potential role of Bregs in the microenvironment of cervical cancer. The study determined that increased Bregs and Tregs were positively linked and negatively correlated with overall survival of patients with cervical cancer. Meanwhile, Bregs participate in transforming CD4+CD25-T cells into Tregs.
Studies on cervical cancer have proved that the proportion of Tregs in peripheral blood of cervical cancer patients is higher than that of healthy people, which is closely related to high risk human papillomavirus infection, clinical stage and lesion degree, and can be used as a biological indicator of lesion progression [Citation7]. Besides, Tregs were highly expressed in positive lymph nodes with lymph node metastasis of cervical cancer, and CD8+T cells/Tregs decreased prominently, increasing the risk of deterioration [Citation20]. This indicates that the proportion of Tregs increases with the continuous tumour progression of cervical cancer, forming immune tolerance and promoting the occurrence of cervical cancer. Moreover, a number of studies believe that with the progression and degeneration of cervical lesions, CD4 +/CD8+ values decline, resulting in poor prognosis [Citation21–23]. In the current work, our data also illustrated that increased Tregs indicated poor survival of patients with cervical cancer. Hence, reasonably weakening the inhibitory effect of Tregs may be a potential strategy for the treatment of human cancers.
In hepatocellular carcinoma, FcγRIIlow/Bregs are selectively accumulated in tumour area, non-tumour area and tumour stroma area. It is noteworthy that the percentage of FcγRIIlow/Bregs in the blood of the patients is higher than that of the normal person, but the amount of FcγRIIlow/Bregs in the liver is the same as that of the normal person [Citation24]. The PD-1/Bregs were found to be mainly concentrated in the border area between tumour tissues and adjacent normal tissues, suggesting that the microenvironment of different local regions of tumour may be regulated by different inducers [Citation25,Citation26]. Furthermore, tumour-associated Bregs show increased IL-10 and IL-35 secretion, which involves multiple pathways [Citation24,Citation25,Citation27]. Although the specific pathway of increased interleukin secretion cannot be fully understood by current studies, it has been confirmed that the increased secretion of IL-10 and IL-35 in local tumours by Bregs is correlated with poor prognosis and relapse of tumour patients [Citation28,Citation29]. Our data also demonstrated that high Bregs were associated with poor survival of cervical cancer patients.
Locally, Bregs binds to the IL-10 receptor on the surface of T cells and the IL-10 signal in the tumour through IL-10 and inhibits the proliferation and function of T cells in vivo, such as inhibiting the secretion of IFN-γ and TNF-α by CD4+T cells. CD8+T cells secreted TNF-α, IFN-γ cells, toxic granase B, and perforin [Citation30]. Furthermore, CD4+T cells can be converted into Tregs in an IL-10-dependent manner, leading to the increase of Tregs in tumour area and even in the whole body. Tumour-associated Tregs inhibit T cells and promote proliferation and progression of tumour cells by secreting IL-10 and mechanisms related to cell-cell contact [Citation29,Citation31]. In current work, we found that Bregs induced Tregs in cervical cancer microenvironment by releasing IL-10, indicating that inhibiting Bregs may be a novel method for the treatment of tumours.
Conclusion
Taken together, Bregs increased in cervical cancer microenvironment and converted resting CD4+T cells to Tregs by IL-10 secretion. Furthermore, high Bregs and Tregs predicted poor prognosis of cervical cancer patients. Bregs-targeted therapy may be a novel method for cervical cancer treatment.
Competing interests
The authors declare that they have no competing interests.
Acknowledgements
The authors thank the patient, who agreed to allow us to publish the clinical data.
Data availability statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Dumauthioz N, Labiano S, Romero P. Tumor resident memory T cells: new players in immune surveillance and therapy. Front Immunol. 2018;9:1.
- Terren I, Orrantia A, Vitalle J, et al. NK cell metabolism and tumor microenvironment. Front Immunol. 2019;10:2278.
- DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19(6):369–8.
- St PM, Ohashi PS. The roles of CD8(+) T cell subsets in antitumor immunity. Trends Cell Biol. 2020;30(9):695–704.
- Kelliher MA, Roderick JE. NOTCH signaling in T-cell-mediated anti-tumor immunity and T-cell-Based immunotherapies. Front Immunol. 2018;9:1718.
- Demaria O, Cornen S, Daeron M, et al. Harnessing innate immunity in cancer therapy. NATURE. 2019;574(7776):45–56.
- Zur HH. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–350.
- Ma Q, Zhao M, Wei X, et al. Expressions of immune negative regulator FoxP3 + treg and PD-L1 protein in the immune microenvironment of cervical lesion. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2017;39:128–132.
- Ma Z, Zhang E, Gao S, et al. Toward a functional cure for hepatitis B: the rationale and challenges for therapeutic targeting of the B cell immune response. Front Immunol. 2019;10:2308.
- Eibel H, Kraus H, Sic H, et al. B cell biology: an overview. Curr Allergy Asthm R. 2014;14:434.
- Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. IMMUNITY. 2015;42(4):607–612.
- Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30(1):221–241.
- Catalan D, Mansilla MA, Ferrier A, et al. Immunosuppressive mechanisms of regulatory B cells. Front Immunol. 2021;12:611795.
- Wang Z, Cheng Q, Tang K, et al. Lipid mediator lipoxin A4 inhibits tumor growth by targeting IL-10-producing regulatory B (breg) cells. Cancer Lett. 2015;364(2):118–124.
- Hu H, Ai X, Lu M, et al. Characterization of intratumoral and circulating IL-10-producing B cells in gastric cancer. Exp Cell Res. 2019;384(2):111652.
- Liu S, Yang L, Jia S, et al. Interleukin-35 suppresses the activity of natural killer-like B cells in patients with hepatocellular carcinoma. Int Immunopharmacol. 2021;100:108161.
- Manna A, Kellett T, Aulakh S, et al. Targeting CD38 is lethal to breg-like chronic lymphocytic leukemia cells and Tregs, but restores CD8+ T-cell responses. Blood Adv. 2020;4(10):2143–2157.
- Alaqla A, Hu Y, Huang S, et al. TLR9 signaling is required for the porphyromonas gingivalis -induced activation of IL-10-expressing B cells. Int J Mol Sci. 2023;24(7):6693.
- Lv Y, Tian W, Teng Y, et al. Tumor-infiltrating mast cells stimulate ICOS(+) regulatory T cells through an IL-33 and IL-2 axis to promote gastric cancer progression. J Adv Res. 2023;S2090-1232(23)00119-4.
- Heeren AM, de Boer E, Bleeker MC, et al. Nodal metastasis in cervical cancer occurs in clearly delineated fields of immune suppression in the pelvic lymph catchment area. Oncotarget. 2015;6(32):32484–32493.
- Das D, Sarkar B, Mukhopadhyay S, et al. An altered ratio of CD4+ and CD8+ T lymphocytes in cervical cancer tissues and peripheral blood – a prognostic clue? Asian Pacific Journal of Cancer Prevention: APJCP. 2018;19:471–478.
- Spinillo A, Dominoni M, Boschi AC, et al. Clinical significance of the interaction between human papillomavirus (HPV) type 16 and other high-risk human papillomaviruses in women with cervical intraepithelial neoplasia (CIN) and invasive cervical cancer. J Oncol. 2020;2020:6508180–6508189.
- McClymont E, Lee M, Elwood C, et al. Cervical cancer screening in immunocompromised women. J Obstet Gynaecol Can. 2019;41(8):1177–1180.
- Ouyang FZ, Wu RQ, Wei Y, et al. Dendritic cell-elicited B-cell activation fosters immune privilege via IL-10 signals in hepatocellular carcinoma. Nat Commun. 2016;7(1):13453.
- Xiao X, Lao XM, Chen MM, et al. PD-1hi identifies a novel regulatory B-cell population in human hepatoma that promotes disease progression. Cancer Discov. 2016;6(5):546–559.
- Shao Y, Lo CM, Ling CC, et al. Regulatory B cells accelerate hepatocellular carcinoma progression via CD40/CD154 signaling pathway. Cancer Lett. 2014;355(2):264–272.
- Zhang L, Tai Y, Ho M, et al. Regulatory B cell-myeloma cell interaction confers immunosuppression and promotes their survival in the bone marrow milieu. Blood Cancer J. 2017;7(3):e547-e547.
- Zhao Z, Chen X, Hao S, et al. Increased interleukin-35 expression in tumor-infiltrating lymphocytes correlates with poor prognosis in patients with breast cancer. CYTOKINE. 2017;89:76–81.
- Zhou X, Su YX, Lao XM, et al. CD19(+)IL-10(+) regulatory B cells affect survival of tongue squamous cell carcinoma patients and induce resting CD4(+) T cells to CD4(+)Foxp3(+) regulatory T cells. Oral Oncol. 2016;53:27–35.
- Li Y, An J, Huang S, et al. Esophageal cancer-derived microvesicles induce regulatory B cells. Cell Biochem Funct. 2015;33(5):308–313.
- Mao FY, Kong H, Zhao YL, et al. Increased tumor-infiltrating CD45RA(-)CCR7(-) regulatory T-cell subset with immunosuppressive properties foster gastric cancer progress. Cell Death Dis. 2017;8(8):e3002–e3002.