Abstract
Objective: Todetect the abnormal distribution of B-lymphocytes between peripheral and bone marrow (BM) compartments and explore the mechanism of abnormal chemotaxis of B-lymphocytes in lupus subjects. Methods: The proportions of CXC chemokine receptor (CXCR)4+ B cells and CFDA-labeled MRL/lpr-derived B cells were detected by flow cytometry. The levels of CXC chemokine ligand (CXCL)12in peripheral blood (PB)were measured by ELISA. The migrated B cells to osteoblasts (OBs) was measured by transwell migration assay. The relative spatial position of B cells, OBs and CXCL12 was presented by Immunofluorescence assay. Results: Firstly, we found that the percentage of CXCR4+ B cells was lower in PB and higher in the BM from both MRL/lpr mice and patientswith Systemic lupus erythematosus (SLE). Secondly, OBs from MRL/lpr mice produced more CXCL12 than that from C57BL/6 mice. Besides, MRL/lpr-derived OBs demonstrated more potent chemotactic ability toward B-lymphocytes than control OBs by vitro an vivo. Additionally, more B-lymphocytes were found to co-localize with OBs within the periosteal zone of bone in MRL/lpr mice. Lastly, the percentages of CXCR4+B cells were found to be negatively correlated with serum Immunoglobulin (Ig) G concentration, moreover, BM CXCL12 levels were found to be positively correlated with SLE disease activity index Score and negatively correlated with serum Complement3 (C3) concentration. Conclusions: our results indicated that there is a shifted distribution of B-lymphocytes between BM and peripheral compartments in both SLE patients and MRL/lpr mice. Besides, the up-regulated levels of CXCL12 in OBs was indicated to contribute to the enhanced chemotactic migration and anchorage of B-lymphocytes to OBs.
Keywords:
Introduction
Systemic lupus erythematosus(SLE)is a multiorgan, multisystem autoimmune disease characterized by the production of autoantibodies, a lack of immunological tolerance, increased levels of inflammatory cytokines, and antibody removal disorders, among other features, which lead to a series of chain reactions that damage target organs and thus cause diseases [Citation1]. B-lymphocytes occupy a central position in the promotion of autoimmunity in SLE and mediate disease not only by producing autoantibodies but also by expanding autoreactive CD4+ T cells via autoantigen presentation through a positive feedback loop with CD4+ T-lymphocytes [Citation2]. The abnormal activation of multiclonal B-lymphocytes may result from inherent high reactivity, ineffective negative selection, deficits in immunomodulation, and an overactive inflammatory environment, among other factors [Citation3]. Considering the important role of B-cell abnormalities in SLE, anti-CD20 monoclonal antibodies such as rituximab have been used with success in the management of refractory SLE. However, the benefits of the addition of rituximab to the treatment of SLE patients remain controversial, and some clinical trials have failed to demonstrate the superiority of rituximab to conventional immunosuppressive therapy. In addition, SLE patients initially responding to rituximab treatment may develop resistance to subsequent rituximab administration [Citation4,Citation5]. All of these factorssuggest the complexity of abnormal B-cell-mediated immunity in SLE.
A previous study found that an abnormal CXC chemokine receptor 4/CXC chemokine ligand 12 (CXCR4/CXCL12) axis plays an important role in the pathogenesis of lupus. For example, CXCR4 is overexpressed in immune cells such as B cells, monocytes and neutrophils, which enhances the migration of immune cells toward the CXCL12 gradient and thereby facilitates cell survival, and plays a pivotal role in disease development [Citation6]. CXCL12, as the ligand for CXCR4, can activate a variety of cell signaling pathways by interacting with its receptor, and these pathways include mechanistic target of rapamycin (mTOR), phosphatidylinositol 3 kinase/protein kinase B (PI3K/AKT), nuclear factor kappa-B (NF-κB) and Janus kinase/signal transduction and activator of transcription (JAK/STAT) pathways [Citation7,Citation8]. Some studies have shown that the levels of CXCL12 in the serum and kidneys of SLE patients are higher than those in controls and correlated with disease activity [Citation9,Citation10]. Although the levels of CXCR4 on antibody-secreting cells are similar between SLE patients and healthy controls [Citation11], some lupus models with active nephritis, such as NZBWF1/J and MRL/lpr mice, have shown conflicting results indicating that the expression of CXCR4 on several peripheral blood (PB) leukocyte subsets is upregulated [Citation6,Citation12,Citation13], which could prolong the B-cell lifespan and augment B-cell chemotaxis. Studies have shown that knockout of CXCL12 is lethal during fetal development in mice and induce a significant reduction in B-lymphocytes in the bone marrow (BM) [Citation14,Citation15] because CXCL12 is involved in the migration of B cells to the BM [Citation16]. In addition to B-cell development, the CXCR4/CXCL12 axis is also reportedly involved in biological processes such as angiogenesis, inflammation and cancer metastasis [Citation17,Citation18].
Our previous studies identified multiple abnormalities within the BM microenvironment in SLE subjects, especially deficit of BM-derived mesenchymal stem cells (BMMSCs) [Citation19,Citation20], which are the progenitor for osteoblasts (OBs). In addition to bone formation, OBs participate in the regulation of hematopoiesis within the BM via the CXCR4/CXCL12 axis [Citation21]. In SLE, whether the CXCR4/CXCL12 axis between OBs and B-lymphocytes is involved in disease pathogenesis is currently unclear. To address this knowledge gap, we investigated the CXCR4/CXCL12 axis between OBs and B-lymphocytes, especially its role in the regulation of the distribution of B-lymphocytes within the BM and peripheral compartments.
Materials and methods
Human samples
Patients diagnosed with SLE according to the 2012 American College of Rheumatology classification criteria and healthy controls were recruited from the Affiliated Hospital of Jiangsu University. All experiments with human samples were approved by the Ethics Committee of the Affiliated Hospital of Jiangsu University (reference number: KY2021K0909). The demographic information of the SLE patients and healthy controls is shown in Supplementary Tables S1 and S2.
Mice
Female MRL/lpr mice (specific pathogen-free (SPF) grade) and female C57/BL6 mice (SPF grade) (both purchased from Shanghai Slack Experimental Animals Limited Liability Company, license number: SCXK (Shanghai) 2017-0005) were fed in the SPF-grade breeding area of the Animal Experimental Center of Jiangsu University. All animals were fed until 12-14 weeks, when the experiment was initiated. All animal experiments were approved by the Animal Experiment Committee of Jiangsu University and conducted in accordance with institutional and national guidelines. All animal experiments were approved by the Animal Ethics Committee of the Jiangsu University Animal Laboratory Center (reference number: UJS-IACUC-AP-2020032539).
Flow cytometry
BM cells were harvested from the femurs and tibias of mice, and PB mononuclear cells were harvested from the inner orbital vein of the mice and processed to obtain single-cell suspensions. Then, 1 × 10^7/mL cells were incubated in 100 µL of dye buffer (phosphate buffered saline (PBS) and 2% fetal bovine serum (FBS)) with antibodies. PB cells were stained with anti-mouse CD19-PE (clone 1D3; BD Biosciences) and anti-CXCR4-FITC (ab1670, ab150129), and BM cells were stained with anti-B220-PE (cloneRA3-6B2; BD Biosciences) and anti-CXCR4-FITC (ab1670, ab150129) in 4 °C. We collected clinical waste BM samples from SLE patients and healthy individuals without autoimmune diseases who underwent lumbar vertebral surgery to obtain BM cells and their supernatants. BM cells were stained with anti-human CD19-PE (clone HIB19; BD Biosciences) and anti-CXCR4-FITC (ab1670, ab150129). All samples were treated and diluted equally before flow cytometry. All flow cytometric data were acquired using a BD LSRFortessa cytometer (BD Biosciences) and analyzed with FlowJo software (BD Biosciences).
RNA extraction and RT–qPCR
The BM, spleen and PB of mice were obtained and processed into single-cell suspensions. RNA was extracted with an RT reagent kit reverse transcription kit (TaKaRa) according to the manufacturer’s instructions. Gene expression was quantified with a Takara STBR Premix Ex Taq kit and a Q5 qRT–PCR instrument (ABI, USA). The primers were as follows: CXCL12 sense, 5′-TCTGAAAATCCTCAACACTCCA-3′, and anti-sense, 5′-CAGGTACTCTTGGATCCACTTT-3′; and β-actin sense, 5′-GTGCTATGTTGCTCTAGACTTCG-3′, and anti-sense, 5′-ATGCCACAGGATTCCATACC-3′.
ELISA
The CXCL12 levels in serum, BM supernatant and culture medium supernatant samples were measured using a human CXCL12 ELISA kit (Multi Sciences) and mouse CXCL12 ELISA kit (Multi Sciences). All samples were treated and diluted equally before ELISA. Sandwich ELISAs for human and mouse CXCL12 were performed according to the manufacturer’s instructions (Multi Sciences).
Cell culture
OB culture
Murine OBs were obtained from published procedures with minor modifications [22]. In brief, the calvariae were removed aseptically from 12-week-old C57 or MRL/lpr mice and subjected to repeated digestion for 10 min at 37 °C with 0.25% type II collagenase (Millipore) in PBS, and the supernatant was then discarded. The bone fragments were added to 0.1% type II collagenase for repeated digestion for 10 min at 37 °C, and the supernatant was discarded. Subsequently, the bone fragments were digested with 0.1% type II collagenase for 20 min, and the supernatant was collected; these steps were repeated four times. Fractions were then collected by centrifugation and cultured in MEM Alpha Modification (α-MEM) containing 10% FBS and 1% penicillin–streptomycin. The cells were cultured to 80% confluence (undifferentiated OBs). In some experiments, the cells were then cultured in differentiation medium (α-MEM containing 10% FBS, 0.05 mmol/L ascorbic acid, 100 mmol/L dexamethasone and 10 mmol/L β-glycerophosphate) for 1 or 2 weeks (differentiated OBs) to obtain OBs-1w and OBs-2w, respectively. The differentiated OBs (OBs2W) were then used for in vitro migration assays.
BM mesenchymal stem cell culture
Mice (aged 12–14 weeks) were euthanized, and their tibias and fibulas were dissected after disinfection. The BM was repeatedly flushed with Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Waltham, MA, USA) and then centrifuged at 1000 rpm for 10 min. The resulting pellet was collected and resuspended in DMEM containing 10% FBS (Gibco) and 1% penicillin–streptomycin (Gibco) in a 100-mm culture dish, which was then placed in a 37 °C humidified incubator with a 5% CO2 atmosphere. Approximately 3 days later, the cells were confluent in the culture dish, and these cells were designated as the first cell culture passage. The cells were then subpassaged at a ratio of 1:1, expanded, purified, and cultured to generate third-passage cells.
In vitro migration assays
B cells were obtained from the spleens of C57 or MRL/lpr mice by magnetic cell sorting (MACS-STEMCELL) with CD45R (B220) magnetic bead (the purities of B cells were 80.4%). The purities of the yields after B cell Microbeads isolation were 91%. The migration of splenic B-cell populations was evaluated in 24-well Co-star Transwell chambers (5-µm pore size, Corning). Briefly, a suspension of 107 B cells or B cells pre-treated with CXCR4 antagonists (AMD3100, MACS-STEMCELL,) in 100 µL of Roswell Park Memorial Institute 1640 (RPMI 1640) medium without FBS was added to each upper chamber, and each lower chamber was seeded with RPMI 1640, CXCL12, OBs or OBs infected with lentiviral short hairpin RNA (shRNA) 340 at the same density. These OBs were osteogenically induced two weeks before the experiment. In some experiments, a CXCL12 neutral ligand(LIT-927, SELLECK) dissolved in dimethyl sulfoxide (DMSO) (66 mg/mL) was added to the lower chamber prior to migration. After 24 h at 37 °C, the B cells in suspension in the lower chamber were harvested and counted with a blood cell counter. The results are expressed as the numbers of B cells that migrated to the lower chamber.
Lentiviral infection
Lentiviruses were packaged and produced according to the manufacturer’s instructions (Gene Pharma). The gene names of the lentiviruses were LV3-CXCL12-Mus-340 (5-CCCGAAATTAAAGTGGATCCA-3′) and LV3-NC (5-TTCTCCGAACGTGTCACGT-3′). OBs (1x105) were split into a 24-well plate and grown to 60–80% confluence after 24 h. The plates were refed medium for OBs, specifically 400 µL of α-MEM (Gibco) without FBS, 2 h prior to transfection. Then, 100 µL of lentivirus solution was added to each well. Twenty-four hours after transfection, the medium was replaced with fresh complete medium (10% α-MEM). The fluorescence (GFP) of OBs was observed under a fluorescence microscope at 72 h after transfection, and GFP-positive OBs were isolated by fluorescence-activated cell sorting (FACS). Subsequently, GFP-positive OBs were induced to undergo osteogenic differentiation for 2 weeks and used for in vitro migration assays. The inhibitory efficiency of shRNA340 against CXCL12 mRNA was monitored by RT–PCR.
B-cell homing assay
CD19+ B cells were recovered from the spleens of MRL/lpr mice by positive selection using a MACS microbead isolation kit (STEMCELL) according to the manufacturer’s instructions. The cells were then resuspended at 1x108 cells/mL in PBS, an equal volume of carboxyfluorescein diacetate succinimidyl ester (CFDA-SE, STEMCELL) was added as a 2X working stock solution to obtain a final concentration of 0.5–10 µM, and the cells were incubated with the dye for 5-10 min in the dark at room temperature. After washing, approximately 1x108 CFDA-stained CD19+ B cells were injected retro-orbitally into 12- to 13-week-old syngeneic C57BL/6 mice, MRL/lpr mice and MRL/lpr mice pre-treated with LIT-927 (ig, 10 mg/kg) [Citation22] for 12 h. The percentage of CFDA-labeled cells in the cell mixture was determined by flow cytometry before injection to measure the number of CFDA-labeled cells transferred. The recipient mice were killed 24 h after injection, and the percentage of CFDA-labeled B cells in the BM was analyzed by flow cytometry.
Immunofluorescence assay
Bones from sacrificed C57BL/6 and MRL/lpr mice were cut into paraffin sections and rehydrated. After antigen retrieval and endogenous peroxidase blockade, we covered the objective tissues with 10% donkey serum or 3% bovine serum albumin (BSA) at room temperature for 30 min. The antibodies used for the immunolabeling of BM sections were anti-B220 (cloneRA3-6B2; BD Biosciences), anti-CXCL12 (GB11624; Servicebio), anti-CD31 (GB12063; Servicebio), and anti-CD51 (GB11293-2; Servicebio). The cells were then incubated with4’,6-diamidino-2′-phenylindole (DAPI) solution at room temperature for 10 min, kept in the dark and cover slipped with antifade mounting medium. Images were collected by fluorescence microscopy in conjunction with a Nikon imaging system. DAPI emits blue fluorescence (emission wavelength: 420 nm) in response to an ultraviolet (UV) excitation wavelength of 330–380 nm, fluorescein isothiocyanate (FITC) emits green fluorescence (emission wavelength: 515–555 nm) in response to an excitation wavelength of 465-495 nm, and cyanine 3 (CY3) emits red fluorescence (emission wavelength: 590 nm) in response to an excitation wavelength of 510–560 nm. Differences between the expression of CXCL12 between C57 and MRL/lpr BM have been analyzed by Image J.
Statistical analysis
All analyses were performed using GraphPad Prism version 8 (USA). The data are expressed as the means ± SDs and were compared by independent Student’s t test or a nonparametric test. The correlation between the percentages of CXCR4+ B cells or the level of CXCL12 in BM and markers of disease activity in patients with SLE was measured by Pearson correlation coefficients. P value < 0.05 was considered to indicate statistical significance.
Results
The BM of both MRL/lpr mice and SLE patients exhibited an higher percentage of CXCR4+ B cells and higher levels of CXCL12
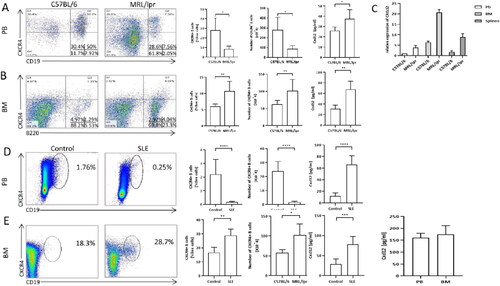
First, the percentages of CXCR4+ B cells in the PB or BM were determined by flow cytometry. Consistent with the findings reported by Wang et al. [Citation6], a lower percentage of CXCR4+ B-lymphocytes in the PB and a higher percentage of these B-lymphocytes in the BM were found in MRL/lpr mice compared with C57 mice (Figure1A, B). Because CXCL12 is the ligand for CXCR4, we subsequently compared the levels of CXCL12in the BM, spleen and PB of MRL/lpr mice and C57 mice. Both the CXCL12 levels in BM and PB were detected markedly higher from MRL/lpr mice than that from C57 control mice, and the highest CXCL12 level was detected in the BM among the three sites either form MRL/lpr mice or C57 mice (). Similar to the results found in lupus mice, a higher percentage of CXCR4+ B cells and higher levels of CXCL12 were detected in the BM of SLE patients than in that from healthy controls, although the difference in the CXCL12 levels was not found significant ().
Figure 1. Differences in the percentage of CXCR4+ B cells and the levels of CXCL12 between control and SLE subjects. A: The percentages (left panel) and absolute number (right panel) of CXCR4 + B cells in peripheral blood of MRL/lpr mice (n = 5) and C57 mice (n = 5) measured by flowmetry, and levels of CXCL12 in peripheral blood of MRL/lpr mice (n = 5) and C57 mice (n = 5) by ELISA. Gating scheme for CXCR4+ B cells that were identified as CD19 + CXCR4+. B: The percentages (left panel) and absolute number (right panel) of CXCR4+ B cells in bone marrow of MRL/lpr mice (n = 5)and C57 mice (n = 5)measured by flowmetry, and levels of CXCL12 in bone marrow of MRL/lpr mice (n = 5) and C57 mice (n = 5) measured by ELISA.Gating scheme for CXCR4+ B cells that were identified as B220 + CXCR4+. C: mRNA expression of CXCL12 in peripheral blood, bone marrow, spleen of MRL/lpr mice (n = 5) and C5 7 mice (n = 5) measured by qRT-PCR. D: The percentages of CXCR4+ B cells and expression of CXCL12 protein in peripheral blood of healthy controls (n = 5) and SLE (n = 10). E: The percentages of CXCR4+ B cells and expression of CXCL12 protein in bone marrow of healthy controls (n = 5) and SLE (n = 10). The last panel is the expression of CXCL12 between PB and BM of SLE (n = 5). Data represented the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, compared with C57BL/6 (A, B, C) and control(D, E).
MRL/lpr-derived OBs produced more CXCL12 than BMMSCs
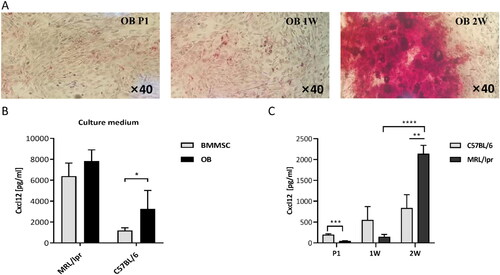
Reportedly, many types of cells in the BM stroma, including MSCs, endothelial cells and OBs, produce CXCL12 [Citation23]. We then successfully obtained OBs from the mouse skull, and their osteogenic potential was validated by alkaline phosphatase (ALP) staining (). Compared to MSCs, OBs were shown to produce higher levels of CXCL12 (). In addition, MRL/lpr-derived OBs were also shown to produce higher levels of CXCL12 than C57-derived cells after 2w culture with osteogenic medium ().
Figure 2. Expression of CXCL12 protein from mice OBs. A: Alkaline phosphatase staining before and after the implement of osteogenic medium. B: Expression of CXCL12 in BMMSCs and OBs from C57 and MRL/lpr mice (n = 5). C: The change of CXCL12 levels in the supernatant of the culture during osteogenic induction. CXCL12 levels were detected by ELISA at the beginning, 1 week (1 W) and 2 weeks (2 W) of induction. Data represented the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, compared with C57.
CXCL12 mediated enhanced chemotactic migration of B-lymphocytes to OBs in SLE
To determine the possible mechanism responsible for the higher percentage of CXCR4+ B cells in the BM of SLE patients, we first investigated the capacity of hematopoietic stem cells (HSCs) to differentiate into B-lymphocytes. We found that the number of CFU-pre-B from MRL/lpr mice was lower than that from C57 mice, which does not support a finding of enhanced B-lymphomagenesis in the BM of SLE mice [S3]. Based on this result, we hypothesized that CXCR4+ B-cell migration to and retention in the BM were likely enhanced and that aberrant chemotaxis plays an important role in this process.
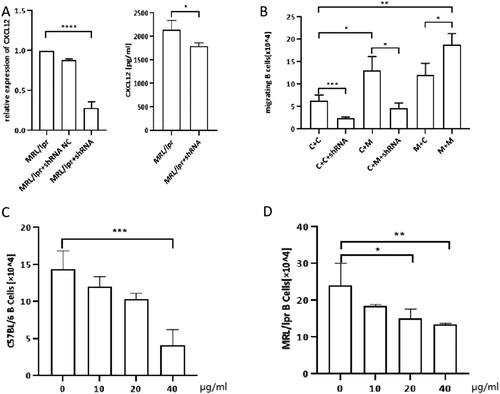
We subsequently investigated the chemotactic migration of B-lymphocytes by a transwell assay. Negative control results determine the basal level of migration of B cells which is due to CXCL12 (Fig. S4). B cells from C57 mice showed greater migration to MRL/lpr-derived OBs than to C57-derived OBs (). This observation implied that MRL/lpr-derived OBs produced more potent and/or higher levels of chemokines to attract B-lymphocytes. To confirm the role of CXCL12 in the chemotactic effect of OBs, we then transfected C57-derived or MRL/lpr-derived OBs with shRNA constructed by lentiviral vectors to downregulate CXCL12 expression. As shown in , CXCL12-shRNA transfection could effectively reduce the level of CXCL12 in the supernatant of OB cultures, as revealed by ELISA. In a subsequent study, we employed CXCL12-shRNA-transfected OBs as coating cells in the lower chamber. Compared with nontransfected cells, the migration of B-lymphocytes toward OBs was significantly suppressed, regardless of whether the OBs were derived from C57 or MRL/lpr mice. Furthermore, the addition of LIT-927 (a neutraligand of CXCL12) to the transwell decreased the migration of both C57- and MRL/lpr-derived B-lymphocytes. In addition, its inhibition of chemotactic migration was shown to occur in a dose-dependent manner ().
Figure 3. The number of transwell migration of B cells to OBs. A: shRNAefficiency was shown by qRT-PCR. shRNA effectively decreases supernatant concentration of CXCL12 in the OBs culture.(MPL/lpr + shRNA: MPL/lpr-derived OBs transinfected with shRNA by lentiviral vector). B: Transwell migration of splenic B cells (upper chamber) to OBs (lower chamber): C + C: C57/BL6 mice B cells (upper chamber) + C57/BL6 mice OBs (lower chamber); C + C + shRNA: C57/BL6 mice B cells (upper chamber) + C57/BL6 mice OBs transfected with shRNA (lower chamber); C + M: C57/BL6 mice B cells (upper chamber) + MRL/lpr mice OBs (lower chamber); C + M + shRNA:C57/BL6 mice B cells (upper chamber) + MRL/lpr mice OBs transfected with shRNA (lower chamber); M + C: MRL/lpr mice B cells (upper chamber) + C57/BL6 mice OBs (lower chamber); M + M: MRL/lpr mice B cells (upper chamber) + MRL/lpr mice OBs (lower chamber). C: Number of C57-derived splenic B cells migrated to MRL/lpr-derived OBs treated with CXCL12 antagonists (LIT-927) at different concentration. D: Number of MRL/lpr-derived splenic B cells migrated to MRL/lpr-derived OBs treated with LIT-927 at different concentration. Data represented the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001.
Higher levels of CXCL12 enhanced the homing of B-lymphocytes to the marrow of MRL/lpr mice in vivo
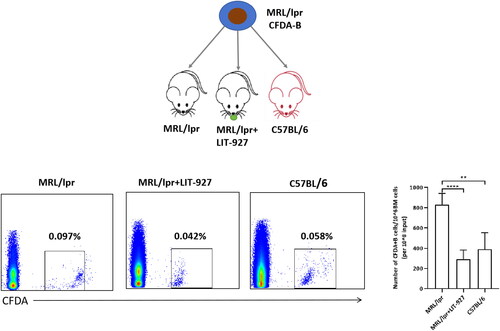
Next, we performed an in vivo experiment to explore the mechanism of abnormal chemotaxis of B-lymphocytes in SLE mice. First, B cells were harvested and separated from the spleens of MRL/lpr mice and labeled these by CFDA. The labeled cells were then intravenously injected into recipient mice. After infusion, the presence of donor-derived B cells in the recipient BM was subsequently measured by flow cytometry. Among all three cohorts, the MRL/lpr cohort exhibited the highest percentage of CFDA-labeled cells. However, if the recipient mice were pretreated with LIT-927, a CXCL12 neutral ligan, the chemotactic capacity of infused B-lymphocytes to the BM was significantly impaired. In addition, no difference in the percentage of CFDA-labeled cells in the marrow was found between the C57 or MRL/lpr cohorts in the presence of LIT-927 (). Taken together, these results once again indicated that marrow-derived CXCL12 is one of the key factors mediating enhanced B-lymphocyte homing to marrow.
Figure 4. The number of CFDA-labeled CD19+ B cells were retro-orbitally injected into 12- to 13-week-old syngenic C57BL/6 (n = 5), MRL/lpr (n = 5) and LIT-927 treated MRL/lpr (n = 5), respectively. The percentage of CFDA-labelled B cells in the bone marrow was analyzed 24 h later post-injection by flow cytometry. Data represented the mean ± SD. **p < 0.01, ****p < 0.0001.
An increasing number of B lymphocytes preferentially colocalized with periosteal OBs in the BM of MRL/lpr mice
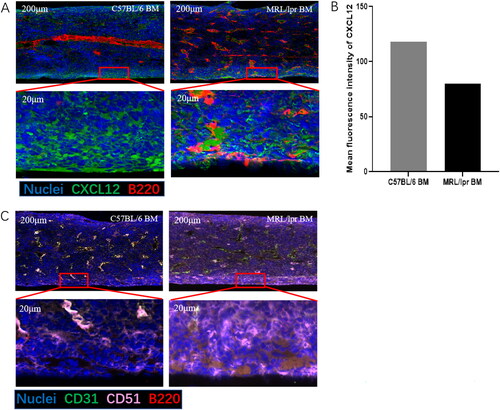
All of the above-mentioned results indicated that OBs overexpressing CXCL12 are more capable of attracting B-lymphocytes to their vicinity. To observe the spatial relationships between OBs and B-lymphocytes in vivo, we then immunostained femoral sections with antibodies against B220 (marker for B-lymphocytes), CD31 (marker for OBs), CD51 (marker for OBs) and CXCL12. Consistent with the flow cytometry results, more B220-positive B-lymphocytes were found in MRL/lpr mice, and the difference in the number of B-lymphocytes between MRL/lpr and C57 mice was more pronounced near the periosteal zone, where markedly fewer periosteal B-lymphocytes were found in C57 mice. We also found higher levels of CXCL12 expression in the MRL/lpr BM compared to C57 BM, which were consistent with the aforementioned research results (). Interestingly, the CXCL12 gradient near the periosteal zone seemed to be more marked in MRL/lpr mice (). In addition, more CD51+CD31- OBs were present in the periosteal zone in MRL/lpr mice. Moreover, the colocalization of B220+ B lymphocytes and CD51+CD31- OBs could readily be observed in MRL/lpr mice ().
Figure 5. Representative image of C57 and MRL/lpr mice. Compared to C57mice, More B cells localize close to periosteal surface (A) and OB cells (B) where contains rich CXCL12 aggregates in MRL/lpr mice. Paraffin sections of mice femurs were assessed for the positivity and position of CXCL12, B cells (B220+ cells) and OB cells(CD31-CD51+)using immunofluorescence under confocal microscopy. The part in the box is magnified tenfold. Sections were counterstained with DAPI to visualize nuclei. A: Blue = DAPI, Red = B220, Green = CXCL12; B: Differences between the expression of CXCL12 between C57 and MRL/lpr BM have been analyzed by Image J. C: Pink = CD51, Green = CD31, Red = B220. Scale bar = 200 μm.
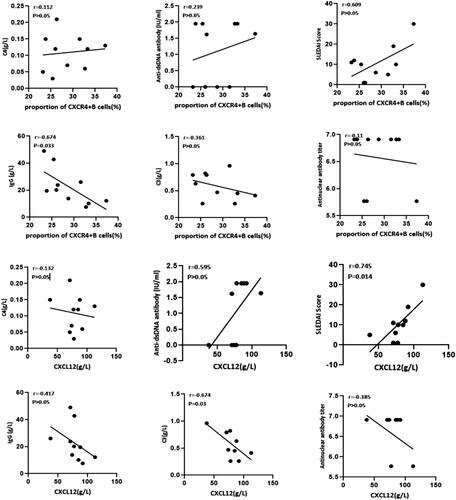
Correlation between percentages of CXCR4+ B cells or level of CXCL12 in BM and markers of disease activity in SLE patients
The disease activity of SLE patients is usually evaluated by the serum levels of complement C3 and C4, anti-dsDNA antibody, anti-nuclear antibody (ANA), serum IgG concentration and systemic lupus erythematosus disease activity index (SLEDAI) score. We finally conducted a correlation analysis between the indicators above and the proportion of CXCR4+ B cells or the concentration of CXCL2 in the patient’s bone marrow. As shown in , the percentages of CXCR4+B cells were negatively correlated with the serum IgG concentration (r= −0.674, p < 0.05). The level of CXCL12 was positively correlated with SLEDAI score (r = 0.745, p < 0.05) and negatively correlated with the complement 3 (C3) concentration (r= −0.674, p < 0.05). These clinical findings once again indicated that the abnormal BM environment characterized by increased levels of CXCR4+B cells and CXCL12 is correlated with SLE disease activity.
Figure 6. The correlation between markers of disease activity of SLE and the percentages of CXCR4+ B cells or the level of CXCL12 in bone marrow. The percentages of CXCR4+ B cells were negatively correlated with IgG concentration (r= −0.674, p < 0.05); The level of CXCL12 was positively correlated with SLEDAI Score (r = 0.745, p < 0.05) and negatively correlated with C3 concentration (r= −0.674, p < 0.05).
Discussion
The pivotal role of B-lymphocyte abnormalities in the pathogenesis of SLE has been widely acknowledged [Citation1,Citation2,Citation5,Citation24]. A conventional perspective on B cells in systemic autoimmunity posits that they contribute to lupus by generating autoantibodies. Upon activation, naïve B lymphocytes differentiate into memory B cells and PCs, both of which are responsible for producing pathogenic antibodies in SLE [Citation24–26]. However, recent studies have revealed that autoimmune disease can occur independently of autoantibody deposition [Citation2,Citation24]. Consequently, therapeutic approaches targeting B-cell depletion using anti-CD20 antibodies like rituximab have been employed for treating SLE in certain clinical settings but with limited success [Citation4,Citation5]. Therefore, it is crucial to comprehensively elucidate the mechanisms underlying self-reactive B-lymphocyte resistance to SLE treatment.
Previous investigations have demonstrated that the microenvironment plays a critical role not only in the development and survival of B lymphocytes [Citation27] but also provides a niche for memory-B cells and PCs’ survival [Citation26,Citation28]. We previously identified an aberrant bone marrow microenvironment in individuals with SLE (19, 20), which is believed to be implicated in the pathogenesis of B-lymphocyte abnormalities. The MRL/lpr mouse model is one of the most commonly used models for studying lupus [Citation29], characterized by its complex genetic background derived from a mixture of LG/J (75%), AKR (12.6%), C3H/Di (12.1%), and C57BL/6 (B6) (0.3%) strains [Citation30]. C57BL/6 mice share similar genetic backgrounds with MRL/lpr mice while possessing strong reproductive capability and extended lifespan; thus serving as constant members within our experimental control group. In this study, a reduced proportion of CXCR4+ B-lymphocytes was observed in the peripheral blood (PB) of MRL/lpr mice compared to C57 control mice, which is consistent with findings in SLE patients. We postulate that the observed decline in CXCR4+ B-lymphocytes in PB may be attributed to either their exhaustion or redistribution.
The CXCL12/CXCR4 axis reportedly mediates the chemotactic migration of various cell types, including neutrophils, monocytes, and B cells [Citation6,Citation8]. CXCL12 plays a crucial role in the accumulation of CXCR4+ immune cells in inflammatory tissues and the chemoattraction of CXCR4+ tumor cells [Citation31]. In NZB/W mice with nephritis manifestation, an elevated level of CXCL12 is hypothesized to be associated with increased renal inflammation and local autoantibody deposition due to inflammatory cell migration [Citation32]. Our study revealed that B lymphocytes from MRL/lpr mice exhibited enhanced migration along the CXCL12 gradient compared to those from C57 mice. Additionally, both C57 and MRL/lpr mice showed higher mRNA levels of CXCL12 in BM than in PB and spleen (). These findings suggest that BM preferentially attracts CXCR4+ B lymphocytes due to a steeper CXCL12 gradient [Citation14,Citation15]. Moreover, we observed a positive correlation between BM levels of CXCL12 and SLEDAI score as well as anti-dsDNA antibody titer. Conversely, there was a negative correlation between BM levels of CXCL12 and serum C3 concentration. Based on these findings, we propose that enhanced migration of CXCR4+ B cells to the BM may contribute to the pathogenesis of SLE.
It is well-established that various cell types in BM, including BMMSCs, vascular endothelial cells, and OBs, secrete CXCL12. In this study, OBs derived from MRL/lpr mice were found to exhibit higher levels of CXCL12 production compared to BMMSCs, which is one of several key factors contributing to enhanced migration of B-lymphocytes both in vitro and in vivo. Apart from its chemotactic effect, the CXCL12/CXCR4 axis has also been demonstrated as crucial for B-cell lymphopoiesis and survival; for instance, mice lacking CXCL12 die perinatally, and mutant embryos show severe reduction in BM B-cell progenitors [Citation6,Citation15]. Previous studies have reported that OBs support multiple cell types residing within the BM by secreting CXCL12 (33) and can maintain HSCs stemness within HSCs niches in the BM [Citation34]. When B cells co-localize with OBs, it is speculated that differentiation of OBs may be inhibited to prevent displacement of B-lymphocytes from their niches [Citation34]. In our current investigation, we observed a higher number of B220-positive B-lymphocytes in the periosteal zone of MRL/lpr mice compared with C57 mice, consistent with an increased population of CD51+CD31- OBs found in the former group. Furthermore, B220-positive B lymphocytes were spatially colocalized with CD51+CD31- OBs in areas where there was aggregation of CXCL12-rich cells, and this colocalization between these two cellular components was more pronounced in MRL/lpr mice. These findings suggest that CXCL12 overexpressing OBs in MRL/lpr mice may play an important role in the localization of B cells within the microenvironment of the BM.
After activation, B lymphocytes undergo transformation into immunoglobulin-producing immunoblasts and subsequently differentiate into PCs. Long-lived PCs are derived from proliferating plasma blasts in the spleen or lymph nodes, which then migrate to the BM guided by a chemokine gradient [Citation25]. Upon reaching the BM, these cells must occupy a specific survival niche for maturation into long-lived PCs. Furthermore, studies have demonstrated the presence of autoantibody-secreting PCs within inflamed kidneys in both SLE patients and murine lupus models [Citation35–37]. Although the number of long-lived PCs in the BM decreases following treatment with targeted agents such as bortezomib [Citation38], it is commonly observed that residual cells exhibit resistance to immunosuppressive agents and B-cell depletion therapy. This resistance is believed to be a major contributing factor to refractoriness/relapse of autoimmune diseases [Citation29,Citation39]. The study demonstrated a preferential docking of long-lived PCs with stromal cells expressing CXCL12 [Citation40]. Dysregulated CXCL12-expressing OBs were found to promote abnormal B-lymphomagenesis in SLE [Citation15,Citation17]. Stromal cell-derived cytokines and adhesion molecules, such as interleukin-6 (IL-6) and CXCL12, provide additional survival signals for long-lived PCs [Citation41,Citation42]. Given the high percentages of CXCR4+ B cells observed in the BM of SLE patients and MRL/lpr mice, which negatively correlate with serum IgG levels in PB, we propose that abnormal OBs in the bone marrow may attract more PCs through the CXCL12/CXCR4 axis leading to their transformation into long-lived PCs. Additionally, it is suggested that migrating CXCR4+B cells to the BM via the CXCR4/CXCL2 axis might represent plasma cells. Furthermore, targeting the CXCL12 antagonist holds potential for better management of SLE. However, further investigation is required to understand the pathology of long-lived PCs in relapse/refractory SLE.
Conclusion
In summary, our study suggests that the aberrant CXCR4/CXCL12 axis is associated with a redistribution of B-lymphocytes between the PB and BM compartments in an SLE mouse model. Importantly, an increased number of B-lymphocytes were observed to co-localize with CXCL12-expressing OBs in the periosteal zone. We propose that dysregulated osteoblastic niches play a pivotal role in the pathogenesis of SLE, warranting further investigation into its clinical significance.
Ethical approval
All experiments with human samples were approved by the Ethics Committee of the Affiliated Hospital of Jiangsu University (reference number: KY2021K0909). All animal experiments were approved by the Animal Ethics Committee of the Jiangsu University Animal Laboratory Centre (reference number: UJS-IACUC-AP-2020032539).
Authors’ contributions
WZ, CM, and MC participated in the conception and design of the study; WZ and WS performed the analysis and interpretation of the data; WZ drafted the manuscript; YT and XF reviewed the manuscript. All the authors read and approved the manuscript.
Supplemental Material
Download MS Word (40.9 KB)Acknowledgments
We thank Dr. Zhongqun Wang for his kind help with this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data (figures and table) used to support the findings of this study are included within the article.
Additional information
Funding
References
- Petri M. Systemic lupus erythematosus: 2006 update. J Clin Rheumatol. 2006;12(1):1–11.
- Chan OT, Madaio MP, Shlomchik MJ. The Central and multiple roles of B cells in lupus pathogenesis. Immunol Rev. 1999;169(1):107–121.
- Sayyed SG, Hägele H, Kulkarni OP, et al. Podocytes produce homeostatic chemokine stromal cell-derived factor-1/CXCL12, which contributes to glomerulosclerosis, podocyte loss and albuminuria in a mouse model of type 2 diabetes. Diabetologia. 2009;52(11):2445–2454.
- Smith KG, Jones RB, Burns SM, et al. Long-term comparison of rituximab treatment for refractory systemic lupus erythematosus and vasculitis: remission, relapse, and re-treatment. Arthritis Rheum. 2006;54(9):2970–2982.
- Arbitman L, Furie R, Vashistha H. B cell-targeted therapies in systemic lupus erythematosus. J Autoimmun. 2022;132:102873.
- Wang A, Fairhurst AM, Tus K, et al. CXCR4/CXCL12 hyperexpression plays a pivotal role in the pathogenesis of lupus. J Immunol . 2009;182(7):4448–4458.
- Chen G, Chen SM, Wang X, et al. Inhibition of chemokine (CXC motif) ligand 12/chemokine (CXC motif) receptor 4 axis (CXCL12/CXCR4)-mediated cell migration by targeting mammalian target of rapamycin (mTOR) pathway in human gastric carcinoma cells. J Biol Chem. 2012;287(15):12132–12141.
- Yi L, Zhou X, Li T, et al. Notch1 signaling pathway promotes invasion, self-renewal and growth of glioma initiating cells via modulating chemokine system CXCL12/CXCR4. J Exp Clin Cancer Res. 2019;38(1):339.
- Hanaoka H, Okazaki Y, Hashiguchi A, et al. Overexpression of CXCR4 on circulating B cells in patients with active systemic lupus erythematosus. Clin. Exp. Rheumatol. 2015;33:863–870.
- Barrera-Vargas A, Gómez-Martín D, Carmona-Rivera C, et al. Differential ubiquitination in NETs regulates macrophage responses in systemic lupus erythematosus. Ann Rheum Dis. 2018;77(6):944–950.
- de la Varga Martínez R, Rodríguez-Bayona B, Añez GA, et al. Clinical relevance of circulating anti-ENA and anti-dsDNA secreting cells from SLE patients and their dependence on STAT-3 activation. Eur J Immunol. 2017;47(7):1211–1219.
- Balabanian K, Couderc J, Bouchet-Delbos L, et al. Role of the chemokine stromal cell-derived factor 1 in autoantibody production and nephritis in murine lupus. J Immunol. 2003;170(6):170: 3392–3400.
- Cheng Q, Khodadadi L, Taddeo A, et al. CXCR4-CXCL12 interaction is important for plasma cell homing and survival in NZB/W mice. Eur J Immunol. 2018;48(6):1020–1029.
- Power CA. Knock out models to dissect chemokine receptor function in vivo. J Immunol Methods. 2003;273(1–2):73–82.
- Nagasawa T, Hirota S, Tachibana K, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382(6592):635–638.
- Allen CD, Ansel KM, Low C, et al. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat Immunol. 2004;5: (9):943–952.
- Ma Q, Jones D, Borghesani PR, et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A. 1998;95(16):9448–9453.
- Janssens R, Struyf S, Proost P. Pathological roles of the homeostatic chemokine CXCL12. Cytokine Growth Factor Rev. 2018;44:51–68.
- Tang Y, Xie H, Chen J, et al. Activated NF-κB in bone marrow mesenchymal stem cells from systemic lupus erythematosus patients inhibits osteogenic differentiation through downregulating smad signaling. Stem Cells Dev. 2013;22(4):668–678.
- Tang Y, Ma X, Zhang H, et al. Gene expression profile reveals abnormalities of multiple signaling pathways in mesenchymal stem cell derived from patients with systemic lupus erythematosus. Clin Dev Immunol. 2012;2012:826182–826112.
- Visnjic D, Kalajzic Z, Rowe DW, et al. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103(9):3258–3264.
- Schall N, Daubeuf F, Marsol C, et al. A selective neutraligand for CXCL12/SDF-1α with beneficial regulatory functions in MRL/Lpr lupus prone mice. Front Pharmacol. 2021;12:752194.
- Semerad CL, Christopher MJ, Liu F, et al. G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood. 2005;106(9):3020–3027.
- Dörner T, Giesecke C, Lipsky PE. Mechanisms of B cell autoimmunity in SLE. Arthritis Res Ther. 2011;13(5):243.
- Dörner T, Radbruch A, Burmester GR. B-cell-directed therapies for autoimmune disease. Nat Rev Rheumatol. 2009;5(8):433–441.
- Malkiel S, Barlev AN, Atisha-Fregoso Y, et al. Plasma cell differentiation pathways in systemic lupus erythematosus. Front Immunol. 2018;9:427.
- Zhu J, Garrett R, Jung Y, et al. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood. 2007;109(9):3706–3712.
- Balakumaran A, Robey PG, Fedarko N, et al. Bone marrow microenvironment in myelomagenesis: its potential role in early diagnosis. Expert Rev Mol Diagn. 2010;10(4):465–480.
- Crampton SP, Morawski PA, Bolland S. Linking susceptibility genes and pathogenesis mechanisms using mouse models of systemic lupus erythematosus. Dis Model Mech. 2014;7(9):1033–1046.
- Santiago-Raber ML, Laporte C, Reininger L, et al. Genetic basis of murine lupus. Autoimmun Rev. 2004;3(1):33–39.
- Kucia M, Jankowski K, Reca R, et al. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol. 2004;35(3):233–245.
- Devarapu SK, Kumar Vr S, Rupanagudi KV, et al. Dual blockade of the pro-inflammatory chemokine CCL2 and the homeostatic chemokine CXCL12 is as effective as high dose cyclophosphamide in murine proliferative lupus nephritis. Clin Immunol. 2016;169:139–147.
- Sugiyama T, Kohara H, Noda M, et al. Maintenance of the hematopoietic stem cell Pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25(6):977–988.
- Sun W, Meednu N, Rosenberg A, et al. B cells inhibit bone formation in rheumatoid arthritis by suppressing osteoblast differentiation. Nat Commun. 2018;9(1):5127.
- Espeli M, Bökers S, Giannico G, et al. Local renal autoantibody production in lupus nephritis. J Am Soc Nephrol. 2011;22(2):296–305.
- Sekine H, Watanabe H, Gilkeson GS. Enrichment of anti-glomerular antigen antibody-producing cells in the kidneys of MRL/MpJ-Fas(lpr) mice. J Immunol . 2004;1950;172(6):3913–3921.
- Starke C, Frey S, Wellmann U, et al. High frequency of autoantibody-secreting cells and long-lived plasma cells within inflamed kidneys of NZB/W F1 lupus mice. Eur J Immunol. 2011;41(7):2107–2112.
- Alexander T, Sarfert R, Klotsche J, et al. The proteasome inhibitior bortezomib depletes plasma cells and ameliorates clinical manifestations of refractory systemic lupus erythematosus. Ann Rheum Dis. 2015;74(7):1474–1478.
- Shachar I, Karin N. The dual roles of inflammatory cytokines and chemokines in the regulation of autoimmune diseases and their clinical implications. J Leukoc Biol. 2013;93(1):51–61.
- Jacquemin C, Augusto JF, Scherlinger M, et al. OX40L/OX40 axis impairs follicular and natural treg function in human SLE. JCI Insight. 2018;3(24):e122167.
- Cassese G, Arce S, Hauser AE, et al. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J Immunol. 2003;171(4):1684–1690.
- Minges Wols HA, Underhill GH, Kansas GS, et al. The role of bone marrow-derived stromal cells in the maintenance of plasma cell longevity. J Immunol. 2002;169(8):4213–4221.

