Abstract
Soil-transmitted helminth (STH) among children aged 24–59 months is one cause of chronic infection that could lead to stunting. The association of 25(OH)D and immune responses during chronic infection in stunted populations has not yet been well established. An association study of case–control data was conducted in Bandung district from October 2019 to January 2023. Sociodemographic factors, stool samples, and serum levels of 25(OH)D, interleukin-4 (IL-4), interleukin-5 (IL-5), and interleukin-13 (IL-13) were assessed. Statistical analysis was performed to evaluate the prevalence and association of 25(OH)D, IL-4, IL-5, and IL-13 with the burden of STH infection in stunted children. In total, 401 stunted children were recruited. A higher burden of STH infection was found for lower levels of IL-5 (r = −0.477; p = 0.004) and IL-13 (r = −0.433; p = 0.028). Thus, 25(OH)D, IL-4, IL-5, and IL-13 play a role in the burden of STH infection.
1. Introduction
Soil-transmitted helminth (STH) infections are the most common disease in the world, with an estimated 1.5 billion infected individuals or 24% of the world’s population [Citation1]. A report by the Ministry of Health of the Republic of Indonesia showed that the prevalence of helminthiasis in Indonesia has reached 28.12%. The prevalence of STH infections in the pre-school age group (24–59 months) ranks second, with a prevalence of around 20–30% [Citation2–4].
The low worm burden at the start of infection causes little harm to the epithelial barrier. While inadequate to elicit a protective Th2 response, this small amount of damage may allow for opportunistic invasion by commensal bacteria, resulting in the production of antimicrobial peptides (AMPs) and IgA. The amount of barrier damage is enhanced by recurrent infections, leading to increased release of alarmins, microRNAs (MiRs), and cysteinyl leukotrienes (CysLTs) from epithelial, mesenchymal, and innate cells. During this period, there is a drop in microbiome diversity, which might be due to immune-mediated control to avoid invasion by opportunistic pathogenic bacteria, or to STH-mediated remodeling. When type 2 signals activate innate cells, type 2 cytokines (interleukin-[IL]4] and IL-13) are released, polarizing CD4+ T cells to a Th2 phenotype. Th2 cells then increase the amount of IL-4 and IL-13 signaling, triggering host-protective responses at the epithelial barrier, such as goblet cell hyperproliferation, mucin synthesis (such as Muc5ac), and increased epithelial cell turnover. These reactions largely affect early larval stages (L1-3), restricting juvenile parasite colonization within the epithelium. As a result, the barrier is rebuilt and the gut microbial populations regenerate [Citation5].
Stunting is an issue worldwide, with a prevalence of 21.3%. In Southeast Asia, the prevalence of stunting in children aged <5 years of age is 24.7%. Indonesia itself reported a prevalence rate of 30.8% in 2018 [Citation1]. Therefore, the prognosis will improve if intervention is performed before age 5.
Stunting in children can result from chronic infections or malnutrition due to deficiencies in macronutrients and micronutrients, such as vitamin D. In chronic STH infection, decreased nutrient intake, malabsorption, and inflammation of the intestinal epithelium can lead to micronutrient deficits, including vitamin D deficiency and growth retardation [Citation6]. The presence of vitamin D deficiency in STH infection decreases the ability of the immune response to increase T-helper-2 (Th2) proliferation and IL-4 and IL-5 synthesis, which contributes to the suppression of the inflammatory response. IL-4 and IL-5 play a role in stimulating the work of eosinophils and macrophages in tissue healing. Furthermore, IL-5 and IL-13 contribute to immunity through the production of histamine by mast cells to expel STH [Citation5–7].
The information provided suggests that 25(OH)D, IL-4, IL-5, and IL-13 could potentially contribute to STH infection, as explained in . The purpose of this study was to evaluate the prevalence of STH infection and the association of 25(OH)D, as an inactive form of vitamin D, IL-4, IL-5, and IL-13 with the burden of STH infection in stunted children aged 24–59 months.
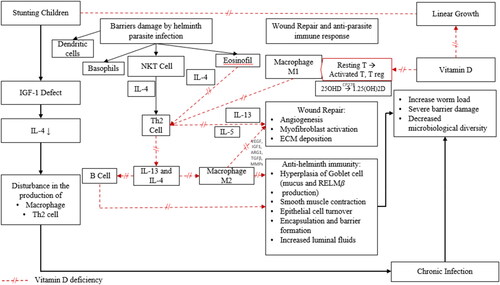
Figure 1. Possible immune responses mechanism in stunted children with STH infection. IGF-1 is the key mediator of the action of the GH axis on linear growth. In stunted children, there is a disturbance in IGF-1. When IGF-1 is disrupted, there is a decrease in IL-4 levels, which can lead to chronic infection. Epithelial and endothelial barriers are damaged by helminth parasite infection, which induces a wound-repair and anti-parasite immune response that is driven by the type 2 cytokines interleukin-4 (IL-4), IL-5, and IL-13. Dendritic cells (DCs), basophils, natural killer T (NKT) cells, eosinophils, and group 2 innate lymphoid cells (ILC2s) function to produce the key TH2 cells. B cells also participate in secondary type 2 responses. Type 2 cytokines, in turn, target epithelial cells, goblet cells, smooth muscle cells, and macrophages, which together coordinate parasite expulsion by increasing fluid and mucus production, encapsulation and barrier formation, epithelial cell turnover, smooth muscle contraction, and the production of anti-parasite effector molecules such as resistin-like molecule-β (RELMβ). In addition to activating several anti-parasite effector mechanisms, the type 2 immune response facilitates wound repair, which is important following infection by these large multicellular tissue-invasive organisms. M2 macrophages are intimately involved in this process as they produce matrix metalloproteinases (MMPs), arginase 1 (ARG1), insulin-like growth factor 1 (IGF1), vascular endothelial growth factor (VEGF), and transforming growth factor-β (TGFβ), which together promote myofibroblast activation, angiogenesis, epithelial cell turnover, and extracellular matrix (ECM) deposition. The helminth-induced type 2 immune response also promotes effective wound healing by suppressing the pro-inflammatory axis that is mediated by M1 macrophages, which could further exacerbate tissue injury if not quickly controlled. M2 macrophages producing TH2 cells have been shown to have important roles in the suppression of this pro-inflammatory axis and can also control potentially harmful type 2 immune responses. Macrophages and mature DCs can induce both adaptive T-lymphocyte-mediated and B-lymphocyte-mediated immunity by internalizing and processing pathogens. Macrophages and mature DCs also express the vitamin-D-activating enzyme CP27B and are thus able to synthesize 1.25(OH)2D from precursor 25OHD. The 1.25(OH)2D synthesized in this way can act in a paracrine fashion on activated B lymphocytes and activated T lymphocytes, which express abundant VDR. The dotted red line shows conditions when vitamin D deficiency occurs, and the purple line indicates when vitamin D is sufficient.
2. Materials and methods
2.1. Study design
A case–control study was designed to evaluate the association of 25(OH)D, IL-4, IL-5, and IL-13 with the burden of STH infection in stunted children aged 24–59 months.
2.2. Study site and population
This study was conducted in Bandung District, West Java, Indonesia, from October 2019 to January 2023. Children aged 24–59 months living in the district were the source population of this study. Across the entire data collection season, children who had a major congenital abnormality or suffered certain syndromes, took deworming drugs in the 6 months prior, or took vitamin D supplements were excluded from the study, as were those whose families moved away from the research location.
2.3. Study size and sampling
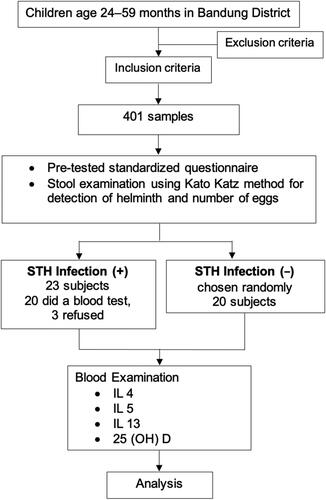
A formula was used to estimate the sample size required in this study for a confidence level of (1 − α) = 95% (Zα = 1.96), an estimated prevalence of helminthiasis of 20%, and a margin of error of 5%. Based on this sample size formula, the minimum sample size was 246 stunted children. Our findings showed that the prevalence of STH infection was 4.7%, with no significant difference in prevalence between stunted and normal-height children. The prevalence of STH infection was much lower (<20%) in Bandung District than in Indonesia as a whole (24.6–26.9%). To evaluate the associations of 25(OH)D, IL-4, IL-5, and IL-13 in stunted children, the sample size was determined using a formula to test the difference between two means, with a statistical significance of 5% (Zα = 1.96) and a power test of 80% (Zβ = 0.84). The minimum required group sizes were 20 for stunting with confirmed STH infection and 20 for stunting without STH infection. Study participants were recruited using a multistage random sampling technique. In the first stage, from the 31 subdistricts, 13 were selected. Second, from these 13 subdistricts, 39 villages were selected. In these 39 villages, we chose 29 primary healthcare centers. Third, a simple random sampling technique was used to select children aged 24–59 months. From 401 stunted children, 23 children tested positive for STH infection in stool examination, and 20 children tested negative for STH infection, among these, 3 children with positive STH infection refused to participate in the study, resulting in 40 stunted children. illustrates the selection process for the study participants.
2.4. Variable measurements
2.4.1. Stunting
Stunting was determined by height-for-age Z scores below minus two, in accordance with the World Health Organization (WHO) growth reference standard.
2.4.2. 25(OH)D level
The concentration of the inactive form of vitamin D, the result of the metabolism of vitamin D2 and D3 by the 25-hydroxylase enzyme found in the liver, was determined using the ELISA method. The classification of 25(OH)D levels according to the Endocrine Society is deficiency at <20 ng/mL, insufficiency at 21–29 ng/mL, and sufficiency at ≥30 ng/mL.
2.4.3. IL-5 level
The IL-5 concentration in serum units was measured using the ELISA method.
2.4.4. IL-13 level
The IL-13 concentration in serum units was measured using the ELISA method.
2.4.5. STH Infection
Infection was identified by the presence of worm eggs (Ascaris lumbricoides, Trichuris trichiura, hookworm) in stool samples. Mild-grade infections of A. lumbricoides range from 1 to 4999 eggs per gram of stool, moderate-grade infections range from 5000 to 49,999 eggs per gram of stool, and severe-grade infections involve more than 50,000 eggs per gram of stool. For T. trichiura, 1–999 eggs per gram of stool indicates a mild grade, 1000–9000 eggs per gram of stool indicates a moderate grade, and more than 10,000 eggs per gram indicates a severe grade. There are 1–1999 eggs per gram of stool in mild-grade hookworm infections, 2000–3999 eggs per gram in moderate-grade infections, and more than 4000 eggs per gram of stool in severe-grade infections.
2.4.6. Height and weight
Children who were at least 2 years old and capable of standing were weighed by themselves using a calibrated digital scale on a flat and solid surface. The measurement findings were read with an accuracy level of 0.1 kg. Height was taken as an assessment of maximum vertical size; body measurements were performed on children ≥2 years old who could stand without assistance. A stadiometer with an adjustable headpiece and a fixed vertical backboard was used to measure height. The stadiometer must be placed on a level ground. Subjects stood upright on a backboard with evenly distributed body weight and both feet flat on the platform. The back of the head, shoulder blades, buttocks, and heels should touch the back board, aligning the head in the Frankfort horizontal plane. The measurement results were read in a position parallel to the meter with an accuracy of 0.1 cm.
2.5. Data management and analysis
Data were obtained using a pre-tested standardized questionnaire and consisted of the sociodemographic characteristics of the subjects and the children’s anthropometric measures. The stool samples were collected in sterile specimen cups after detailed instruction by a trained field worker. Peripheral venous blood samples were taken from subjects to examine the serum levels of 25(OH)D, IL-5, and IL-13. The blood sample and stool analyses were performed by an accredited laboratory at Dr. Hasan Sadikin Bandung General Hospital. The Kato–Katz method for the detection of common helminth species such as A. lumbricoides, T. trichiura, and hookworm, and the numbers of eggs from these species, was performed on the stool samples. Euroimmune® and Elabscience® kits were used to examine 25(OH)D, IL-5, and IL-13 levels in the blood samples.
The data were verified, coded, and entered into IBM SPSS version 29 (SPSS Inc., Chicago, IL). Statistical analysis was performed using the chi-square test, Exact Fisher test, Kruskal–Wallis test, F test, Mann–Whitney test, unpaired T test, Spearman correlation, and logistic double regression. Descriptive analysis was carried out to identify the characteristics of the subjects. Bivariate analysis was performed to determine the association of serum levels of 25(OH)D, IL-5, and IL-13 with the burden of STH infection.
3. Results
3.1. Participant characteristics
Between October 2019 and January 2023, 401 children were included in this study, consisting of 23 (5.74%) stunted children with STH infection and 378 (94.26%) stunted children without STH infection. The characteristics of the participants are summarized in . There were significant differences in paternal education, water sources, distant between water sources, and cleanliness of toenails.
Table 1. Characteristics of stunted children aged 24–59 months with or without soil-transmitted helminth (STH) infection.
Most of the cases were found in children who sourced water from an electric water pump. There was a higher proportion of STH infection in situations where the distance between a water source and the toilet was less than 10 m. There were also a higher proportion of stunted children with STH infection who had dirty toenails.
Among the 23 cases of helminthiasis in stunted children, 16 were mild cases, 4 were moderate cases, and only 3 were severe cases. A. lumbricoides was the most common STH identified, with a prevalence of 95.7% (22 children), meanwhile, only one subject was infected with two types of worms, namely, A. lumbricoides and hookworm. We also detected other species in one sample with a mild T. trichiura infection, and one sample with a mild Necator americanus infection in normal height children. In this study, we compared the severity levels between normal children and stunted children, by analyzing the average number of eggs in each group, a statistical was conducted to evaluate the variation in severity levels between the two groups. The findings indicate that there were no significant differences between the two groups (p > 0.05).
3.2. Comparison of 25(OH)D, IL-4, IL-5, and IL-13 levels according to the severity of STH infection in stunted children
From the 23 stunted children with STH infection, only 20 children completed the blood examination. Their 25(OH)D levels were measured, and the results are presented in . The table shows the 25(OH)D levels of the participants in each group that were considered deficient, insufficient, and sufficient. The findings show that among the stunted children with STH infection, there was no significant differentiation observed between vitamin D insufficiency compared with the control group (45% vs. 35%).
Table 2. Serum 25(OH)D levels of stunted children aged 24–59 months with soil-transmitted helminth (STH) infection.
The results of the comparison of 25(OH)D, IL-4, IL-5, and IL-13 levels can be seen in . The result shows there is no significant difference between the level of IL-4 and 25(OH)D and STH infection based on the severity of the infection. Meanwhile a significant difference was observed between IL-5 and IL-13 levels based on the severity of STH infection, with p values of 0.038 and 0.028, respectively. We clarify the significance value within the and .
Figure 3. Differences in interleukin-5 levels based on the severity of soil-transmitted helminth (STH) infection in stunted children aged 24–59 months.
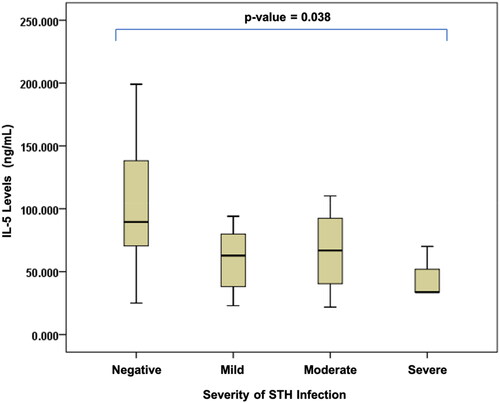
Figure 4. Differences in interleukin-13 levels based on the severity of soil-transmitted helminth (STH) infection in stunted children aged 24–59 months.
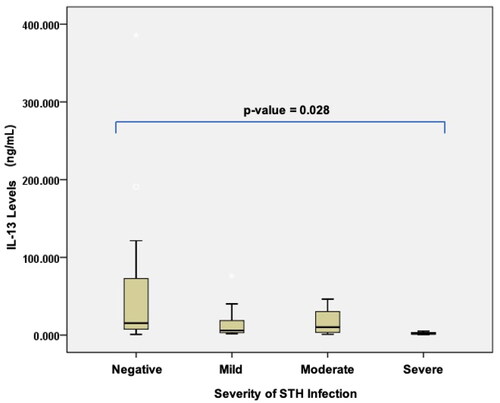
Table 3. Comparison of 25(OH)D, interleukin-4, interleukin-5, and interleukin-13 levels based on the severity of soil-transmitted helminth (STH) infection in stunted children aged 24–59 months.
In stunted children with severe STH infections, the levels of IL-5 and IL-13 were lowest, as compared to those in other degrees of infection. The severity of STH infection was negatively correlated with the levels of IL-5 and IL-13: the lower the levels of IL-5 (r = 0.477; p = 0.004) and IL-13 (r = 0.443; p = 0.004), the more severe the infection ( and Citation4).
3.3. Correlations between IL-4, IL-5, and IL-13 levels and vitamin D
An analysis was carried out to examine the immune response and correlation between cytokines and vitamin D. The results are shown in .
Table 4. Correlations of interleukin-4, interleukin-5, and interleukin-13 with vitamin D during soil-transmitted helminth (STH) infection in stunted children aged 24–59 months.
The relationships between these cytokines and vitamin D revealed a significant unidirectional association (p = 0.005) between IL-13 and vitamin D levels.
4. Discussion
In the findings of this study, A. lumbricoides infection was the most common infection. This conclusion is in line with studies carried out in North Sumatra by Pasaribu et al. as well as reports by Wang et al. in China [Citation8,Citation9]. A. lumbricoides infection is linked to the highest burden of STH infection worldwide and affects children more frequently.
Meanwhile, sociodemographic factors play a role in STH infection. This study showed that the prevalence of STH infection was influenced by the father’s educational level (p = 0.010). Stunted children who have fathers with lower levels of education are more likely to suffer STH infection. In a study conducted by Monazza Aslam et al. in Pakistan, children’s health was affected by parental education; this is related to parental literacy, greater exposure to the media, and the ability to read and write, which leads to increased awareness and knowledge of health matters. In this cultural context, a father’s education is positively related to health behaviors because decisions about children’s health are usually made by the father. Therefore, health education and knowledge are important, particularly for fathers, to allow them to make wise decisions about the health of their children [Citation10].
In this study, 25(OH)D, IL-4, IL-5, and IL-13 levels were measured to observe their relationship with STH infection. Although there was no correlation between 25(OH)D levels and the severity of STH infection in this study, according to the Endocrine Society classification, 25(OH)D levels in the moderate and severe groups showed quantitative insufficiency (median moderate degree: 24.19 ng/mL and median severe degree: 29.24 ng/mL). Meanwhile, the mild and uninfected groups showed sufficient 25(OH)D levels (median mild degree: 31.42 ng/mL and median uninfected: 31.84 ng/mL). This means that infections may worsen if the level of 25(OH)D is insufficient or even deficient.
Vitamin D is synthesized from sunlight; the skin absorbs ultraviolet radiation, then converts it to previtamin D3, which is isomerized into vitamin D3 [Citation11]. In this research, the subjects were children who live in tropical areas with quite intense exposure to sunlight. This may be why some of the subjects in the case group had 25(OH)D levels above 29 ng/mL.
Some subjects in the case group had 25(OH)D levels greater than 30 ng/mL, which may have been related to the children’s habit of playing outside. According to studies by Pasaribu et al. playing outside or on the ground can increase the risk of STH infection by as much as 7.53 times [Citation9]. This research shows that exposure to risky environmental factors influences the incidence of STH infection even at normal levels of 25(OH)D. Therefore, higher levels of 25(OH)D may be required to protect children from STH infection.
In this study, the relationship between helminthiasis and IL-5 and IL-13 levels was investigated. Th2 and mast cells produce the cytokine protein IL-5, which is crucial for the growth and activation of eosinophils, a type of white blood cell involved in the immune response to parasites and allergens. IL-5 stimulates eosinophil proliferation, increasing the number of eosinophils in the blood and tissues. Increased proliferation of eosinophils can improve the ability of a cell to combat infection and react to inflammatory reactions [Citation8,Citation12–14].
IL-13 is the main cytokine in the immune response to allergens. Such cytokines help to activate Th2 cells and encourage B cells to produce IgE. Under normal conditions, IL-13 helps maintain the balance of the immune response to foreign allergens, but in those with allergies, IL-13 production can increase noticeably and cause excessive allergic reactions. Furthermore, IL-13 can promote the alternative type of macrophage (M2), which plays a role in controlling inflammatory reactions and tissue repair [Citation7,Citation8,Citation12].
In this study, significant inverse correlations were found between the degree of STH infection and the levels of IL-5 (p = 0.038; r = −0.477) and IL-13 (p = 0.028; r = −0.443). This can be explained as follows: Helminth infection stimulates the inflammatory process by inducing a Th2 cytokine response via the production of IL-5 and IL-13 to prevent the development of larval epithelium, restore the integrity of the mucosal barrier, and play a role in controlled immune response suppression to reduce the burden. The study findings show that the more severe the degree of infection, the lower the levels of IL-5 and IL-13.
Furthermore, the analysis of the relationship between cytokines and vitamin D revealed a significant unidirectional association between IL-13 and vitamin D levels. Meanwhile, based on the results in , an inverse correlation was found between IL-13 levels (r = −0.443; p = 0.004) and the degree of infection. This opens opportunities for the role of vitamin D through the activation of the cytokine IL-13, which could be used as a therapeutic supplement to reduce the degree of STH infection. Other research has been conducted on the effect of vitamin D on parasitic infections. A prior study showed that L. mexicana infection in mice receiving vitamin D treatment resulted in fewer lesions than that in rats without vitamin D therapy [Citation14].
Maintaining 25(OH)D at a certain level would provide benefits to IL-13 and help to maintain the balance of the immune response to allergens. This supports evidence of the role of controlled suppression of the STH immune response, thus reducing the helminthic burden, inhibiting the formation of the larval epithelium, activating the Th2 barrier response, and restoring the integrity of the mucosal barrier and diversity of the microbiome. The importance of IL-13 and Th2 memory cells becomes clear: when these processes are disrupted, the helminthic load increases, the mucosal barrier is damaged, and tissue healing is prevented [Citation5].
5. Conclusions
In this study, the prevalence of STH infection in stunted children was found to be 5.74%. Of the various characteristics studied, social and environmental factors such as the father’s educational level, water source, and distance from a water source to the toilet were the significant factors that influenced the incidence of STH infection.
In stunted children, an association was observed for IL-5 and IL-13 levels with the burden of STH infection. Higher levels of Th2 cytokine response via the production of IL-5 and IL-13 would protect against a higher burden of STH infection. Therefore, pediatricians should consider checking the levels of IL-5 and IL-13 in stunted children with STH infection to determine the presence of a higher burden of disease that might affect child development. The unidirectional association between IL-13 and vitamin D levels opens opportunities for pediatricians to give vitamin D supplements as an adjuvant therapy for STH infection. Pediatricians should consider educating and encouraging parents to practice clean and healthy living behaviors with their children, including the supplementation of vitamin D to increase 25(OH)D levels as a preventive measure against infection, especially STH.
5.1. Limitations of this study
In this study, 25(OH)D, IL-4, IL-5, and IL-13 levels were not measured serially after therapy to observe the differences in post-therapy levels. Furthermore, other factors such as parental height, secondary infections, genetic disorders, etc., that might influence the incidence of stunting were not examined.
Institutional Review Board statement
Ethical clearance was obtained from Institutional Review Board (or Research Ethics Committee) of the Faculty of Medicine of Universitas Padjadjaran, with ethical number 825/UN6.KEP/EC/2021. The committee provided ethical clearance approval for all components of the study protocol submitted to the Bandung District Health Authority to conduct this study in primary healthcare centres in the Bandung District area.
Informed consent statement
Informed consent was obtained from all participants involved in the study.
Acknowledgments
The author expresses their gratitude to Masahiro Umezaki from the University of Tokyo for reviewing this article, and to the field surveyors, the staff of the primary health care centres, and the cadres of the Bandung district. Many thanks to the data collector, Hadyana Sukandar, for statistical analysis, and to Hijriyanti Amd, Ak. and laboratory staff, and others who supported this study.
Disclosure statement
The authors report there are no competing interests to declare.
Additional information
Funding
References
- World Health Organization. Reducing stunting in children: equity considerations for achieving the global nutrition targets 2025. Geneva, Switzerland: World Health Organization; 2018.
- Cruz-Cruz C, López-Hernández D, Hernández-Shilón JA, et al. Stunting and intestinal parasites in school children from high marginalized localities at the Mexican southeast. J Infect Dev Ctries. 2018;12(11):1–8.
- Shang Y, Tang LH, Zhou SS, et al. Stunting and soil-transmitted-helminth infections among School-Age pupils in rural areas of Southern China. Parasites Vectors. 2010;3(1):97.
- Oliveira D, Ferreira FS, Atouguia J, et al. Infection by intestinal parasites, stunting and anemia in School-Aged children from Southern Angola. PLoS One. 2015;10(9):e0137327.
- Colombo SAP, Grencis RK. Immunity to soil-transmitted helminths: evidence from the field and laboratory models. Front Immunol. 2020;11:1286.
- Maizels RM, McSorley HJ. Regulation of the host immune system by helminth parasites. J Allergy Clin Immunol. 2016;138(3):666–675.
- Easton AV, Mayra R-A, Victor L, et al. Immune response and microbiota profiles during coinfection with Plasmodium Vivax and soil-transmitted helminths. MBio. 2020;11(5):10–1128.
- Wang X, Zhang L, Luo R, et al. Soil-Transmitted helminth infections and correlated risk factors in preschool and School-Aged children in rural southwest China. PLoS One. 2012;7(9):e45939.
- Pasaribu AP, Alam A, Sembiring K, et al. Prevalence and risk factors of soil-transmitted helminthiasis among school children living in an agricultural area of North Sumatera, Indonesia. BMC Public Health. 2019;19(1):1066.
- Aslam M, Kingdon GG. Parental education and child health-Understanding the pathways of impact in Pakistan. World Dev. 2012;40(10):2014–2032.
- Wacker M, Holick MF. Sunlight and vitamin D: a global perspective for health. Dermatoendocrinol. 2013;5(1):51–108.
- Parija SC. Textbook of microbiology and immunology. Berlin, Heidelberg, Germany: Springer; 2023.
- Chard AN, Baker KK, Tsai K, et al. Associations between soil-transmitted helminthiasis and viral, bacterial, and protozoal enteroinfections: a Cross-Sectional study in rural laos. Parasites Vectors. 2019;12(1):216.
- Whitcomb JP, Deagostino M, Ballentine M, et al. The role of vitamin D and vitamin D receptor in immunity to Leishmania Major infection. J Parasitol Res. 2012;2012:1–10.