Abstract
Autoimmune diseases (AIDs) alter the placental immune environment leading to fetal loss. This study investigated the effects of AIDs on pregnancy and the placenta in AID-prone MRL/MpJ-Faslpr/lpr mice and wild-type MRL/MpJ, which were mated with male MRL/MpJ and MRL/MpJ-Faslpr/lpr at five months and defined as moLpr and moMpJ, respectively. AID indices (spleen weight and serum autoantibody levels) and fertility status (number and size of fetuses, morphology, and comprehensive gene expression of placentas) were evaluated on gestational day 15.5. Both strains showed equivalent fertility, but moLpr showed lighter placentas and fetuses than moMpJ, and decreased fertility with AID severity. moLpr placentas had a higher number of T cells, higher expression of genes associated with T helper 2 and T follicular helper functions, and altered expression of genes (Krt15, Slc7a3, Sprr2a3) that significantly regulate pregnancy or immunity. The gene expression of T cell migration-associated chemokines (Ccl5, Cxcl9) was significantly increased in moLpr placentas, and CCL5 and CXCL9 were detected in moLpr placentas, particularly in T cells and placenta-component cells, respectively. Thus, AID altered placental morphofunction and fertility in mice; however, fertility was maintained at the examined time points. This study enhances our understanding of placental alterations and gestational risk due to AIDs.
Introduction
Placenta serves as a structural and immunological barrier during pregnancy and is composed of heterogeneous cells derived from both the mother and fetus and plays crucial roles in maintaining pregnancy from implantation to parturition by exchanging metabolites [Citation1]. Placental abruption increases the risk of fetal loss in mammals [Citation2]. Since the placenta is composed of autologous or allogeneic cells, it must maintain a specific immunological environment during pregnancy. Briefly, the placenta has several inhibitory mechanisms against immunological rejection responses between the mother and fetus, including placenta-specific natural killer (NK) cells, which selectively attack trophoblasts that excessively invade maternal tissues and produce cytokines to maintain pregnancy [Citation3,Citation4]. In addition, the progression of pregnancy alters macrophage subsets and T helper (Th) 1/Th2 balance in the placenta, promoting implantation and immune tolerance in the fetus [Citation5]. Fetal cells express ligands that promote maternal T-cell apoptosis and do not express antigens recognized by maternal immune cells; these mechanisms contribute to the evasion of maternal immunity [Citation6,Citation7]. Regulatory T cells (Tregs) in the decidua (Dc) of the mouse placenta are also important for implantation and maintenance of pregnancy[Citation8].
Maternal autoimmune diseases (AIDs) are considered as risk factors for pregnancy in humans. In neonatal lupus, mother-derived autoantibodies attack the fetus via the placenta [Citation9,Citation10]. In addition, an altered maternal immune status due to pregnancy can exacerbate or alleviate the onset of AIDs, suggesting the possibility of an altered immune balance in the placenta or fetus in these cases[Citation11,Citation12]. Preeclampsia is a representative complication during pregnancy that is associated with substantial maternal and fetal morbidity and mortality and presents with hypertension and proteinuria in the mother, shallow placentation, and intrauterine growth restriction [Citation13]. Immunological factors, such as T cell and NK cell imbalance, have also been suggested as pathogenic factors in preeclampsia [Citation13]. Clinical cases of AIDs have been reported not only in humans but also in several other animals [Citation14,Citation15]. Importantly, placental structures differ among species. The transfer of antibodies via the placenta between the mother and fetus is commonly observed in animals, such as dogs and cats [Citation16,Citation17], which also develop AIDs with the production of autoantibodies [Citation14,Citation15]. The risk of immune imbalance in the placenta and fetus has been investigated mainly in the field of infectious diseases in animals. However, there have been few reports on the relationship between AIDs, changes in placental gestational immunity, and their effects on fetal phenotypes and placental morpho-function.
Species-specific differences in placental morphology complicate the analysis of the relationship between AIDs and pregnancy in mammals. Most spontaneous animal models of AIDs have been established in rodents, which have hemochorial placentas similar to those of primates. The mouse placenta is mainly composed of a heterogeneous labyrinth (Lb), a junctional zone (Jz), and maternal tissue-derived Dc, which functions as a structural boundary between the mother and foetus [Citation18]. In particular, trophoblasts surrounding the maternal blood in the Lb form the blood-placental barrier, which is an immunological barrier between the mother and foetus. Through these barriers, the experimental transfer of antibodies from the mother to the foetus via the placenta has been reported in mice [Citation19]. The MRL/MpJ-Faslpr/lpr (Lpr) mouse, a representative AID model carrying a mutant apoptosis-related TNF receptor superfamily member 6 (Fas), shows an increase in lymphocytes, mainly T cells [Citation20], and autoantibody production. MRL/MpJ (MpJ) mice used as controls in this study carried normal Fas. Lpr mice show systemic lupus erythematosus (SLE)-like symptoms, including lymphadenopathy, splenomegaly, and nephritis, as observed in humans and other animals [Citation14,Citation21,Citation22]. Previous studies have also suggested that Lpr causes reproductive disorders, such as age-related disturbances of the estrous cycle, decreased numbers of ovarian follicles, and impaired oocyte pick-up by the infundibulum of the oviducts [Citation23,Citation24]. Additionally, Lpr mice show high serum autoantibody levels during pregnancy [Citation25], one of which is associated with reduced fertility and placental and fetal growth [Citation26].
To investigate the effects of maternal AIDs on the establishment and maintenance of pregnancy, and the morphology and function of the placenta, we evaluated maternal fertility and placental morphology in Lpr mice. The data obtained in this study will contribute to our understanding of the pathogenesis of unexplained pregnancy abnormalities in animal and human AIDs cases.
Materials and methods
Animal ethics
All animal experiments were approved by the Institutional Animal Care and Use Committee of the Faculty of Veterinary Medicine, Hokkaido University (approval no. 21-0008). Animals were handled in accordance with the Guide for the Care and Use of Laboratory Animals, Faculty of Veterinary Medicine, Hokkaido University (approved by the Association for Assessment and Accreditation of Laboratory Animal Care International).
Animals, sample preparation, serological analysis, and histological analysis
At Japan SLC, Inc. (Hamamatsu, Japan), female Lpr mice were mated with male MpJ mice at 20 weeks of age, and pregnant mice were defined as moLpr (). As healthy controls, female MpJ mice were mated with male Lpr mice at 20 weeks of age, and pregnant mice were defined as moMpJ. A vaginal plug was observed on gestational day (GD) 0.5. These pregnant mice were purchased from Japan SLC, Inc. and maintained under specific pathogen-free conditions at 23 ± 1 °C with a constant humidity of 55 ± 5% under 12 h light-dark cycle in our facility.
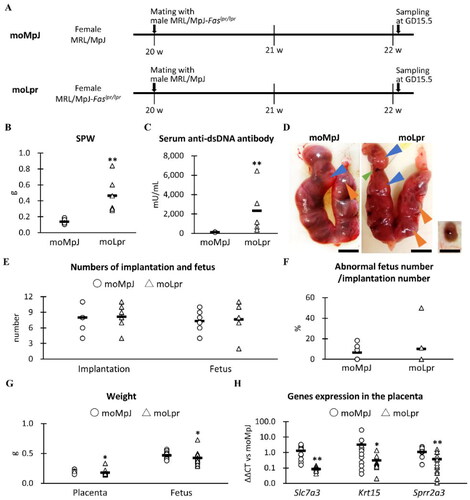
Figure 1. Experimental protocol, indices for systemic immunity and fertility, and placental gene expression associated with pregnancy.
(A) Experimental protocol. (B) Maternal spleen weight (SPW). (C) Maternal serum levels of anti-double-stranded (ds) DNA antibodies. (D) The uterus, placenta, and fetus of MRL/MpJ (MpJ) and MRL/MpJ-Faslpr/lpr (Lpr) mice. The uterus includes the placenta (orange arrowheads) and the fetus (blue arrowheads). The green arrowhead shows an absorption scar observed in the uterus. The right panel shows a placental remnant and an absorbed fetus in Lpr. Bars = 1 cm. (E) Number of implantations and fetuses. The number of implantations was defined as the total number of fetuses, abnormal fetuses, absorption scars, and placental remnants. (F) The ratio of the number of abnormal fetuses to the number of implants. (G) Placental and fetal weights. (H) Pregnancy-associated placental mRNA expression moMpJ: MRL/MpJ (MpJ) mother mated with Lpr father. moLpr: Lpr mother mated with MpJ father. Slc7a3: solute carrier family 7 (cationic amino acid transporter, y + system) member 3. Krt15: keratin 15. Sprr2a3: small proline-rich protein 2A1. Each bar represents the mean of each group. The number of animals or organs analyzed is summarized in . Significance for other strains at the same age and gestational day (GD) (Mann-Whitney U-test, *: P < 0.05, ** P < 0.01).
On GD 15.5, pregnant mice were euthanized by cutting the carotid arteries under deep anesthesia using an intraperitoneal injection of a mixture of medetomidine (0.3 mg/kg), midazolam (4 mg/kg), and butorphanol (5 mg/kg). The numbers of mice and their organs used in this study are summarized in . The serum, uterus, foetus, and spleen were collected. To evaluate systemic immune condition of each mother, the spleen weight (SPW) and serum levels of anti-double strand DNA (dsDNA) antibody were quantified using LBIS® Anti-dsDNA-mouse ELISA Kit (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan). After separating the fetuses from the uterus, the number of fetuses (No.Fe) was counted, and absorption scars and placental remnants were defined as abnormal fetuses. The number of implantations (No.Im) was defined as the sum of the total of No.Fe and number of abnormal fetuses. From these values, the ratio of abnormal fetal number to No.Im (AbFR) was calculated. The mean values of placental weight (PW) and fetal weight (FW) were calculated using 1–4 fetuses from each mother. These values were used as fertility indices. Furthermore, placentas were fixed with 4% paraformaldehyde at 4 °C overnight. For RNA analysis, placentas with measured PW were stored in RNAlater (Thermo Fisher Scientific, Waltham, MA, USA). Fixed placentas were embedded in paraffin and cut into sections (3 μm-thick) including the cross-section of the placenta, which was perpendicular to the plane proximate to the fetus. Deparaffinized sections were stained with hematoxylin (HE) or analyzed by immunohistochemistry (IHC) or immunofluorescence (IF).
Table 1. The number of samples used in this study.
IHC and IF
IHC for vimentin (Vim), Gr-1, IBA1, B220, and CD3 was performed to identify the three placental layers and to detect neutrophils, macrophages, B cells, and T cells, respectively. Additionally, an anti-chemokine (C-X-C motif) ligand 9 (CXCL9) antibody was used to detect target chemokines. IHC and IF analyses were performed as described previously [Citation27]. Briefly, the sections were deparaffinized, and antigen retrieval was performed. Subsequently, to block internal peroxidase activity, the sections were soaked in methanol containing 0.3% H2O2 for 20 min at 25 °C. After washing thrice in phosphate-buffered saline (PBS), the sections were incubated with a blocking serum for 1 h at 25 °C to block the nonspecific reaction. Subsequently, sections were incubated with primary antibodies overnight at 4 °C. The sections were then washed three times in PBS and incubated with secondary antibodies for 30 min at 25 °C and washed three times in PBS. The sections were then incubated with streptavidin-conjugated horseradish peroxidase (SABPO® kit; Nichirei, Tokyo, Japan) for 30 min at 25 °C, washed three times in PBS, and the immune positive reaction was visualized with 3,3′-diaminobenzidine tetrahydrochloride in 0.05 M Tris-HCl buffer-H2O2 solution. Finally, the sections were stained with hematoxylin. IF was performed to detect the C-C motif chemokine 5 (CCL5). Paraffin sections were deparaffinized and antigens were retrieved and blocked using normal serum for IHC. After overnight incubation with primary antibody at 4 °C, the sections were incubated with secondary antibody for 1 h at 25 °C. Sections were observed under a fluorescence microscope (BZX-710; Keyence, Osaka, Japan). Details of antigen retrieval, dilution, and antibodies used are listed in Supplemental Table 1.
Histoplanimetry
Each layer of placentas including Lb, Jz, and Dc was analyzed by using more than two sections for Vim, having the 100 μm distance with the estimated midline. Since Vim-positive (+) Lb and Dc were clearly separated by the Vim-negative (–) Jz, the area of each layer was traced and measured. The ratio of the area of each layer to that of the entire placental section was calculated. Moreover, immune cells, including Gr-1+ neutrophils, IBA1+ macrophages, B220+ B cells, CD3+ T cells, and NK cells, which showed cytoplasmic granules on HE-stained sections, were counted in four sections. NDP.view 2 (Hamamatsu Photonics, Shizuoka, Japan) was used for tracing, measuring, and counting.
Microarray analysis
Total RNA was purified from placental tissues in RNAlater solution (Thermo Fisher Scientific) using TRIzol® Reagent (Thermo Fisher Scientific) following the manufacturer’s protocol. Eight RNA samples from four pregnant MpJ mice and five RNA samples from three pregnant Lpr mice were collected and purified using the RNeasy Micro Kit (QIAGEN, Hilden, Germany) following the manufacturer’s protocol. One pooled sample from each group was prepared and RNA quality was determined using an Agilent 2100 Bioanalyzer series II (Agilent; Santa Clara, CA, USA). Complementary DNA (cDNA) was synthesized and complementary RNA (cRNA) was labeled using a Low Input Quick Amp Labeling Kit (Agilent). Hybridization was performed using a Gene Expression Hybridization Kit and SurePrint G3 Mouse 8 × 60K ver.2.0 (Agilent). Scanning, quantification, and normalization were performed using an Agilent Technologies Microarray Scanner (Agilent), Agilent Feature Extraction 12.0.3.1 (Agilent), and GeneSpring GX 14.9 (Agilent), respectively. STRING (ELIXIR, Cambridgeshire, UK) was used for gene ontology (GO) analysis, focusing on biological processes.
Quantitative polymerase chain reaction (qPCR)
Total RNA was purified from placental tissues in RNAlater solution using TRIzol Reagent following the manufacturer’s protocol. RNA samples from 14 placentas from six pregnant MpJ and five pregnant Lpr mice were collected, purified, and used as templates for cDNA synthesis using ReverTra Ace qPCR RT Master Mix (Toyobo Co., Ltd, Osaka, Japan). QPCR analysis was performed on the cDNA (20 ng/μL) using THUNDERBIRD® SYBR® qPCR Mix (Toyobo Co., Ltd.) and the gene-specific primers listed in Supplemental Table 2. Specific primer pairs were designed and selected based on the following criteria: 1) the primer pairs did not amplify a product of less than 1000 bp derived from nonspecific genes, even if primer target sequences had several complementarities between target genes and others, and 2) more than five base sequences of primers were different from the nonspecific gene-derived sequences. The qPCR cycling conditions were: 95 °C for 1 min, (95 °C for 15 s and 60 °C for 45 s [40 cycles]). Data were normalized to the values of actin beta (Actb) and moMpJ at five months GD15.5) using the delta-delta Ct method.
Statistical analysis
The results are expressed as the mean ± standard error (SE) and were statistically analyzed in a non-parametric manner. The significance between the two groups was analyzed using the Mann-Whitney U-test (p < 0.05). The correlation between the two parameters was analyzed using Spearman’s correlation test (p < 0.05).
Results
Systemic immune condition, fertility, and expressed genes in the placenta
In this study, moMpJ and moLpr were synthesized. SPW and serum anti-dsDNA antibodies were evaluated as indices of systemic immunity; moLpr mice showed significantly higher values for both indices than moMRL mice ( and ).
For fertility indices, No.Im and No.Fe were visually counted, as shown in , and then AbFR was calculated. Although resorption scars of foetuses and placental remnants were observed in the uteri of both strains, there were no significant differences in Im, Fe, and AbFR between moMpJ and moLpr mice ( and ). PW and FW were also compared separately, resulting in significantly lower values for moLpr than for moMpJ ().
To clarify the changes in gene expression in the placenta caused by AID, we performed a comprehensive gene expression analysis. In particular, we focused on ten of the most up- and down-regulated genes in the placentas of moLpr on GD15.5, compared with those of moMpJ (Supplemental Figure 1). No significant strain differences were detected in the genes upregulated by qPCR (Supplemental Figure 1A). However, significantly decreased expression was confirmed in solute carrier family 7 (cationic amino acid transporter, y+ system), member 3 (Slc7a3), keratin 15 (Krt15), and small proline-rich protein 2A3 (Sprr2a3) for the placentas of moLpr compared to moMRL by qPCR among the downregulated sets of genes examined (Supplemental Figure 1B, ).
summarizes the correlations between the indices of systemic immune conditions, fertility, and gene expression, as shown in . Serum SPW and anti-dsDNA antibody levels in moMpJ and moLpr mice were positively correlated with PW. Furthermore, anti-dsDNA antibody serum levels in moLpr mice correlated negatively with No.Im and No.Fe and positively with AbFR. Furthermore, a negative correlation was found between SPW and serum levels of anti-dsDNA antibody, Slc7a3 and Krt15 expression in the placenta in all mice, SPW and Sprr2a3 in all mice, and between anti-dsDNA antibody and Krt15 in moLpr. In contrast, the placental expression of Krt15 in moMpJ and moLpr, or that of Sprr2a3 in moMpJ, was negatively correlated with AbFR. The expression of Krt15 and Sprr2a3 in all mice and moMpJ was positively correlated with PW.
Table 2. The correlations between indices for maternal autoimmune disease and fertility.
Histological characteristics of the placenta
The placentas of moMRL and moLpr mice consisted of the Lb, Jz, and Dc from the fetal side (). To identify these layers more clearly, IHC was performed for Vim (). To quantify the three-layer ratio, Lb was the largest layer in both strains, and no significant differences in each layer were observed between the two strains ().
Figure 2. Morphological analysis of the placenta.
(A) Placental histology with the labyrinth (Lb), junctional zone (Jz), and decidua (Dc) observed on the fetal side. Hematoxylin and eosin staining (HE). Bars = 100 μm. (B) Immunohistochemistry (IHC) of placental vimentin (vim). Lb and Dc were positive (+) for Vim but negative for Jz. Bars = 500 μm. (C) The ratio of each layer area to the total placental area. (D) The histology of each placental layer. Abundant maternal blood was observed (asterisk) in the Lb, which was surrounded by trophoblast cells (blue arrowheads). Several nucleated fetal red blood cells were also observed (orange arrowheads). In IHC for Vim, fetal vascular endothelial (Ve) cells were Vim+. In Jz, glycogen cells (asterisks), spongy trophoblast cells (orange arrowheads), and giant trophoblast cells (blue arrowheads) were observed; however, no Vim+ cells were observed. In Dc, abundant natural killer (NK) cells (black arrowheads) and decidual cells (orange arrowheads) were observed. In IHC, decidual cells (orange arrowheads) and maternal Ve were Vim+. HE stain and IHC. Bars = 100 μm. moMpJ: MRL/MpJ (MpJ) mice mated with MRL/MpJ-Faslpr/lpr (Lpr) mice. moLpr: Lpr mother mated with MpJ father. Each bar represents the mean of each group. The number of animals or organs analyzed is summarized in .
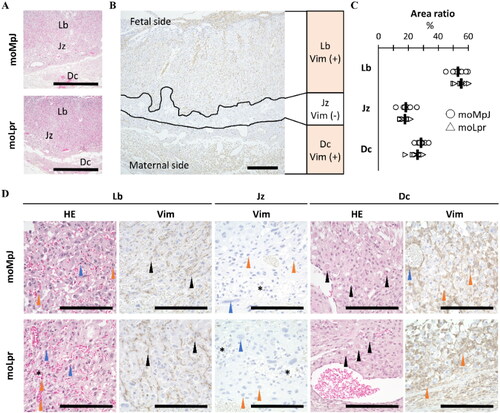
As shown in , Lb in both strains contained abundant maternal and fetal blood vessels and trophoblasts, whereas fetal vascular endothelial (Ve) cells showed a Vim+ reaction. Glycogen cells, spongy trophoblasts, and trophoblast giant cells were observed in the Jz of both strains; however, no Vim+ cells were detected. Dc cells mainly originate from maternal tissues and both strains contain abundant NK cells, Vim+ decidual cells, and Vim+ maternal Ve cells. However, no remarkable differences were observed in the structures of the strains.
Immune cells found in the placenta
Histological observations using HE- or IHC-stained sections detected NK cells mainly in the extravascular area of Dc, Gr-1+ neutrophils in both extravascular regions and blood vessels throughout the entire placenta, B220+ B cells in the blood vessels of Lb, and CD3+ T cells in both extravascular and intravascular spaces of Lb (). Histoplanimetry to quantify these immune cells showed that the number of lymphocytes tended to be higher in moLpr mice than in moMpJ mice, resulting in a significant difference in T cells ().
Figure 3. Immune cells observed in the placenta.
(A) Histological analysis of placental immune cells. In the decidua (Dc), natural killer (NK) cells contained eosinophilic granules (black arrowheads). Immunohistochemistry (IHC) revealed Gr-1-positive (+) neutrophils in the extravascular space and blood vessels of the Dc. B220+ B cells in blood vessels (black arrowheads), CD3+ T cells in the extravascular area (orange arrowheads), and blood vessels (blue arrowheads) in the labyrinth (Lb). Squares show high-magnification images of each positive cell. Hematoxylin and eosin staining. IHC. Bars = 100 μm. (B) The number of immune cells in the placenta. (C) The mRNA expression of genes associated with T cells. moMpJ: MRL/MpJ (MpJ) mice mated with MRL/MpJ-Faslpr/lpr (Lpr) mice. moLpr: Lpr mother mated with MpJ father. Th: T helper cells. Tc: T killer cells. Tfh: T follicular helper cells. Tregs: Regulatory T cells. Each bar represents the mean of each group. The number of animals or organs analyzed is summarized in . Significance for another strain at the same age and gestational age (Mann-Whitney U-test, *: P < 0.05, ** P < 0.01).
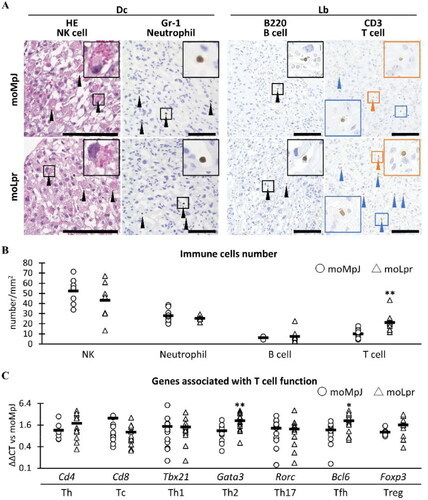
Based on the histoplanimetric data of T cells shown in , the expression of genes associated with T cell function in the placentas was examined by qPCR (). The expression of GATA-binding protein 3 (Gata3) and B cell leukemia/lymphoma 6 (Bcl6), genes associated with the function of Th2 cells and T follicular helper (Tfh) cells, respectively, was significantly higher in moLpr than in moMRL.
Immune-associated genes expressed in the placenta
Supplemental Figure 2 summarizes the identified GO associated with immunological processes using 2-fold upregulated genes in the microarray analysis of moLpr placentas: defense response (GO:0006952, 28 genes, false discovery rate [FDR] = 0.0025), immune response (GO:0006955, 25 genes, FDR = 0.0034), and defense response to other organisms (GO:0098542, 20, FDR = 0.0295).
Based on the results associated with placental T cells shown in , we focused on Ccl5 and Cxcl9, genes related to T cell chemotaxis and function that were commonly included as the top 10 genes in the three immune-associated GO terms (Supplemental Figure 2). The expression of Ccl5 and Cxcl9 was significantly increased in the placentas of moLpr mice compared to that in moMpJ qPCR analysis ().
Figure 4. Placental gene expression and protein location associated with T cells.
mRNA expression associated with T cells in the placenta. (B) CCL5 localization in the moLpr placenta. (C) CXCL9 localization in the placenta. moMpJ: MpJ mother mated with Lpr father. moLpr: Lpr mother mated with MpJ father. ND: not detected. CCL5: C-C motif chemokine 5. CXCL9: Chemokine (C-X-C motif) ligand 9. Each bar represents the mean of each group. The number of animals or organs analyzed is summarized in Table 1. Significance for another strain at the same age and gestational age (Mann-Whitney U-test, *: P < 0.05, ** P < 0.01).
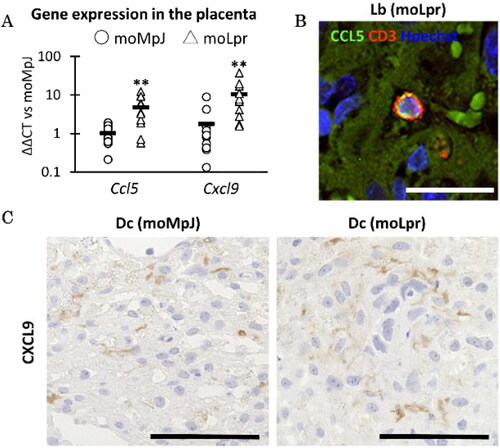
Furthermore, we examined the localisation of CCL5 and CXCL9 in the placenta. A small number of CCL5+ cells were localised in the blood vessels of Lb mice and co-localised with CD3+ T cells in moLpr mice () but not in moMpJ mice. CXCL9 was localised to the placenta-containing cells in the Dc of both strains ().
summarises the correlations among systemic immune conditions, fertility indices, and the number of immune cells. The neutrophil number in the placenta was positively correlated with SPW, PW, and FW in moLpr and AbFR in all mice and moMpJ, and negatively correlated with Im and Fe in moLpr mice. The number of B cells in the placentas correlated positively with serum anti-dsDNA antibody levels examined in both strains and negatively with No.Im and No.Fe examined in all mice and moLpr. Furthermore, the T cell number in the placenta positively correlated with both the systemic immune condition indices examined in all mice and FW examined by moLpr.
Table 3. The correlations among indices for autoimmune disease, fertility, immune cell quantities, and T cell.
For placental gene expression associated with T cell function, Gata3 in all mice correlated positively with SPW and serum anti-dsDNA antibody levels and negatively correlated with FW. Bcl6 in all mice positively correlated with serum anti-dsDNA antibody levels. Ccl5 was positively correlated with SPW in all mice, moLpr, serum anti-dsDNA antibody in all mice, AbFR, PW, and FW in moLpr, and negatively correlated with FW in moMpJ. Cxcl9 expression positively correlated with systemic immune condition indices in all mice.
Discussion
In the present study, fertility and placental morpho-function were compared between moMpJ and moLpr mice. As a result, moLpr mice maintained fertility equivalent to that of moMpJ mice. However, the severity of AIDs was associated with decreased fertility. Furthermore, moLpr placentas showed an increase in T cells, and the present analysis suggested changes in gene expression related to T cell differentiation, chemotaxis, and maintenance of pregnancy. These results indicate that while maternal immune abnormalities affect fertility and placental morphology, a compensatory mechanism maintains pregnancy in mice.
Although moLpr showed significantly higher AID indices than moMpJ, the two strains had similar No.Im, No.Fe, and AbFR values, suggesting that there was no significant difference in fertility. However, the placentas and fetuses in moLpr mice were significantly lighter than those in moMpJ, and the indices of reproductive ability including No.Im, No.Fe, and AbFR were significantly correlated with the serum autoantibody levels in moLpr. In humans, some pregnant patients with SLE tend to lose their fetus, and it has been considered that one of the causes is the transfer of maternal serum autoantibodies to the fetus via the placenta [Citation9,Citation10]; experimental transfer of antibodies from mother to foetus via the placenta has been reported in mice [Citation19]. In addition, altered morphology and function of the ovary, including T cell infiltration, oestrus cycle irregularity, and luteal deficiency, as well as reduced oocyte pick-up rate by the infundibulum of the oviducts, have been reported in Lpr mice [Citation23,Citation24], which might also affect their altered phenotypes during pregnancy.
In the present study, there were no significant morphological differences between the examined placenta-composing layers and cells; however, the weight of the moLpr placentas was significantly lower than that of the moMpJ placentas. In humans, complications of maternal AIDs and fetal heart disease can cause fetal circulatory failure, including placental edema and fetal hydrops [Citation28–30]. Shallow placentation and intrauterine growth restriction have been reported in preeclampsia [Citation13]. In the present study, moLpr mice showed decreased placental weight with increasing severity of AID, but the fetuses did not show any phenotypes, suggesting increased or congested blood flow. Although further analysis is needed to clarify the details of these pathological events, we performed a histological analysis on GD15.5, which may have occurred before the appearance of placental pathological changes. Furthermore, in moLpr mice, nutrition for lost fetuses may have been compensated by other fetuses.
This study also investigated changes in gene expression in the placentas of AID mice. The gene expression of Slc7a3, Krt15, and Sprr2a3 was lower in moLpr placentas than in moMpJ placentas. Both SLC7A3 and KRT15 are associated with implantation [Citation31]. In particular, SLC7A3, which is involved in the transport of arginine, which is important for fetal growth after implantation in pigs, and in the secretion of interferon tau, a pregnancy recognizer, in ruminants [Citation32,Citation33]. Thus, the downregulation of Krt15 and Slc7a3 in the placenta may affect reproductive mechanisms. The role of SPRR2A-producing cells are unclear in the placenta but are known to be involved in epidermal protection and epithelial repair. SPRR2A is induced by estrogen in the uterus, because its expression increases during estrus [Citation34]. Importantly, the Lpr strain exhibits functional changes in estrogen receptors, including decreased DNA-binding activity[Citation35]. Thus, altered gene expression in moLpr placentas suggests modified gene expression in placental or immune cells and subsequent functional changes associated with low fertility, light placentas, or maintenance of pregnancy with AIDs.
In the mammalian placenta, various immune cells maintain a specific immune environment during pregnancy and an immune balance that can affect pregnancy status [Citation3,Citation4]. In the present study, neutrophils and lymphocytes were observed in the placenta of both strains. While we observed placental NK cells, their quantitative values and gene expression of NK cell markers (Supplemental Figure 3) did not differ remarkably between moMpJ and moLpr mice. Macrophages expressing IBA1 or CD163 have also been reported in rat placentas [Citation36]. IBA1+ macrophages were also detected in mouse placentas, but their quantitative values and the expression of macrophage marker-related genes (Cd163, Cd1d1, Cd1d2, Cd209a, Cd68, Cd80, Cd86, Cxcr4, Fcgr3, Itgam, Itgax, Ky, Mrc1, Tlr2, and Tlr4) examined by microarray (these molecules are listed in another study [Citation37]) in the placenta did not differ between moMpJ and moLpr (Supplemental Figures 3 and 4). The neutrophil number was also comparable among the strains, but correlated negatively with fertility indices and positively with PW and FW in moLpr mice. These data suggest that moLpr mice had lighter placentas and fetuses compared to moMpJ mice, but individual values of moLpr increased with the infiltration of neutrophils into the placenta. Furthermore, the placental neutrophil number also showed a positive correlation with AbFR. Consistent with these results, mating between mouse strains, (CBA/J) and (DBA/2J), an abortion-prone model, also showed an increase in placental neutrophils and an inflammatory response between the mother and fetus associated with fetal death [Citation38].
Importantly, the number of T cells was higher in moLpr placentas than in moMpJ placentas. The Lpr strain showed a systematic increase in lymphocytes, mainly T cells, due to the mutation of Fas. Another [Citation20] study suggested the expansion of splenic Tfh cells, which contributes to the progression of AIDs in Lpr mice [Citation39]. In fact, in the present study, moLpr placentas showed higher expression of genes related to Th2 and Tfh, and Th2 and Tfh enhanced AID by promoting autoantibody production [Citation39–41]. These results suggest that increased placental T cells are associated with AIDs progression and an altered local immune system in moLpr placentas.
Placental expression of Ccl5 and Cxcl9 increased in moLpr cells, although their expression was suppressed in a healthy state [Citation42]. CCL5 and CXCL9 were expressed in T cells and placental component cells, respectively, in the placenta of moLpr, but not in moMpJ. These results reflect altered T cell function in the placenta, particularly migration, because CCL5 and CXCL9 are involved in the chemotaxis and/or differentiation of Th2 and Th1 cells, respectively [Citation43,Citation44]. Th2 predominance is maintained during pregnancy [Citation45]; Th2 enhances CCL5 production, whereas Th1 enhances CXCL9 production [Citation46,Citation47]. Thus, the Th1/Th2 balance contributes to the maintenance of pregnancy; however, the moLpr results might indicate an imbalance in AID. Moreover, the upregulation of CXCL9 suggests an association with abortion[Citation48].
In humans, AIDs has a significant effect on mothers during and after pregnancy. In particular, preeclampsia, which occurs during pregnancy, is characterized by hypertension and proteinuria [Citation13], and its pathology has not been fully elucidated, although the involvement of endoglin has been reported [Citation49]. In addition, autoimmune responses against angiotensin receptors [Citation50] and the elevation of genes associated with AIDs in the placenta [Citation51] have been suggested in preeclampsia. AIDs can affect mothers after childbirth [Citation52]; autoimmune thrombocytopenia is associated with an increased risk of severe postpartum bleeding [Citation53]. Recently, justification for prenatal diagnosis of these diseases has also been advocated, and various methods using maternal blood, amniotic fluid, and placental cells are currently being attempted for prenatal risk assessment [Citation54,Citation55]. In particular, Lpr mice carry an identified gene mutation; therefore, an experimental challenge using moLpr and targeting the mutated Fas gene may be useful for evaluating the methodology of prenatal diagnosis and gene therapy for AID-related reproductive abnormalities.
In conclusion, this study showed that the development of AIDs in female mice diminished fertility in correlation with disease severity and affected immune cell distribution and immune- and pregnancy-associated gene expression, particularly those of T cells, although fertility remained similar to that of healthy mice. These data suggest that AIDs can cause reproductive disorders during pregnancy and may be crucial for understanding previously unexplained cases.
Supplemental Material
Download Zip (390.1 KB)Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data supporting the findings of this study are available from the corresponding author upon request.
Additional information
Funding
References
- Hill PMM, Young M. Placental transfer of free amino acids against varying concentrations. J Physiol. 1973;235(2):1–11.
- Baschat AA, Hecher K. Fetal growth restriction due to placental disease. Semin Perinatol. 2004;28(1):67–80.
- Colucci F, Boulenouar S, Kieckbusch J, et al. How does variability of immune system genes affect placentation? Placenta. 2011;32(8):539–545.
- Hanna J, Goldman-Wohl D, Hamani Y, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12(9):1065–1074.
- Lin H, Mosmann TR, Guilbert L, et al. Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J Immunol. 1993;151(9):4562–4573.
- Abrahams VM, Straszewski-Chavez SL, Guller S, et al. First trimester trophoblast cells secrete fas ligand which induces immune cell apoptosis. Mol Hum Reprod. 2004;10(1):55–63.
- Apps R, Murphy SP, Fernando R, et al. Human leucocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology. 2009;127(1):26–39.
- Zenclussen AC, Kökény G, Thimm O, et al. Mechanisms behind flare of renal lupus during murine pregnancy. Reprod Biomed Online. 2008;17(1):114–126.
- Buyon JP, Waltuck J, Caldwell K, et al. Relationship between maternal and neonatal levels of antibodies to 48 kDa SSB(La), 52 kDa SSA(Ro), and 60 kDa SSA(Ro) in pregnancies complicated by congenital heart block. J Rheumatol. 1994;21:1943–1950.
- Petri M, Orbai A-M, Alarcón GS, et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64(8):2677–2686.
- de Man YA, Dolhain RJEM, van de Geijn FE, et al. Disease activity of rheumatoid arthritis during pregnancy: results from a nationwide prospective study. Arthritis Rheum. 2008;59(9):1241–1248.
- Petri M, Howard D, Repke J. Frequency of lupus flare in pregnancy. The Hopkins lupus pregnancy center experience. Arthritis Rheum. 1991;34(12):1538–1545.
- Phipps EA, Thadhani R, Benzing T, et al. Pre-eclampsia: pathogenesis, novel diagnostics and therapies. Nat Rev Nephrol. 2019;15(5):275–289.
- Bennett D. Immune-based non-erosive inflammatory joint disease of the dog. 1. Canine systemic lupus erythematosus. J Small Anim Pract. 1987;28(10):871–889.
- Ellis J, Bell R, Barnes DC, et al. Prevalence and disease associations in feline thrombocytopenia: a retrospective study of 194 cases. J Small Anim Pract. 2018;59(9):531–538.
- Harding SK, Bruner DW, Bryant IW. The transfer of antibodies from the mother cat to her newborn kittens. Cornell Vet. 1961; 51: 535–539.
- Stoffel MH, Friess AE, Hartmann SH. Ultrastructural evidence of transplacental transport of immunoglobulin G in bitches. J Reprod Fertil. 2000;118(2):315–326.
- Elmore SA, Cochran RZ, Bolon B, et al. Histology atlas of the developing mouse placenta. Toxicol Pathol. 2022;50(1):60–117.
- Paoletti LC, Pinel J, Kennedy RC, et al. Maternal antibody transfer in baboons and mice vaccinated with a group B streptococcal polysaccharide conjugate. J Infect Dis. 2000;181(2):653–658.
- Watanabe-Fukunaga R, Brannan CI, Copeland NG, et al. Lymphoproliferation disorder in mice explained by defects in fas antigen that mediates apoptosis. Nature. 1992;356(6367):314–317.
- Fields ML, Sokol CL, Eaton-Bassiri A, et al. Fas/fas ligand deficiency results in altered localization of anti-double-stranded DNA B cells and dendritic cells. J Immunol. 2001;167(4):2370–2378.
- Hewicker M, Kromschröder E, Trautwein G. Detection of circulating immune complexes in MRL mice with different forms of glomerulonephritis. Z Versuchstierkd. 1990;33(4):149–156.
- Hosotani M, Ichii O, Nakamura T, et al. Autoimmune abnormality affects ovulation and oocyte-pick-up in MRL/MpJ-Fas lpr/lpr mice. Lupus. 2018;27(1):82–94.
- Otani Y, Ichii O, Otsuka-Kanazawa S, et al. MRL/MpJ-Fas(lpr) mice show abnormalities in ovarian function and morphology with the progression of autoimmune disease. Autoimmunity. 2015;48(6):402–411.
- Isonishi S, Kanai Y. Antibody to poly (ADP-ribose) as a predictor of obstetric complications in autoimmune MRL/Mp-lpr/lpr mice: basis for its application to pregnant patients with systemic lupus erythematosus. Immunol Lett. 1988;18(1):61–66.
- Cohen J, Bakimer R, Blank M, et al. Pathogenic natural anti-cardiolipin antibodies: the experience from monoclonal gammopathy. Clin Exp Immunol. 1994;97(2):181–186.
- Yamakawa T, Ichii O, Nakamura T, et al. Modified foreign body reaction to silicone imbedded in subcutaneous tissues by different mouse systemic immune conditions. J Biomed Mater Res A. 2022;110(12):1921–1931.
- Hoda M, Scott W, Sharma K, et al. Reversal of fetal heart block in antibody-positive mother after hydroxychloroquine and dexamethasone. Pediatr Cardiol. 2023;44(3):727–731.
- Vidaeff AC, Pschirrer ER, Mastrobattista JM, et al. Mirror syndrome. J Reprod Med. 2002;47(9):770–774.
- Zon EM, Nik Lah NAZ, Hoo PS. Late-onset mirror syndrome. Malays Fam Physician. 2021;16(1):129–132.
- Nuño-Ayala M, Guillén N, Arnal C, et al. Cystathionine β-synthase deficiency causes infertility by impairing decidualization and gene expression networks in uterus implantation sites. Physiol Genomics. 2012;44(14):702–716.
- Gao H, Wu G, Spencer TE, et al. Select nutrients in the ovine uterine lumen. III. Cationic amino acid transporters in the ovine uterus and peri-implantation conceptuses. Biol Reprod. 2009;80(3):602–609.
- Steinhauser CB, Wing TT, Gao H, et al. Identification of appropriate reference genes for qPCR analyses of placental expression of SLC7A3 and induction of SLC5A1 in porcine endometrium. Placenta. 2017;52:1–9.
- Tan YF, Sun XY, Li FX, et al. Gene expression pattern and hormonal regulation of small proline-rich protein 2 family members in the female mouse reproductive system during the estrous cycle and pregnancy. Reprod Nutr Dev. 2006;46(6):641–655.
- Thomas T, Gunnia UB, Seibold JR, et al. Restoration of the DNA binding activity of estrogen receptor in MRL-lpr/lpr mice by a polyamine biosynthesis inhibitor. Arthritis Rheum. 1991;34(1):55–62.
- Hume DA, Teakle N, Keshvari S, et al. Macrophage deficiency in CSF1R-knockout rat embryos does not compromise placental or embryo development. J Leukoc Biol. 2023;114(5):421–433.
- Mezouar S, Katsogiannou M, Ben Amara A, et al. Placental macrophages: origin, heterogeneity, function and role in pregnancy-associated infections. Placenta. 2021;103:94–103.
- Hosseini MS, Ali-Hassanzadeh M, Nadimi E, et al. Stereological study of the placental structure in abortion-prone mice model (CBA/J × DBA/2J). Ann Anat. 2020;230:151508.
- Yang X, Yang J, Chu Y, et al. T follicular helper cells mediate expansion of regulatory B cells via IL-21 in lupus-prone MRL/lpr mice. PLOS One. 2013;8(5):e62855.
- Charles N, Hardwick D, Daugas E, et al. Basophils and the T helper 2 environment can promote the development of lupus nephritis. Nat Med. 2010;16(6):701–707.
- Yamashita M, Ukai-Tadenuma M, Miyamoto T, et al. Essential role of GATA3 for the maintenance of type 2 helper T (Th2) cytokine production and chromatin remodeling at the Th2 cytokine gene loci. J Biol Chem. 2004;279(26):26983–26990.
- Silasi M, Mor G. Decidual stromal cells as regulators of T-cell access to the maternal-fetal interface. Am J Reprod Immunol. 2012;68(4):279–281.
- Thomas MS, Kunkel SL, Lukacs NW. Regulation of cockroach antigen-induced allergic airway hyperreactivity by the CXCR3 ligand CXCL9. J Immunol. 2004;173(1):615–623.
- Zhang Q, Qin J, Zhong L, et al. CCL5-Mediated Th2 immune polarization promotes metastasis in luminal breast cancer. Cancer Res. 2015;75(20):4312–4321.
- Saoudi A, Kuhn J, Huygen K, et al. TH2 activated cells prevent experimental autoimmune uveoretinitis, a TH1-dependent autoimmune disease. Eur J Immunol. 1993;23(12):3096–3103.
- Farber JM. A macrophage mRNA selectively induced by gamma-interferon encodes a member of the platelet factor 4 family of cytokines. Proc Natl Acad Sci U S A. 1990;87(14):5238–5242.
- Meyer-Hoffert U, Lezcano-Meza D, Bartels J, et al. Th2- and to a lesser extent Th1-type cytokines upregulate the production of both CXC (IL-8 and gro-alpha) and CC (RANTES, eotaxin, eotaxin-2, MCP-3 and MCP-4) chemokines in human airway epithelial cells. Int Arch Allergy Immunol. 2003;131(4):264–271.
- Spathakis M, Filidou E, Pappa C, et al. Spontaneous abortion is associated with differentially expressed angiogenic chemokines in placenta and decidua. Arch Gynecol Obstet. 2023;308(3):821–830.
- Margioula-Siarkou G, Margioula-Siarkou C, Petousis S, et al. The role of endoglin and its soluble form in pathogenesis of preeclampsia. Mol Cell Biochem. 2022;477(2):479–491.
- Xia Y, Kellems RE. Is preeclampsia an autoimmune disease? Clin Immunol. 2009;133(1):1–12.
- Tsai S, Hardison NE, James AH, et al. Transcriptional profiling of human placentas from pregnancies complicated by preeclampsia reveals disregulation of sialic acid acetylesterase and immune signalling pathways. Placenta. 2011;32(2):175–182.
- Privitera AA, Fiore M, Valenti G, et al. The role of serum potassium and sodium levels in the development of postpartum hemorrhage. A retrospective study. It Journ Gyn Obs. 2020;32(02):126–135.
- Care A, Pavord S, Knight M, et al. Severe primary autoimmune thrombocytopenia in pregnancy: a national cohort study. BJOG. 2018;125(5):604–612.
- Gullo G, Scaglione M, Buzzaccarini G, et al. Cell-free fetal DNA and non-invasive prenatal diagnosis of chromosomopathies and pediatric monogenic diseases: a critical appraisal and medicolegal remarks. J Pers Med. 2022;13(1):1.
- Medenica S, Abazovic D, Ljubić A, et al. The role of cell and gene therapies in the treatment of infertility in patients with thyroid autoimmunity. Int J Endocrinol. 2022;2022:4842316–4842310.

