Abstract
Circular RNA (circRNA) has been found to be differentially expressed and involved in regulating the processes of human diseases, including thoracic aortic dissection (TAD). However, the role and mechanism of circNRIP1 in the TAD process are still unclear. GEO database was used to screen the differentially expressed circRNA and mRNA in type A TAD patients and age-matched normal donors. Angiotensin II (Ang II)-induced human aortic vascular smooth muscle cells (HA-VSMCs) were used to construct TAD cell models. The expression levels of circNRIP1, NRIP1, CXC-motif chemokine 5 (CXCL5) and IGF2BP1 were detected by quantitative real-time PCR. Cell proliferation and migration were determined by EdU assay, transwell assay and wound healing assay. The protein levels of synthetic phenotype markers, contractile phenotype markers, CXCL5 and IGF2BP1 were tested by western blot analysis. The interaction between IGF2BP1 and circNRIP1/CXCL5 was confirmed by RIP assay, and CXCL5 mRNA stability was assessed by actinomycin D assay. CircNRIP1 was upregulated in TAD patients and Ang II-induced HA-VSMCs. Knockdown of circNRIP1 suppressed Ang II-induced proliferation, migration and phenotypic switch of HA-VSMCs. Also, high expression of CXCL5 was observed in TAD patients, and its knockdown could inhibit Ang II-induced HA-VSMCs proliferation, migration and phenotypic switch. Moreover, CXCL5 overexpression reversed the regulation of circNRIP1 knockdown on Ang II-induced HA-VSMCs functions. Mechanistically, circNRIP1 could competitively bind to IGF2BP1 and subsequently enhance CXCL5 mRNA stability. CircNRIP1 might contribute to TAD progression by promoting CXCL5 mRNA stability via recruiting IGF2BP1.
Introduction
Thoracic aortic dissection (TAD) is one of the deadliest aortic diseases because of its acute onset, fast progression, and high rate of aortic rupture [Citation1,Citation2]. The pathological condition of TAD is mechanical stress and degradation of the inner wall of the aorta, in which vascular smooth muscle cells (VSMCs) are involved in this process [Citation3]. VSMCs are the main cell type of the aortic media, which is essential for maintaining the structural and functional integrity of the aortic wall [Citation4]. Under various pathologic conditions, VSMCs change from normal contractile phenotype to synthetic phenotype with high proliferation and migration, and this phenotypic switch is considered to be the root cause of vascular disease [Citation5,Citation6]. Currently, angiotensin II (Ang II)-induced VSMCs is widely used to construct TAD cell models in vitro [Citation7,Citation8]. Therefore, it is essential to investigate the molecular mechanisms affecting Ang II-induced VSMCs proliferation, migration and phenotypic switch in order to find more effective molecular targets for TAD treatment.
Circular RNAs (circRNAs), a class of non-coding RNAs with a closed-loop structure [Citation9–11], have been shown to be strongly associated with vascular disease [Citation12,Citation13]. The dysregulation of circRNAs has also been reported in TAD [Citation14]. Xu et al. showed that circ_TGFBR2 was lowly expressed in TAD patients, which suppressed VSMCs proliferation, migration and phenotypic switch to restrain TAD progression [Citation15]. Here, we analysed differentially expressed circRNAs between normal donors and TAD patients in GEO database (GSE97745), and found that circNRIP1 expression was significantly increased in TAD patients. However, the role and mechanism of circNRIP1 in TAD progression have not been reported.
CXC-motif chemokine 5 (CXCL5) is a member of the CXC-class chemokine family and has granulocyte chemotaxis and infiltration [Citation16,Citation17]. Previous studies have shown that CXCL5 expression is associated with a variety of diseases, such as neonatal platelet transfusion [Citation18], multiple sclerosis [Citation19], and cerebral ischaemic injury [Citation20]. It had been indicated that CXCL5 inhibition decreased MMP9 expression and neutrophil infiltration to alleviate acute AD development [Citation21]. Therefore, CXCL5 may be a potential therapeutic target for TAD. In this, we found that circNRIP1 could interact with RNA binding protein IGF2BP1 to regulate CXCL5 mRNA stability. However, whether circNRIP1 mediates Ang II-induced VSMCs functions by regulating IGF2BP1/CXCL5 axis is unclear.
The aim of this study was to investigate the role of circNRIP1 in TAD progression and its underlying mechanism. Through analysing, we proposed the following hypothesis and verified that circNRIP1 increased CXCL5 mRNA stability through the recruitment of IGF2BP1, thereby promoting Ang II-induced VSMCs proliferation, migration, and phenotypic switch.
Materials and methods
GEO database
In GEO database (accession GSE97745), we screened the differentially expressed circRNA in type A TAD patients (n = 3) and age-matched normal donors (NA; n = 3) using GEO2R analysis tool (|log2FC|>1 and p < 0.05). Besides, the differentially expression of mRNA in TAD patients and NA was analysed in GSE107844 dataset using GEO2R analysis tool (|log2FC|>1 and p < 0.05). The results were expressed as volcano plots.
Samples collection
After surgical resection, aortic specimens were obtained from 33 type A TAD patients (undergoing aortic replacement) and 33 non-TAD normal donors (NA) between October 2019 and May 2021 at Sinopharm Dongfeng General Hospital, Hubei University of Medicine. The clinical and demographic characteristics of the patients with TAD and NA were shown as . Each participate gave informed signed consent before donating tissue. This study was approved by the Ethics Committee of Sinopharm Dongfeng General Hospital, Hubei University of Medicine.
Table 1. The clinical and demographic characteristics of the patients with TAD and NA.
Cell culture, treatment and transfection
Human aortic-VSMCs (HA-VSMCs, ATCC, Manassas, VA, USA) were cultured in a complete medium composed of F-12K medium (ATCC) according to the manufacturer’s recommendation. For mimic TAD cell models in vitro, HA-VSMCs were induced with 0.1 μM Ang II (Yuanye Bio-Technology, Shanghai, China) for 24 h. HA-VSMCs were transfected with circNRIP1 siRNAs (si-circNRIP1#1: 5′-GACAGACGGAAGTGTTTGGATdTdT-3′; si-circNRIP1#2: 5′-ACAGACGGAAGTGTTTGGATTdTdT-3′), CXCL5 siRNAs (si-CXCL5#1: 5′-GAGAGAGCTGCGTTGCGTTTGTTTA-3′; si-CXCL5#2: 5′-GAGAGCTGCGTTGCGTTTGTTTACA-3′), CXCL5 overexpression vector (oe-CXCL5), IGF2BP1 siRNA (si-IGF2BP1: 5′-CCGGGAAAGTAGAATTACAAGGAAA-3′) and their controls (si-NC and vector) (RiboBio, Guangzhou, China) by Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA).
Quantitative real-time PCR (qRT-PCR)
Total RNAs were extracted by TRIzol reagent (Invitrogen) and then used for reverse transcription by PrimeScript RT reagent Kit (Takara, Dalian, China). PCR reaction was performed using SYBR Green with specific primers (). Fold changes of circNRIP1, NRIP1, CXCL5, IGF2BP1, GAPDH and U6 were calculated by 2−ΔΔCt method.
Table 2. Primer sequences used for qRT-PCR.
Identification of circRNA
The PCR amplification products of circNRIP1 from cDNA or gDNA using divergent primers or convergent primers were used for performing agarose gel electrophoresis. Besides, RNA extracted from HA-VSMCs was incubated with or without RNase R and then used for qRT-PCR. Additionally, the nuclear and cytoplasm RNAs of HA-VSMCs were isolated by PARIS Kit (Invitrogen) and then used for qRT-PCR.
EdU assay
HA-VSMCs in 96-well plates were incubated with EdU and stained by DAPI solution using EdU Assay Kit (Ribobio). Fluorescence images were captured under the microscope to calculate EdU positive cell rate (%).
Transwell assay
HA-VSMCs with serum-free medium were added in the upper of transwell chambers (Corning Inc., Corning, NY, USA). After cultured 24 h under the condition of lower component filled with completed medium, cells were fixed with 4% paraformaldehyde and stained by crystal violet. Migrated cell number was counted using a microscope.
Wound healing assay
HA-VSMCs were grown in 12-well plates and cultured until 90 confluences. Cell layers were scraped using 200 μL pipette tips, and then the cells were cultured with serum-free medium for 24 h. Images were captured under a microscope at 0 and 24 h to count wound confluence rate (%).
Western blot (WB) analysis
Proteins were isolated by RIPA lysis buffer (Beyotime, Shanghai, China), and then the protein samples were separated by SDS-PAGE gel and transferred onto PVDF membranes. After blockage, membrane was incubated with primary antibodies (Abcam, Cambridge, MA, USA) targeting MMP9 (1:5000, ab76003), CX43 (1:2000, ab11370), α-SMA (1:1000, ab5694), SM22α (1:1000, ab14106), CXCL5 (1:1000, ab126763), IGF2BP1 (1:1000, ab290736) or GAPDH (1:2500, ab9485). After incubated with secondary antibody (1:50000, ab205718), protein bands were visualised using ECL reagent (Millipore, Billerica, MA, USA).
RIP assay
HA-VSMCs were transfected with or without si-NC/si-circNRIP1, followed by treated with or without Ang II. The lysates of HA-VSMCs were incubated with magnetic beads (Millipore) pre-coated with anti-IgG and anti-IGF2BP1. Then, the immunoprecipitated RNA was collected for qRT-PCR to measure the enrichments of circNRIP1 and CXCL5 mRNA.
mRNA stability assay
HA-VSMCs transfected with si-NC/si-circNRIP1/si-IGF2BP1 were incubated with Actinomycin D reagent (Sigma-Aldrich, St. Louis, MO, USA). At each indicated time point (0, 4, 8 and 12 h), cells were collected for detecting CXCL5 mRNA expression using qRT-PCR.
Statistical analysis
All data were stated as mean ± SD, and GraphPad Prism 7.0 was utilised for data analysis. The differences between groups were compared by Student’s t-test or ANOVA. The correlation between circNRIP1 and CXCL5 expression was analysed by Pearson correlation coefficient. The difference at p < 0.05 was considered statistically significant.
Results
CircNRIP1 was upregulated in TAD patients and Ang II-induced HA-VSMCs
In GSE97745 dataset, volcano plots showed the greater upregulation of circNRIP1 expression in the TAD group than in the NA group (). Through analysing, circNRIP1 is located at chr21 and is formed by back-splicing of the exons 2-3 of NRIP1 gene (). After amplified by the cDNA or gDNA using convergent primers or divergent primers, we found that circNRIP1 could be amplified by divergent primers in cDNA but not in gDNA (). Besides, circNRIP1 could resist to RNase R digestion (), and was mainly distributed in the cytoplasm (). These results confirmed that circNRIP1 was indeed a circRNA with a circular structure. Additionally, we detected that circNRIP1 was highly expressed in the aortic specimens of TAD patients and Ang II-induced HA-VSMCs (). Through analysing the correlation of circNRIP1 expression with clinicopathologic features in patients of TAD, we found that high circNRIP1 expression was associated with the hypertension and D-dimer of TAD patients (). These data suggested that circNRIP1 might participate in regulating TAD progression.
Figure 1. CircNRIP1 expression in TAD patients and Ang II-induced HA-VSMCs. (A) Volcano plots showed the differentially expressed circRNAs in TAD patients and NA in GSE97745 dataset. (B) The basic information of circNRIP1. (C) After amplified by the cDNA or gDNA using convergent primers or divergent primers, PCR products were used for agarose gel electrophoresis. (D) RNase R treatment was used to detect the resistance of circNRIP1 on RNase R digestion. (E) Subcellular localisation assay was used to analyse circNRIP1 expression in cytoplasm and nucleus. (F) CircNRIP1 expression in the aortic specimens of TAD patients and NA was analysed by qRT-PCR. (G) CircNRIP1 expression in HA-VSMCs treated with or without Ang II was detected by qRT-PCR. *p < 0.05, **p < 0.01, ***p < 0.001.
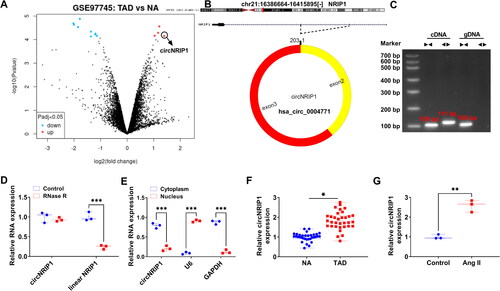
Table 3. Correlation of circNRIP1 expression with clinicopathologic features in patients of TAD.
CircNRIP1 knockdown suppressed Ang II-induced proliferation, migration and phenotypic switch of HA-VSMCs
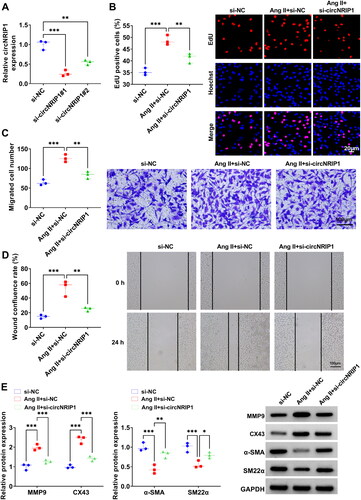
To explore circNRIP1 roles in TAD progression, we constructed the siRNAs (si-circNRIP1#1/#2) of circNRIP1 to silence circNRIP1 in HA-VSMCs. We found that both siRNAs significantly inhibited circNRIP1 expression without affecting NRIP1 mRNA expression, with si-circNRIP1#1 showing the best knocking down efficiency for circNRIP1 expression ( and Supplementary Figure 1A). Therefore, si-circNRIP1#1 (abbreviated si-circNRIP1) was used for subsequent functional studies. After Ang II treatment, we confirmed that the transfection of si-circNRIP1 markedly reduced circNRIP1 expression without affecting NRIP1 mRNA expression in HA-VSMCs (Supplementary Figure 1B and C). Functional experiments showed that Ang II treatment promoted EdU positive cell rate, migrated cell numbers and wound healing rate of HA-VSMCs, while circNRIP1 knockdown repressed Ang II-induced proliferation and migration in HA-VSMCs (). Besides, Ang II treatment could enhance the protein levels of synthetic phenotype markers (MMP9 and CX43) and reduce the protein levels of contractile phenotype markers (α-SMA and SM22α), whereas circNRIP1 knockdown also inhibited Ang II-induced phenotypic switch of HA-VSMCs (). These data revealed that circNRIP1 might promote Ang II-induced HA-VSMCs proliferation, migration and phenotypic switch.
Figure 2. Effect of si-circNRIP1 knockdown on Ang II-induced HA-VSMCs functions. (A) The transfection efficiencies of si-circNRIP1#1 and si-circNRIP1#2 were detected by qRT-PCR. (B-E) HA-VSMCs were transfected with si-NC/si-circNRIP1 followed by induced with Ang II. EdU assay (B), transwell assay (C) and wound healing assay (D) were used to measure cell proliferation and migration. (E) Protein levels of MMP9, CX43, α-SMA and SM22α were assessed by WB analysis. *p < 0.05, **p < 0.01, ***p < 0.001.
CXCL5 was highly expressed in TAD and was positively regulated by circNRIP1
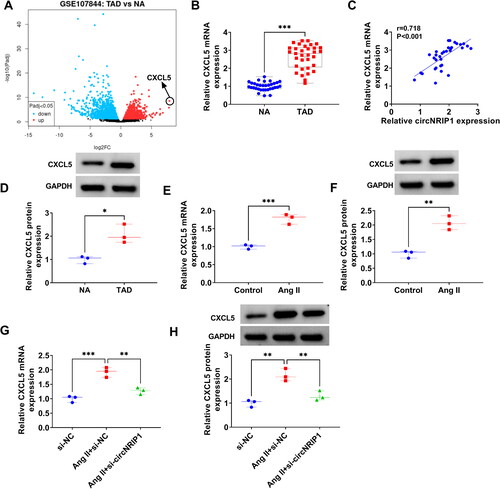
Volcano plots showed the differentially expressed mRNAs in GSE107844 dataset, in which CXCL5 expression was the most upregulated in TAD patients (). In the aortic specimens of TAD patients, CXCL5 was markedly overexpressed, and Pearson correlation coefficient confirmed that its expression was positively correlated with circNRIP1 expression (). At the protein level, we also observed the high expression of CXCL5 in TAD patients (). Also, CXCL5 expression was enhanced in Ang II-induced HA-VSMCs at the mRNA and protein levels (). In addition, we found that si-circNRIP1 could reduce CXCL5 mRNA and protein levels in Ang II-induced HA-VSMCs (), confirming that CXCL5 expression was positively regulated by circNRIP1.
Figure 3. CXCL5 expression in TAD patients and Ang II-induced HA-VSMCs. (A) Volcano plots showed the differentially expressed mRNAs in TAD patients and NA in GSE107844 dataset. (B) CXCL5 mRNA expression in the aortic specimens of TAD patients and NA was detected by qRT-PCR. (C) Pearson correlation coefficient was used for assessing the correlation of CXCL5 mRNA expression and circNRIP1 expression in TAD patients. (D) CXCL5 protein expression was detected by WB analysis in the aortic specimens of TAD patients and NA. (E-F) CXCL5 mRNA and protein expression in HA-VSMCs treated with or without Ang II was examined by qRT-PCR and WB analysis. (G-H) CXCL5 mRNA and protein expression was tested by qRT-PCR and WB analysis in Ang II-induced HA-VSMCs transfected with si-NC/si-circNRIP1. *p < 0.05, **p < 0.01, ***p < 0.001.
Silencing of CXCL5 inhibited Ang II-induced HA-VSMCs proliferation, migration and phenotypic switch
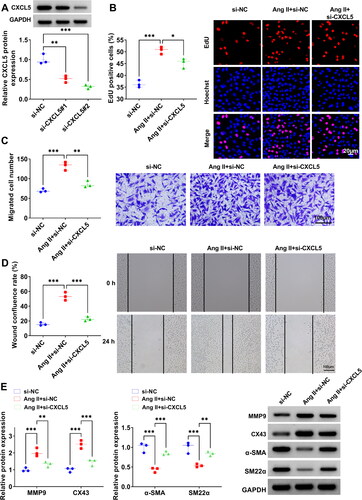
To investigate the function of CXCL5 in TAD progression, the siRNAs of CXCL5 (si-CXCL5#1/#2) were constructed and then transfected into HA-VSMCs. By evaluating the knocking down efficiency, we determined that si-CXCL5#2 had the best inhibition effect on CXCL5 protein expression (), so si-CXCL5#2 (abbreviated si-CXCL5) was used for functional studies. As shown in , CXCL5 knockdown inhibited EdU positive cell rate, migrated cell numbers and wound healing rate in Ang II-induced HA-VSMCs. WB analysis revealed that CXCL5 knockdown reduced MMP9 and CX43 protein levels, while promoted α-SMA and SM22α protein levels in Ang II-induced HA-VSMCs (). These results suggested that CXCL5 might contribute to Ang II-induced HA-VSMCs functions.
Figure 4. Effect of si-CXCL5 on Ang II-induced HA-VSMCs functions. (A) The transfection efficiencies of si-CXCL5#1 and si-CXCL5#2 were assessed by WB analysis. (B-E) HA-VSMCs were transfected with si-NC/si-CXCL5 followed by induced with Ang II. Cell proliferation and migration were determined by EdU assay (B), transwell assay (C) and wound healing assay (D). (E) WB analysis was used to test the protein levels of MMP9, CX43, α-SMA and SM22α. *p < 0.05, **p < 0.01, ***p < 0.001.
CircNRIP1 mediated Ang II-induced HA-VSMCs functions by regulating CXCL5 expression
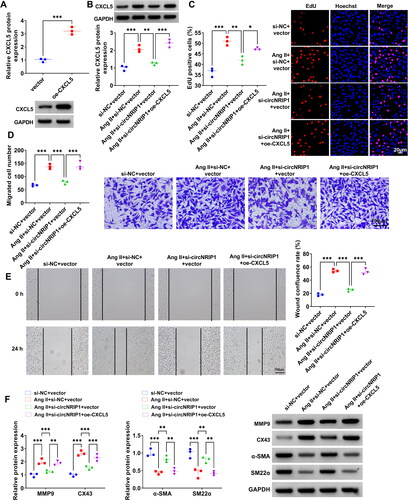
Further analysis was performed to explore whether circNRIP1 regulated Ang II-induced HA-VSMCs functions by CXCL5. In this, oe-CXCL5 was used to enhance CXCL5 protein expression in HA-VSMCs (). Then, HA-VSMCs were co-transfected with si-circNRIP1 and oe-CXCL5 followed by induced with Ang II. The detection of CXCL5 protein expression indicated that si-circNRIP1 significantly reduced CXCL5 protein expression in Ang II-induced HA-VSMCs, and this effect was abolished by oe-CXCL5 (). Functional experiments suggested that the suppressive effect of si-circNRIP1 on EdU positive cell rate, migrated cell numbers and wound healing rate in Ang II-induced HA-VSMCs could be eliminated by CXCL5 overexpression (). Moreover, downregulation of circNRIP1 also decreased synthetic phenotype markers (MMP9 and CX43) protein levels and increased contractile phenotype markers (α-SMA and SM22α) protein levels in Ang II-induced HA-VSMCs, while these effects were reversed by overexpressing CXCL5 (). Above all, circNRIP1 knockdown repressed Ang II-induced HA-VSMCs functions via reducing CXCL5 expression.
Figure 5. Effects of si-circNRIP1 and oe-CXCL5 on Ang II-induced HA-VSMCs functions. (A) The transfection efficiency of oe-CXCL5 was evaluated by WB analysis. (B-F) HA-VSMCs were transfected with si-NC/si-circNRIP1/vector/oe-CXCL5 followed by induced with Ang II. (B) CXCL5 protein expression was examined by WB analysis. EdU assay (C), transwell assay (D) and wound healing assay (E) were performed to detect cell proliferation and migration. (F) Protein levels of MMP9, CX43, α-SMA and SM22α were measured using WB analysis. *p < 0.05, **p < 0.01, ***p < 0.001.
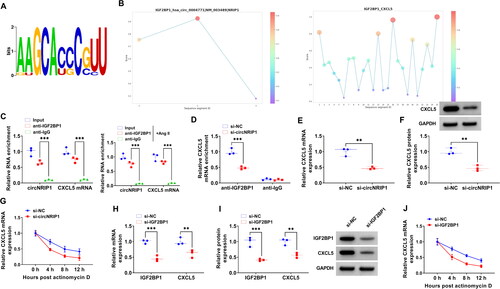
CircNRIP1 promoted CXCL5 mRNA stability by binding with IGF2BP1
IGF2BP1 is an RNA-binding protein whose known motif is shown in . Through RBPsuite predicting, we found that IGF2BP1 protein had binding sites to circNRIP1 and CXCL5 mRNA (). In RIP experiment, circNRIP1 and CXCL5 mRNA levels were significantly enriched in anti-IGF2BP1 under the Ang II treated and untreated conditions, indicating that IGF2BP1 protein could bind to circNRIP1 and CXCL5 mRNA (). Also, circNRIP1 knockdown could reduce the binding of IGF2BP1 protein to CXCL5 mRNA (). Moreover, we confirmed that CXCL5 mRNA and protein levels could be reduced by si-circNRIP1 (), and Actinomycin D assay showed that circNRIP1 knockdown could significantly reduce CXCL5 mRNA stability (). After silenced IGF2BP1 using si-IGF2BP1, we found that CXCL5 mRNA and protein levels were significantly decreased (). Additionally, knockdown of IGF2BP1 also suppressed the mRNA stability of CXCL5 (). Above data revealed that circNRIP1 bound with IGF2BP1 to enhance CXCL5 mRNA stability.
Figure 6. Effect of circNRIP1 and IGF2BP1 on CXCL5 mRNA stability. (A) The known motif of IGF2BP1. (B) RBPsuite predicted the binding sites between IGF2BP1 and circNRIP1/CXCL5. (C-D) RIP assay was used to confirm the interaction between IGF2BP1 and circNRIP1/CXCL5. (E-F) CXCL5 mRNA and protein expression in HA-VSMCs transfected with si-NC/si-circNRIP1 was tested by qRT-PCR and WB analysis. (G) Actinomycin D assay was used to detect the mRNA stability of CXCL5 in HA-VSMCs transfected with si-NC/si-circNRIP1. (H-I) The mRNA and protein levels of IGF2BP1 and CXCL5 were measured by qRT-PCR and WB analysis in HA-VSMCs transfected with si-NC/si-IGF2BP1. (J) The mRNA stability of CXCL5 in HA-VSMCs transfected with si-NC/si-IGF2BP1 was assessed by Actinomycin D assay. **p < 0.01, ***p < 0.001.
Discussion
TAD is a fatal condition in which blood from the aorta enters the media of the blood vessel through a ruptured lining, forming 2 spaces inside and outside the vessel [Citation22]. The dysfunction of VSMCs is associated with TAD [Citation23,Citation24]. Increasing evidence suggests that circRNAs play an important role in TAD. For example, Liang et al. suggested that circPTGR1, circNOX4, circAMN1 and circUSP3 were highly expressed in TAD patients and might be used as the potential biomarkers for TAD diagnosis [Citation25]. Hsa_circ_0007386 could induce VSMCs apoptosis to aggravate TAD progression through regulating miR-1271-5P/IGF1R/AKT axis [Citation26]. circ_TGFBR2 inhibited VSMCs phenotypic switch to restrain TAD progression by sponging miR-29a [Citation15]. Nevertheless, there are still many circRNAs, whose roles in TAD have not been revealed. Through GEO database analysis, we found that circNRIP1 was significantly overexpressed in TAD patients, but its role and mechanism in TAD progression remains unclear. Our study suggested that circNRIP1 indeed had elevated expression in TAD patients, and its knockdown repressed Ang II-induced HA-VSMCs proliferation, migration and phenotypic switch through IGF2BP1/CXCL5 axis. These data confirmed that circNRIP1 might contribute to TAD progression, and targeting circNRIP1/IGF2BP1/CXCL5 axis might be an effective measure for the treatment of TAD.
Previous study indicated that CXCL5 could serve as a potentially treatable target for acute AD [Citation21]. Furthermore, Sun et al. revealed that CXCL5 had a promotion effect on VSMCs migration [Citation27]. Our study demonstrated that CXCL5 was significantly overexpressed in TAD patients, and its expression was positively regulated by circNRIP1. Besides, we confirmed that CXCL5 knockdown inhibited Ang II-induced proliferation, migration and phenotypic switch in HA-VSMCs, suggesting that targeted CXCL5 might be a potential method for TAD treatment consistent with previous study [Citation21]. In addition, CXCL5 overexpression reversed the regulation of si-circNRIP1 on Ang II-induced HA-VSMCs functions, confirming that circNRIP1 accelerated TAD progression via increasing CXCL5 expression.
IGF2BP1 is a post-transcriptional fine-tuner that plays a crucial role in both physiological and pathological states [Citation28]. CircRNAs have been found to bind to IGF2BP1 to regulate downregulate mRNA stability [Citation29,Citation30]. Peng et al. reported that circCRIM1 suppressed lung cancer immune evasion by disrupting HLA-F mRNA stability through binding to IGF2BP1 [Citation31]. Chen et al. found that circUHRF2 enhanced DDX27 mRNA stability by recruiting IGF2BP1, thereby promoting colorectal cancer cell stemness and metastasis [Citation32]. By RBPsuite prediction and experimental verification, we found that IGF2BP1 protein had binding sites to circNRIP1 and CXCL5 mRNA, and CXCL5 expression was negatively regulated by circNRIP1 and IGF2BP1. Further experimental analysis revealed that si-circNRIP1 could inhibit the binding of IGF2BP1 protein to CXCL5 mRNA. Additionally, circNRIP1 silencing could reduce CXCL5 mRNA stability, indicating that circNRIP1 bound with IGF2BP1 to enhance CXCL5 mRNA stability.
In conclusion, our study revealed a novel mechanism by which circNRIP1 promoted TAD progression. Our findings suggested that circNRIP1 facilitated Ang II-induced HA-VSMCs proliferation, migration and phenotypic switch by increasing CXCL5 mRNA stability through the recruitment of IGF2BP1. These findings provide potential molecular targets for the treatment of TAD.
Supplemental Material
Download MS Word (134.7 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Zheng HQ, Rong JB, Ye FM, et al. Induction of thoracic aortic dissection: a mini-review of beta-aminopropionitrile-related mouse models. J Zhejiang Univ Sci B. 2020;21(8):1–10.
- Saremi F, Hassani C, Lin LM, et al. Image predictors of treatment outcome after thoracic aortic dissection repair. Radiographics. 2018;38(7):1949–1972.
- Sen I, Erben YM, Franco-Mesa C, et al. Epidemiology of aortic dissection. Semin Vasc Surg. 2021;34(1):10–17.
- Cao G, Xuan X, Hu J, et al. How vascular smooth muscle cell phenotype switching contributes to vascular disease. Cell Commun Signal. 2022;20(1):180.
- Fernández-Villabrille S, Martín-Carro B, Martín-Vírgala J, et al. Phosphorus may induce phenotypic transdifferentiation of vascular smooth muscle cells through the reduction of microRNA-145. Nutrients. 2023;15(13):2918.
- Shi J, Yang Y, Cheng A, et al. Metabolism of vascular smooth muscle cells in vascular diseases. Am J Physiol Heart Circ Physiol. 2020;319(3):H613–H631.
- Zhang S, Zhao S, Han X, et al. Lnc-C2orf63-4-1 confers VSMC homeostasis and prevents aortic dissection formation via STAT3 interaction. Front Cell Dev Biol. 2021;9:792051.
- Yang Y, Jiao X, Li L, et al. Increased circulating Angiopoietin-Like protein 8 levels are associated with thoracic aortic dissection and higher inflammatory conditions. Cardiovasc Drugs Ther. 2020;34(1):65–77.
- Li Y, Chen J, Xie M, et al. Identification of a circRNA-miRNA-mRNA network to explore the effects of circRNAs on renal injury in systemic lupus erythematosus. Autoimmunity. 2023;56(1):2193361.
- Mei HY, Liu J, Shen XP, et al. A novel circRNA, circRACGAP1, hampers the progression of systemic lupus erythematosus via miR-22-3p-mediated AKT signalling. Autoimmunity. 2022;55(6):360–370.
- Wang F, Zhang F, Tian Q, et al. CircVMA21 ameliorates lipopolysaccharide (LPS)-induced HK-2 cell injury depending on the regulation of miR-7-5p/PPARA. Autoimmunity. 2022;55(2):136–146.
- Zhang X, Lu J, Zhang Q, et al. CircRNA RSF1 regulated ox-LDL induced vascular endothelial cells proliferation, apoptosis and inflammation through modulating miR-135b-5p/HDAC1 axis in atherosclerosis. Biol Res. 2021;54(1):11.
- Ding S, Zhu Y, Liang Y, et al. Circular RNAs in vascular functions and diseases. Adv Exp Med Biol. 2018;1087:287–297.
- Zou M, Huang C, Li X, et al. Circular RNA expression profile and potential function of hsa_circRNA_101238 in human thoracic aortic dissection. Oncotarget. 2017;8(47):81825–81837.
- Xu Z, Zhong K, Guo G, et al. Circ_TGFBR2 inhibits vascular smooth muscle cells phenotypic switch and suppresses aortic dissection progression by sponging miR-29a. J Inflamm Res. 2021;14:5877–5890.
- Zhou SL, Dai Z, Zhou ZJ, et al. Overexpression of CXCL5 mediates neutrophil infiltration and indicates poor prognosis for hepatocellular carcinoma. Hepatology. 2012;56(6):2242–2254.
- Metzemaekers M, Mortier A, Vacchini A, et al. Endogenous modification of the chemoattractant CXCL5 alters receptor usage and enhances its activity toward neutrophils and monocytes. Sci Signal. 2021;14(673):eaax3053.
- Moore CM, O’Reilly D, McCallion N, et al. Changes in inflammatory proteins following platelet transfusion in a neonatal population. Pediatr Res. 2023;94(6):1973–1977.
- Karaaslan Z, Yilmaz V, Yuceer H, et al. Serum CXCL5 as a biomarker in multiple sclerosis and neuromyelitis optica spectrum disorder. North Clin Istanb. 2023;10:341–344.
- Cao Q, Chen J, Zhang Z, et al. Astrocytic CXCL5 hinders microglial phagocytosis of myelin debris and aggravates white matter injury in chronic cerebral ischemia. J Neuroinflammation. 2023;20(1):105.
- Chang TT, Liao LY, Chen JW. Inhibition on CXCL5 reduces aortic matrix metalloproteinase 9 expression and protects against acute aortic dissection. Vascul Pharmacol. 2021;141:106926.
- Goldfinger JZ, Halperin JL, Marin ML, et al. Thoracic aortic aneurysm and dissection. J Am Coll Cardiol. 2014;64(16):1725–1739.
- Zhi K, Yin R, Guo H, et al. PUM2 regulates the formation of thoracic aortic dissection through EFEMP1. Exp Cell Res. 2023;427(2):113602.
- An Z, Liu Y, Song ZG, et al. Mechanisms of aortic dissection smooth muscle cell phenotype switch. J Thorac Cardiovasc Surg. 2017;154(5):1511–1521.e6. e1516.
- Liang Q, Zhou Z, Li H, et al. Identification of pathological-related and diagnostic potential circular RNAs in Stanford type a aortic dissection. Front Cardiovasc Med. 2022;9:1074835.
- Xie X, Hong X, Hong S, et al. Progression of thoracic aortic dissection is aggravated by the hsa_circ_0007386/miR-1271-5P/IGF1R/AKT axis via induction of arterial smooth muscle cell apoptosis. Biomedicines. 2023;11(2):571.
- Sun N, Chu B, Choi DH, et al. ETV2 enhances CXCL5 secretion from endothelial cells, leading to the promotion of vascular smooth muscle cell migration. Int J Mol Sci. 2023;24(12):9904.
- Jiang X, Liu B, Nie Z, et al. The role of m6A modification in the biological functions and diseases. Signal Transduct Target Ther. 2021;6:74.
- Xie F, Huang C, Liu F, et al. CircPTPRA blocks the recognition of RNA N(6)-methyladenosine through interacting with IGF2BP1 to suppress bladder cancer progression. Mol Cancer. 2021;20(1):68.
- Lv J, Li K, Yu H, et al. HNRNPL induced circFAM13B increased bladder cancer immunotherapy sensitivity via inhibiting glycolysis through IGF2BP1/PKM2 pathway. J Exp Clin Cancer Res. 2023;42(1):41.
- Peng W, Ye L, Xue Q, et al. Silencing of circCRIM1 drives IGF2BP1-Mediated NSCLC immune evasion. Cells. 2023;12(2):273.
- Chen M, Tian B, Hu G, et al. METTL3-Modulated circUHRF2 promotes colorectal cancer stemness and metastasis through increasing DDX27 mRNA stability by recruiting IGF2BP1. Cancers (Basel). 2023;15(12):3148.

