Abstract
Thymoma is closely associated with myasthenia gravis (MG). However, due to the heterogeneity of thymoma and the intricate pathogenesis of MG, it remains unclear why some patients with thymoma develop MG and others do not. In this study, we conducted a comparative phenotype analysis of thymocytes in type B thymomas in patients with MG (MG (+) thymomas) and without MG (MG (−) thymomas) via fluorescence-activated cell sorting (FACS). Our results show that the developmental stages defined by the expression of CD3, CD4, and CD8 were largely maintained in both MG (+) and MG (−) thymomas, with CD4+CD8+ cells constituting the majority of thymocytes in type B thymoma, and no significant difference between this cell population was observed in MG (+) and MG (−) thymomas.We discovered that CD4+CD8+ thymocytes in MG (+) thymomas expressed low levels of αβ TCR and high levels of IL-7 receptor α (IL-7Rα), whereas in MG (−) thymomas, CD4+CD8+ thymocytes exhibited the opposite pattern of αβ TCR and IL-7Rα expression. These results suggest that the positive and negative selection processes of CD4+CD8+ thymocytes might differ between MG (+) thymomas and MG (−) thymomas. The expression of the Helios transcription factor is induced during negative selection and marks a group of T cells that have undergone negative selection and are likely to be deleted due to strong TCR binding with self-peptides/MHC ligands. We observed that the percentage of Helios-positive CD4SP T cells was greater in MG (−) than in MG (+) thymomas. Thus, the differentially regulated selection process of CD4+CD8+ thymocytes, which involves TCR and IL-7/IL-7Rα signaling, is associated with the presence of MG in type B thymomas.
Introduction
The thymus is a central lymphatic organ that is responsible for the production of αβ TCR T cells, including the generation of regulatory T cells (Tregs); therefore, the thymus is crucial for immune homeostasis and self-tolerance. Although thymic function declines with age, the thymus can function in adults even at an advanced age [Citation1]. The stages of T cell development in the human thymus can typically be distinguished by the expression of CD3, CD4, and CD8, which progress from CD3−CD4−CD8− triple-negative (TN) to immature CD4+CD8+ double-positive (DP) cells after passing β-selection. DP cells undergo positive selection, in which thymocytes bind to self-peptide/MHC molecules, and mature into CD4+CD8− single-positive (CD4SP) or CD4−CD8+ single-positive (CD8SP) T cells. Negative selection occurs during the development of DP and SP cells to selectively delete T cells that bind with high affinity to self-peptide/MHC molecules. As the intensity and duration of TCR signaling are the major determinants of selection [Citation2], defects in the TCR signaling component led to abnormal T cell development and cause autoimmunity [Citation3,Citation4].
The generation of a functional T cell repertoire in the thymus is orchestrated mainly by thymic epithelial cells, which provide thymocytes with cues for their proliferation, differentiation, and survival [Citation5]. The association between thymoma and autoimmune diseases, particularly myasthenia gravis (MG), which can occur in up to 44% of patients [Citation6,Citation7], strongly indicates the importance of thymic epithelial cells in shaping the normal development of the thymus and maintaining immune homeostasis. Thymomas are uncommon neoplasias originating from the epithelial cell population in the thymus. Thymomas are classified by histological features as type A thymoma, type AB thymoma, type B thymoma (separated into B1, B2, and B3 thymomas), micronodular thymoma with lymphoid stroma, and metaplastic thymoma [Citation8]. The etiology of autoimmune diseases involves a complex interplay of genetic and environmental factors. Immature T cells in the thymus play an important role in the development of many autoimmune diseases [Citation9–11].
The most likely explanation for autoimmunity related to thymomas is that the damage induced by tumor growth within the thymus diminishes the ability of the thymus to maintain self-tolerance [Citation12]. For instance, decreased expression of the autoimmune regulator (AIRE) protein by medullary thymic epithelial cells in thymomas may contribute to defective negative selection of autoreactive T cells and the development of autoimmune diseases [Citation13]. Thymocyte development and negative selection are also subject to regulation by cytokine signaling. IL-7 signaling has been extensively investigated and shown to play an important role in survival at all stages of thymocyte development [Citation14,Citation15]. IL-7 becomes dispensable as thymocytes mature to the DP stage, where IL-7Rα expression is low [Citation16], which enables the programming of these DP thymocytes for possible subsequent cell death. It has been demonstrated that IL-7Rα downregulation is due to the upregulation of TCR signaling [Citation17,Citation18]. However, the role of IL-7 signaling in thymoma and its association with MG have not been elucidated.
Thymoma-associated autoimmune diseases also involve alterations in circulating T cell subsets [Citation9]. The primary T cell abnormality appears to be related to the acquisition of the CD45RA+ phenotype in naïve CD4+ T cells during terminal intratumoral thymopoiesis followed by the export of these CD4+ T cells into the circulation [Citation19,Citation20]. Several studies have shown that the development of Tregs is disrupted in thymomas [Citation21]. However, abnormalities in Tregs in thymomas occur irrespective of MG status [Citation22]. Thus, the relevance of defective Treg development in the context of MG remains to be established.
Type B thymomas primarily exhibit a cortical epithelial immunophenotype [Citation23]. Clinical studies have shown that patients with type B thymoma have a greater incidence of autoimmune diseases than do patients with type A thymoma [Citation12,Citation24]. In this study, we conducted a comparative phenotype analysis of thymocytes in type B thymomas in patients with MG (MG (+) thymomas) and in those without autoimmune diseases, including MG (MG (−) thymomas). Our results showed that the majority of thymocytes in type B thymomas were DP cells. The developmental stages did not significantly differ between MG (+) and MG (−) thymomas. DP cells in MG (+) thymomas expressed low levels of αβ TCR and increased levels of IL-7Rα compared to DP thymocytes in MG (−) thymomas. IL-7/IL-7Rα signaling might be involved in the development of MG in patients with type B thymoma.
Materials and methods
Patients
The records of patients who were diagnosed with thymoma and thymic hyperplasia and who underwent thymectomy in our department between March 2016 and August 2020 were collected. The diagnosis of thymoma was suggested preoperatively from the radiological appearance and computed tomography (CT) findings and was confirmed in all patients by histology of the resected specimens. There were 145 patients enrolled in this retrospective study (). The types of thymoma were classified histologically according to the WHO thymoma classification (2021 revision). The diagnosis of MG was made by the neurology department according to the clinical features, decrement testing with 3 Hz serial stimulation, and detection of anti-AChR antibodies.
Table 1. Clinical data of thymoma and thymic hyperplaisa patients.
Human samples and cell preparation
A total of 15 tumor samples were obtained from patients with type B thymomas who underwent thymectomy between September 2021 and May 2024 at Tongji Hospital. The clinical characteristics of the patients are presented in Supplementary Table 1. Fifteen patients who were postoperatively diagnosed with thymoma via pathological examination, excluding those with autoimmune diseases other than MG. The use of human tissue samples was approved by the Huazhong University of Science and Technology Ethics Committee. The fresh tumor samples were manually cut into 3–5 mm3 fragments, ground and filtered through a 70 µm strainer, and subjected to centrifugation over a two-step gradient of 40% and 70% Percoll (Cytiva, Danaher, Sweden). Lymphocytes were collected between the two layers for subsequent flow cytometry analysis.
Immunohistochemical staining and quantification
Tissue samples were fixed in 10% formalin and processed for paraffin embedding. Sections (4 µm) were deparaffinized, rehydrated, and subjected to microwave antigen retrieval in EDTA buffer (pH 9). After blocking endogenous peroxidase activity with 3% H2O2 for 10 min, the slides were incubated with primary antibodies against IL-7 (1:400, PA5-119844, Invitrogen, USA) overnight at 4 °C. Then, the slides were incubated with HRP-linked secondary antibodies. Immunoreactivity was visualized using the DAB method (GK600505, GeneTech, Shanghai, China). Slides incubated without the primary antibody were used as the negative control.
To assess the immunostaining intensity of IL-7 in thymomas, the average integrated optical density (average IOD) of IL-7 staining was measured in a total of 3 areas (400× magnification, Olympus microscope). Images were opened with Image-Pro Plus software, and the average IOD was calculated automatically using the hue-saturation-intensity (HSI) threshold (60, 255, 255) in the randomly selected area of interest. The average IOD for each patient was determined by the mean value of three images.
Flow cytometry
For cell surface staining, thymocytes (1 × 106 cells/sample) were stained with the indicated mAbs at 4 °C for 30 min. For intracellular staining, thymocytes (1 × 106 cells/sample) were surface stained with the indicated antibodies and subsequently fixed and permeabilized using a FoxP3/Transcription Factor Staining Buffer Kit (eBioscience, San Diego, California, USA). Information on the antibodies used for surface and intracellular staining is provided in Supplementary Table 2. Dead cells were excluded by using Fixable Viability Dye eFluor 780 (eBioscience). Flow cytometric analysis was performed on an Attune NxT Acoustic cytometer (Thermo Fisher Scientific, Waltham, Massachusetts, USA). The data were analyzed using FlowJo software V10 (BD Bioscience).
Statistical analysis
Unpaired two-tailed Student’s t test and one-way ANOVA were used for comparisons between two groups or among multiple groups, respectively. GraphPad Prism 8.0 was used for statistical analysis. The statistical significance threshold was set at p < .05. In all the bar graphs shown, the statistical means ± SDs are depicted.
Results
Analysis of the developmental stages of MG (+) versus MG (−) thymomas
A total of 145 hospitalized patients with thymomas or thymic hyperplasia were enrolled at Tongji Hospital between March 2016 and August 2020 (). Among the enrolled subjects, type B thymoma was the most prevalent entity (55.17%), and patients with type B thymoma were more likely to exhibit MG. A total of 22 (75.86%) patients with type B thymoma were diagnosed with MG, which was consistent with reports by others [Citation25,Citation26]. Based on the observed characteristics of the thymoma patients, we focused our study on type B thymoma and aimed to determine why some patients with type B thymoma develop MG and others do not. We enrolled 15 type B thymoma patients who underwent mediastinal mass resection at our department between June 2021 and May 2024 (Table S1). Among them, 8 patients had MG, and 7 patients did not have MG.
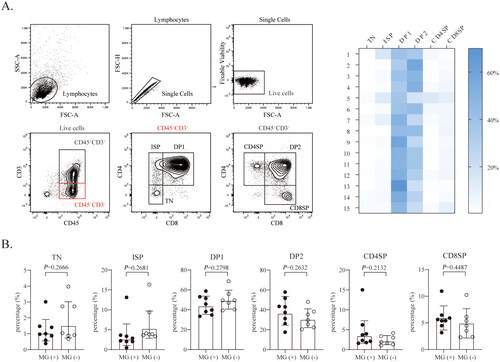
We first assessed the status of thymic developmental stages in type B thymomas. As shown in , the developmental stages included CD3−CD4−CD8− triple negative (TN), CD3-CD4+CD8− immature single positive (ISP), CD3−CD4+CD8+ double positive (DP1), CD3+CD4+CD8+ double positive (DP2), mature CD3+CD4+CD8− single positive (CD4SP) and mature CD3+CD4-CD8+ single positive (CD8SP), similar to the stages observed during normal thymic development, as previously reported [Citation27]. By analyzing thymocytes from 15 type B thymomas, we found that CD4+CD8+ thymocytes were the dominant component. The developmental stages were largely maintained, and the development of all stages of the CD4 and CD8 lineages showed no significant differences between MG (+) and MG (−) thymomas ().
Figure 1. Analysis of the developmental stages of MG (+) versus MG (−) thymomas. (A) Representative flow cytometry contour plots and heatmaps showing the gating strategy used to analyze thymocytes from type B thymomas and the stage distribution of each patient (n = 15). (B) The percentage of thymocytes at the indicated developmental stages in MG (+) thymomas (n = 8) and MG (−) thymomas (n = 7). The data are shown as the means ± SDs. Statistical significance was determined by using Student’s t test for B.
Proportion of Thpok+ CD4SP cells is higher in MG (+) thymomas
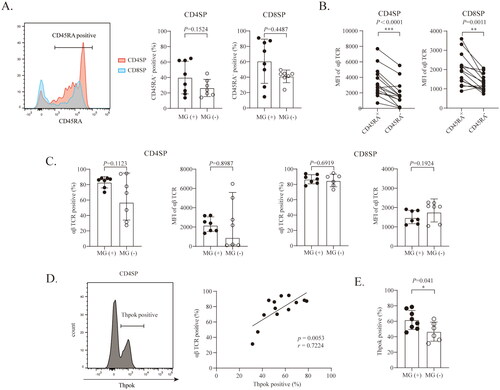
CD4SP or CD8SP cells with CD45RA expression in the thymus represent the most mature thymocytes, and cells with this expression pattern are indistinguishable from their peripheral T cell counterparts [Citation28]. Thus, we examined CD45RA expression on CD4SP and CD8SP cells. As shown in , the proportions of CD45RA+CD4+ T cells among the total CD4SP and of CD45RA+CD8+ T cells among the total CD8SP were comparable between MG (+) thymomas and MG (−) thymomas. To further characterize the features of CD45RA+CD4SP cells and CD45RA+CD8SP cells in type B thymomas, we examined the expression levels of the αβ TCR in CD4SP and CD8P cells. As shown in , the expression level of αβ TCR in CD45RA+CD4SP cells was significantly greater than that in CD45RA−CD4SP cells. A similar expression pattern was also observed in CD8SP cells. The level of αβ TCR expression did not differ in CD4SP cells or CD8SP cells, between MG (−) thymomas and MG (+) thymomas (). TCR signals play a critical role in determining the development of the CD4 helper T cell lineage regulated by the transcription factor Thpok [Citation29–31]. Interestingly, as shown in , in type B thymomas, Thpok expression levels were positively correlated with αβ TCR expression levels in CD4SP cells. The proportion of Thpok-positive CD4SP cells was significantly greater in MG (+) thymomas than in MG (−) thymomas ().
Figure 2. Proportion of Thpok+ CD4SP cells is higher in MG (+) thymomas. (A) The percentages of CD45RA+ cells in the CD4SP or CD8SP population in MG (+) thymomas (n = 8) and MG (−) thymomas (n = 7). (B) The MFI of the αβ TCR in CD45RA+ and CD45RA− thymocytes in the CD4SP and CD8SP stages (n = 14) (data on the αβ TCR of patient #9 were lost due to a technical error). (C) The expression of the αβ TCR in CD4SP and CD8SP thymocytes in MG (+) thymomas (n = 7) and MG (−) thymomas (n = 7). (D) Correlation between the levels of αβ TCR and Thpok expression in CD4SP cells (n = 13) (r = 0.7224, p = .0053) (data from patient #1 was lost due to a technical error). (E) The percentages of Thpok+CD4SP cells among CD4SP cells in MG (+) thymomas (n = 7) and MG (−) thymomas(n = 6). The data are shown as the means ± SDs. Statistical significance was determined by using Student’s t test for A, B, C, and E. *p < .05, **p < .01, ***p <.001.
DP cells in MG (+) thymomas display αβ TCR and IL-7 receptor α expression patterns opposite those observed in their counterparts in MG (−) thymomas
In addition to assessing TCR expression in CD4SP and CD8SP cells, we also assessed TCR expression in thymocytes at other developmental stages. As shown in , αβ TCR expression was almost undetectable in thymocytes before DP1. A substantial fraction of DP2 thymocytes, which are positive for CD3, CD4, and CD8, express the αβ TCR in type B thymomas. Interestingly, unlike that in CD4SP cells, the level of αβ TCR expression was higher in MG (−) DP2 cells than in MG (+) DP cells (). Several studies have shown that DP2 thymocytes that are committed to the CD4 T cell lineage lose CD8 surface protein expression and appear to be CD4+CD8INT transitional cells [Citation32,Citation33]. Notably, these transitional cells can also differentiate into CD8SP cells [Citation34]. As shown in , based on the CD8 expression level, DP2 cells were classified into CD4+CD8HI (R-DP2) and CD4+CD8INT subsets. We found that the percentage of αβ TCR positive cells and the TCR expression level among R-DP2 and CD4+CD8INT cells was greater in MG (−) thymomas than in MG (+) thymomas.
Figure 3. DP cells from MG (+) thymomas display opposite αβ TCR and IL-7Rα expression patterns to those from MG (−) thymomas. (A) The expression of αβ TCR thymocytes in the indicated developmental stages in type B thymomas. (B) The expression of the αβ TCR in DP2 thymocytes in MG (+) thymomas (n = 7) and MG (−) thymomas (n = 7). (C) The expression of the αβ TCR in R-DP2 and CD4+CD8INT thymocytes in MG (+) thymomas (n = 7) and MG (−) thymomas (n = 7). (D) The expression of IL-7Rα in thymocytes at the indicated developmental stages from type B thymomas (n = 13). (E) The MFI of IL-7Rα in R-DP2 and CD4+CD8INT thymocytes in MG (+) thymomas (n = 6) (the IL7Rα data of patient #9 and #10 were lost due to a technical error) and MG (−) thymomas (n = 7). (F) The immunoreactivity of IL-7 in type B thymomas was calculated as the average IOD. The statistical diagram shows the expression of IL-7 in MG (+) (n = 4) and MG (−) (n = 5) thymomas. (G) The percentage of Helios+ CD4SP cells in MG (+) thymomas (n = 7) and MG (−) thymomas (n = 7). The data are shown as the means ± SDs. Statistical significance was determined by using Student’s t test. *p < .05. **p<.01.
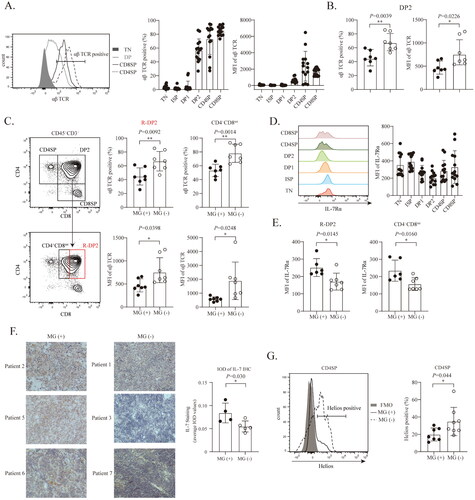
To understand the consequence of reduced expression of the αβ TCR in DP2 cells in MG (+) thymomas, we examined IL-7Rα expression in these cells. IL-7/IL-7R signaling is important for the survival, proliferation and differentiation of immature thymocytes [Citation35]. IL-7Rα expression is upregulated at the TN stage, coinciding with TCR β-selection of thymocytes [Citation35,Citation36]. IL-7Rα expression is significantly reduced at the later stage of thymic development and even terminates at the DP stage. Redundant IL-7 signaling in DP thymocytes must be prevented, thereby ensuring correct selection [Citation5,Citation37]. As shown in , DP thymocytes expressed lower levels of IL-7Rα than thymocytes did at other developmental stages. Interestingly, in contrast to the αβ TCR expression pattern in MG (+) and MG (−) thymomas, IL-7Rα expression in R-DP2 and CD4+CD8INT thymocytes was higher in MG (+) than in MG (−) thymomas ().
IL-7 is mainly produced by epithelial cells in the thymus [Citation38,Citation39]. We assessed intratumoral IL-7 expression in type B thymomas by using immunohistochemistry. The intensity of IL-7 immunoreactivity was measured by the IOD. Positive staining for IL-7 was observed in type B thymomas. The average IOD of IL-7 in MG (+) thymomas was higher than that in MG (−) thymomas (). These results strongly suggest that in MG (+) thymomas, a higher level of IL-7Rα expression in DP2 cells might result in the transduction of excessive IL-7 signaling, thereby interfering with the selection process of DP2 cells.
The transcription factor Helios marks a group of T cells that undergo negative selection. These cells are capable of strongly binding with self-peptide/MHC ligands and are likely to be deleted [Citation40,Citation41]. As shown in , the percentage of Helios-positive cells in the CD4SP population was higher in MG (−) thymomas than in MG (+) thymomas. Our data indicate that more immature thymocytes in MG (+) thymomas than in MG (−) thymomas escape negative selection and mature into CD4SP cells with high αβ TCR expression.
The proportion of intratumoral CD25+Foxp3+CD4+ T cells does not differ between MG (+) and MG (−) thymomas
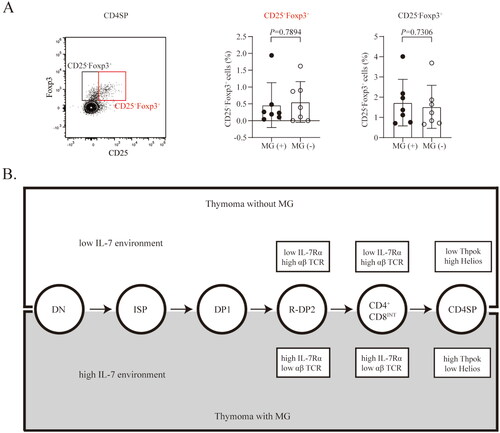
Several studies have shown that the proportion of regulatory T (Treg) cells is lower in thymomas than in hyperplastic or normal thymus [Citation42,Citation43]. However, others have shown that the percentage of Treg cells among CD4SP thymocytes did not differ between thymoma patients with MG and those without MG [Citation22]. Thus far, the role of thymic Treg cells in the association of thymoma with MG is still debatable. We thus assessed Treg cells, defined by the expression of CD25, Foxp3, and CD4 in the thymomas. As shown in , the percentage of CD25+Foxp3+ cells in the total CD4SP population was the same in patients with B type thymoma regardless of MG status.
Figure 4. The proportion of intratumoral CD25+Foxp3+CD4+ T cells did not differ between MG (+) and MG (−) thymomas. (A) The percentage of CD25-Foxp3+ and CD25-Foxp3+ CD4SP cells in MG (+) (n = 7) and MG (−) (n = 7) thymomas. The data are shown as the means ± SDs. Statistical significance was determined by using Student’s t test. (B) Schematic depicting the different environments and phenotypes of thymocytes in MG (+) and MG (−) thymomas.
Discussion
Although the association between thymoma and MG has been well recognized clinically, it remains unclear why some patients with thymoma develop MG but others do not. Moreover, the mechanisms underlying the dysregulation of thymic development in thymomas are also unclear. Type B thymomas contain a high abundance of infiltrated immature thymocytes and are strongly linked to MG [Citation44,Citation45]. In our study, we analyzed the phenotypic properties of intratumoral thymocytes in type B thymomas and investigated the association between type B thymoma and MG. Our data showed that CD4+CD8+ thymocytes were the dominant population in type B thymomas. We also showed that immature DP cells in MG (+) thymomas express reduced levels of TCR than do those in MG (−) thymomas. However, in MG (+) thymomas, these cells expressed increased levels of IL7Rα.
Several studies have shown that CD3-CD4-CD8- cells express high levels of the IL-7 receptor to support their proliferation and survival [Citation46]. Our data also confirmed that TN cells in type B thymomas expressed high levels of IL-7Rα. Because IL-7 is expressed at low levels in the thymus, the strength of IL-7 signaling is determined by the expression level of IL-7Rα [Citation14]. IL-7Rα expression in DP thymocytes is dramatically reduced during the preselection period [Citation16]. During the DP stage, thymocytes are subjected to selection, resulting in either expansion/differentiation into SP cells (positive selection) or deletion (negative selection). IL-7R expression is critically regulated in developing thymocytes; IL-7R expression is downregulated in thymocytes that fail the positive selection process but is upregulated or maintained in those undergoing positive selection [Citation47]. Our data showed that in MG (+) thymomas, R-DP2 and CD4+CD8INT cells with upregulated IL-7R might represent DP cells that underwent positive selection.
Downregulation of IL-7R in DP cells also plays a critical role in negative selection. Downregulation of IL-7R in DP cells during negative selection is believed to occur due to enhanced TCR signaling [Citation17,Citation48], which is supported by our results. Moreover, IL-7Rα was highly expressed in CD4+CD8INT thymocytes in MG (+) but not MG (−) thymomas, indicating that abnormal survival signals mediated by IL-7R are transduced to those cells in MG (+) thymomas and lead to unsuccessful negative selection and the accumulation of mature CD4SP cells. Although abnormal negative selection in the thymus is associated with the occurrence of autoimmune diseases [Citation49,Citation50], our results revealed for the first time that MG (+) and MG (−) thymomas exhibit distinct patterns of TCR and IL-7Rα expression on DP thymocytes, including CD3+CD4+CD8+ cells and CD3+CD4+CD8INT cells. In addition, negative selection of DP thymocytes can occur in the cortex of the thymus, which is relatively deficient in IL-7-producing cells [Citation14,Citation50]. In our study, we found that IL-7 expression was greater in MG (+) thymomas than in MG (−) thymomas, further indicating that the selection process of DP thymocytes in MG (+) thymomas differs from that in MG (−) thymomas and is influenced by IL-7/IL7R signaling.
We acknowledge that the data acquired in this study were from a limited sample size. However, these findings advance the field one step further towards understanding why some thymoma patients develop MG but others do not. Dysregulation of IL-7/IL-7R signaling at the DP stage in thymomas might be associated with the development of MG ().
Authors contributions
Shengling Fu and Lequn Li conceived and designed the present study. Tianlai Wang and Boyu Wang performed the experiments, analyzed the data, and drafted the article. Xiaowu Fan and Yixin Cai critically revised the manuscript. All the authors have read and approved the final manuscript.
Acknowledgements
We thank the Experimental Medicine Center of Tongji Hospital for the equipment support.
Disclosure statement
No potential conflicts of interest were reported by the author(s).
Data availability statement
All data are available in the main text.
Additional information
Funding
References
- Spits H. Development of alphabeta T cells in the human thymus. Nat Rev Immunol. 2002;2(10):1–9.
- Gascoigne NR, Rybakin V, Acuto O, et al. TCR signal strength and T cell development. Annu Rev Cell Dev Biol. 2016;32(1):327–348.
- Fu G, Chen Y, Yu M, et al. Phospholipase Cgamma1 is essential for T cell development, activation, and tolerance. J Exp Med. 2010;207(2):309–318.
- Sakaguchi N, Takahashi T, Hata H, et al. Altered thymic T cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature. 2003;426(6965):454–460.
- Kadouri N, Nevo S, Goldfarb Y, et al. Thymic epithelial cell heterogeneity: TEC by TEC. Nat Rev Immunol. 2020;20(4):239–253.
- Souadjian JV, Enriquez P, Silverstein MN, et al. The spectrum of diseases associated with thymoma. Arch Intern Med. 1974;134(2):374–379.
- Marx A, Willcox N, Leite MI, et al. Thymoma and paraneoplastic myasthenia gravis. Autoimmunity. 2010;43(5-6):413–427.
- Marx A, Chan J, Chalabreysse L, et al. The 2021 WHO classification of tumors of the thymus and mediastinum: what is new in thymic epithelial, germ cell, and mesenchymal tumors? J Thorac Oncol. 2022;17(2):200–213.
- Hoffacker V, Schultz A, Tiesinga JJ, et al. Thymomas alter the T cell subset composition in the blood: a potential mechanism for thymoma-associated autoimmune disease. Blood. 2000;96(12):3872–3879.
- Vincent A, Willcox N. The role of T cells in the initiation of autoantibody responses in thymoma patients. Pathol Res Pract. 1999;195(8):535–540.
- Wang L, Wang FS, Gershwin ME. Human autoimmune diseases: a comprehensive update. J Intern Med. 2015;278(4):369–395.
- Shelly S, Agmon-Levin N, Altman A, et al. Thymoma and autoimmunity. Cell Mol Immunol. 2011;8(3):199–202.
- Kisand K, Lilic D, Casanova JL, et al. Mucocutaneous candidiasis and autoimmunity against cytokines in APECED and thymoma patients: clinical and pathogenetic implications. Eur J Immunol. 2011;41(6):1517–1527.
- Hong C, Luckey MA, Park JH. Intrathymic IL-7: the where, when, and why of IL-7 signaling during T cell development. Semin Immunol. 2012;24(3):151–158.
- Tani-Ichi S, Shimba A, Wagatsuma K, et al. Interleukin-7 receptor controls development and maturation of late stages of thymocyte subpopulations. Proc Natl Acad Sci U S A. 2013;110(2):612–617.
- Ribeiro AR, Rodrigues PM, Meireles C, et al. Thymocyte selection regulates the homeostasis of IL-7-expressing thymic cortical epithelial cells in vivo. J Immunol. 2013;191(3):1200–1209.
- Carrette F, Surh CD. IL-7 signaling and CD127 receptor regulation in the control of T cell homeostasis. Semin Immunol. 2012;24(3):209–217.
- Doan LL, Kitay MK, Yu Q, et al. Growth factor independence-1B expression leads to defects in T cell activation, IL-7 receptor alpha expression, and T cell lineage commitment. J Immunol. 2003;170(5):2356–2366.
- Ströbel P, Helmreich M, Menioudakis G, et al. Paraneoplastic myasthenia gravis correlates with generation of mature naive CD4(+) T cells in thymomas. Blood. 2002;100(1):159–166.
- Shimizu H, Ichikawa Y, Yoshida M, et al. Lymphocyte subsets of the peripheral blood in myasthenia gravis determined by two-color flow cytometry. AUTOIMMUNITY. 1990;6(3):173–182.
- Tao Z, Jiang Y, Xia S. Regulation of thymic T regulatory cell differentiation by TECs in health and disease. Scand J Immunol. 2021;94(4):e13094.
- Fattorossi A, Battaglia A, Buzzonetti A, et al. Thymopoiesis, regulatory T cells, and TCRVbeta expression in thymoma with and without myasthenia gravis, and modulatory effects of steroid therapy. J Clin Immunol. 2008;28(2):194–206.
- Li H, Ren B, Yu S, et al. The clinicopathological significance of thymic epithelial markers expression in thymoma and thymic carcinoma. BMC Cancer. 2023;23(1):161.
- Weksler B, Lu B. Alterations of the immune system in thymic malignancies. J Thorac Oncol. 2014;9(Suppl 2):S137–S142.
- Evoli A, Minisci C, Di Schino C, et al. Thymoma in patients with MG: characteristics and long-term outcome. Neurology. 2002;59(12):1844–1850.
- Tang M, Shao Y, Dong J, et al. Risk factors for postoperative myasthenia gravis in patients with thymoma without myasthenia gravis: a systematic review and meta-analysis. Front Oncol. 2023;13:1061264.
- Weerkamp F, Pike-Overzet K, Staal FJ. T-sing progenitors to commit. Trends Immunol. 2006;27(3):125–131.
- Goff LK, Huby RD. Characterization of constitutive and strain-dependent subsets of CD45RA + cells in the thymus. Int Immunol. 1992;4(11):1303–1311.
- Taniuchi I. CD4 helper and CD8 cytotoxic T cell differentiation. Annu Rev Immunol. 2018;36(1):579–601.
- Zeidan N, Damen H, Roy DC, et al. Critical role for TCR signal strength and MHC specificity in ThPOK-Induced CD4 helper lineage choice. J Immunol. 2019;202(11):3211–3225.
- Tokunaga T, Hayashi A, Kadota Y, et al. Regulation of Th-POK and Runx3 in T cell development in human thymoma. Autoimmunity (Chur, Switzerland. 2009;42(8):653–660.
- Bosselut R. CD4/CD8-lineage differentiation in the thymus: from nuclear effectors to membrane signals. Nat Rev Immunol. 2004;4(7):529–540.
- Bosselut R, Guinter TI, Sharrow SO, et al. Unraveling a revealing paradox: why major histocompatibility complex I-signaled thymocytes “paradoxically” appear as CD4 + 8lo transitional cells during positive selection of CD8+ T cells. J Exp Med. 2003;197(12):1709–1719.
- Brugnera E, Bhandoola A, Cibotti R, et al. Coreceptor reversal in the thymus: signaled CD4 + 8+ thymocytes initially terminate CD8 transcription even when differentiating into CD8+ T cells. Immunity. 2000;13(1):59–71.
- Yassai M, Gorski J. Thymocyte maturation: selection for in-frame TCR alpha-chain rearrangement is followed by selection for shorter TCR beta-chain complementarity-determining region 3. J Immunol. 2000;165(7):3706–3712.
- Seddon B. Thymic IL-7 signaling goes beyond survival. Nat Immunol. 2015;16(4):337–338.
- Munitic I, Williams JA, Yang Y, et al. Dynamic regulation of IL-7 receptor expression is required for normal thymopoiesis. BLOOD. 2004;104(13):4165–4172.
- Shitara S, Hara T, Liang B, et al. IL-7 produced by thymic epithelial cells plays a major role in the development of thymocytes and TCRgammadelta + intraepithelial lymphocytes. J Immunol. 2013;190(12):6173–6179.
- Zamisch M, Moore-Scott B, Su DM, et al. Ontogeny and regulation of IL-7-expressing thymic epithelial cells. J Immunol. 2005;174(1):60–67.
- Daley SR, Hu DY, Goodnow CC. Helios marks strongly autoreactive CD4+ T cells in two major waves of thymic deletion distinguished by induction of PD-1 or NF-kappaB. J Exp Med. 2013;210(2):269–285.
- Ross EM, Bourges D, Hogan TV, et al. Helios defines T cells being driven to tolerance in the periphery and thymus. Eur J Immunol. 2014;44(7):2048–2058.
- Kohler S, Keil T, Hoffmann S, et al. CD4(+) FoxP3(+) T regulatory cell subsets in myasthenia gravis patients. Clin Immunol. 2017;179:40–46.
- Luther C, Poeschel S, Varga M, et al. Decreased frequency of intrathymic regulatory T cells in patients with myasthenia-associated thymoma. J Neuroimmunol. 2005;164(1–2):124–128.
- Furuya T, Ishihara S, Ogi H, et al. Characteristic differences in the abundance of tumor-infiltrating lymphocytes and intratumoral developing T cells in thymoma, with special reference to PD-1 expression. Cancer Immunol Immunother. 2023;72(8):2585–2596.
- Nacu A, Andersen JB, Lisnic V, et al. Complicating autoimmune diseases in myasthenia gravis: a review. Autoimmunity. 2015;48(6):362–368.
- Boudil A, Matei IR, Shih HY, et al. IL-7 coordinates proliferation, differentiation and Tcra recombination during thymocyte beta-selection. Nat Immunol. 2015;16(4):397–405.
- Akashi K, Kondo M, Weissman IL. Role of interleukin-7 in T cell development from hematopoietic stem cells. Immunol Rev. 1998;165(1):13–28.
- Katz G, Pobezinsky LA, Jeurling S, et al. T cell receptor stimulation impairs IL-7 receptor signaling by inducing expression of the microRNA miR-17 to target janus kinase 1. Sci Signal. 2014;7(340):ra83.
- Rose NR. Negative selection, epitope mimicry and autoimmunity. Curr Opin Immunol. 2017;49:51–55.
- Teshima T, Reddy P, Liu C, et al. Impaired thymic negative selection causes autoimmune graft-versus-host disease. Blood. 2003;102(2):429–435.

