Abstract
Purpose
To analyze the factors affecting the elevation of serum procalcitonin (PCT) in patients with extensive burns, and explore its potential value in evaluating the severity and prognosis.
Methods
Clinical data of 139 patients with extensive burns admitted to our burn center from January 2014 to December 2019 were retrospectively analyzed. Spearman’s Rank correlation coefficient was used to analyze the factors influencing the elevated PCT levels. The predictive power of PCT for death was evaluated by receiver operating characteristic (ROC) and multiple logistic regression analysis.
Results
72 cases exhibited elevated serum PCT concentrations during the shock phase, but none of them had obvious signs of infection. PCT level in the shock phase was positively correlated with burn area, depth, degree of inhalation injury, delay in fluid resuscitation, APACHE II, and SOFA scores. The peak values of PCT during shock and infection phases were significantly higher in the non-survivors than in the survivors. The areas under the ROC curve for predicting death were 0.788 and 0.926, respectively, and 5.4 ng/mL (OR = 5.33) and 8.5 ng/mL (OR = 14.49) were the high-risk thresholds for death prediction.
Conclusions
Serum PCT level in the shock phase is a potential indicator for evaluating the severity of burns, while the PCT level during the infection period can be used as an early warning indicator for severe systemic infection. High levels of PCT peaks during the shock and infection periods indicate an increased risk of poor prognosis, and targeted treatment is required accordingly.
Keywords:
Introduction
A series of pathophysiological changes such as extensive skin injury, shock, severe stress response, and ischemia-reperfusion injury in the early stage of burns lead to the imbalance of the body’s environmental homeostasis and severe damage to organ functions [Citation1]. Severe burns progress rapidly, and if not effectively controlled, may further develop into sepsis and multiple organ dysfunction, with a high fatality rate [Citation2]. The evaluation of the severity of burns is of great significance in formulating treatment protocols. However, in addition to the area and depth of the burn, the condition of burn patients is also related to many factors, such as the etiology of the injury, whether the early treatment is correct, whether there is a combined injury or poisoning, as well as age and pre-injury health, etc., making the injury and prognostic assessment more complicated [Citation3, Citation4]. With better healthcare providers and improved therapeutics for burns, classification standards for burn severity should also be adapted to changes in various factors.
In 1993, Assicot et al. [Citation5] reported for the first time that serum procalcitonin (PCT) levels were elevated in patients with systemic bacterial infections. Since then, PCT has been widely used as a new biological indicator for identifying infectious diseases [Citation6–8]. In severe bacterial infections, the body’s innate immune system recognizes pathogen-related molecular modules (PAMPs) carried in lipopolysaccharides on the pathogen’s surface, thereby causing systemic inflammation. Under the stimulation of endotoxin and inflammatory cytokines, the expression of calcitonin genes in a variety of tissue cells other than the thyroid is up-regulated, resulting in a large amount of PCT [Citation9]. Studies have also shown that the increase in PCT is not only related to infection, and it also rises remarkably in noninfectious situations such as trauma, major surgery, acute graft rejection, post-resuscitation syndrome, acute pancreatitis, and subarachnoid hemorrhage [Citation10–16]. The possible mechanism underlying this phenomenon is that severe tissue organ damage can lead to a large number of cell disintegration and necrosis, mitochondria rupture, and release of damage-related molecular modules (DAMPs) that have similar host responses to PAMPs, which can also be recognized by module recognition receptors on the surface of immune cells, causing systemic inflammatory responses similar to severe infections, which in turn leads to an increase in PCT [Citation17, Citation18].
In clinical practice, a significant increase in serum PCT levels is common in the shock stage of extensive burns, but the influencing factors that lead to the increase remain unclear. The purpose of this study is to analyze the influencing factors of elevated serum PCT in burn patients, and explore its value in the treatment of critical burns, to provide new standards for the classification of burn severity and provide a new theoretical basis for early warning of adverse prognosis risks.
Patients and methods
Patients
The subjects were composed of burn patients admitted in the burn center of the Fourth Medical Center of PLA General Hospital consecutively from January 2014 to December 2019. The data of burned patients were retrieved from the database of our burn center. The study was approved by the Clinical Trial Medical Ethics Committee of our hospital (2020KY025-KS001). Informed consent was not required in this study. The patients were included and excluded according to the following criteria.
Inclusion criteria
Patients with major burn injuries (≥50% total body surface area, TBSA); routine treatment methods such as fluid resuscitation, surgical removal of necrotic tissue, immune conditioning, metabolic conditioning, nutritional support and organ function protection were carried out according to the guidelines [Citation19, Citation20]; urgent laboratory testing was performed within 48 hours after injury, including serum PCT, blood routine test, hepatic and renal function, electrolyte levels, coagulation function electrolytes, and blood gas analysis.
Exclusion criteria
Obvious signs of infection before burns; vital organ injuries or severe preexisting diseases; incomplete clinical data records; transferred to other hospital or gave up treatment during the period.
Clinical indicators
The clinical data collected from the patients’ medical records included their age, gender, height, body weight, burn area (including total burn area and areas of partial thickness and full thickness burns), cause of burn injury, degree of inhalation injury, delayed recovery time, vital signs, mental status, and outcomes.
Wound secretions, sputum, and urine specimens were collected for microbial culture. Blood samples were collected from each patient for routine blood tests, hepatic and renal function tests, electrolyte tests, coagulation function, blood gas analysis, and blood culture tests. Serum PCT was measured using an electrochemical immunoanalyzer (COBAS® e411, Roche, Mannheim, Germany) and dedicated reagents (Elecsys BRAHMS PCT, Roche, Mannheim, Germany) according to the manufacturer’s instructions. The principle of this assay was based on the enzyme-linked immunosorbent assay, also known as the sandwich method. The detection range of this assay was 0.02–100 ng/mL. The maximum value of PCT on each day was used for statistical analysis.
Definitions
Burn severity of skin tissue was evaluated using the burn index, which was calculated as follows: burn index = full thickness TBSA + 1/2 partial thickness TBSA [Citation21]. The diagnosis of sepsis was based on the American Burn Association (ABA) criteria [Citation22]. Patients with a PCT concentration ≥ 2.00 ng/ml were highly suspected of sepsis [Citation23–25]. The degree of inhalation injury was determined on the patient medical history and the results of fiberoptic bronchoscopy, and were classified as no, mild, moderate, or severe injury [Citation26, Citation27]. Acute Physiology and Chronic Health Evaluation II (APACHE II) consisted of age score, acute physiology score (APS) and chronic health status score (CPS) [Citation28]. Sequential Organ Failure Assessment (SOFA) scores reflect organ function of the following six systems: respiratory, hepatic, cardiovascular, neural, renal and coagulation [Citation29, Citation30].
Statistical analysis
Quantitative variables were presented as median (interquartile range) and the Mann-Whitney U test was performed. Categorical variables were presented as frequencies (percentages) and were compared using a chi-squared test. Spearman’s rank correlation coefficient was used to analyze the correlation between PCT and other factors of patients with major burns. The prognostic accuracy of the selected variables was expressed as the area under the receiver operating characteristic curve (AUC-ROC). The optimal threshold value was assessed using Youden’s index, and the sensitivity, specificity in terms of mortality projection were calculated. Logistic regression analysis was performed to identify the independent predictors for prognosis. Statistical analysis was performed using SPSS 23.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA). Two-tailed P values were used in this study and a P-value < 0.05 was considered significant. The confidence intervals are reported with a 95% confidence level.
Results
Patient demographic characteristics
A total of 139 severely burned patients with a burn area of 50% TBSA were included in this study. The demographic data were presented in . The median age of all the included cases was 39.0 years old, the majority of which (75.5%) were male. The most common cause of injury was flame burns (70.5%), and 112 patients (80.6%) sustained inhalation injury. The fatality rate observed in this cohort during the hospitalization was 19.4%. Compared with the survivors, the non-survivors had higher age, burn index, APACHE II score and SOFA score, combined with more serious inhalation injury, and a longer delay in fluid resuscitation.
Table 1. Patient demographic characteristics.
Factors correlated with elevated PCT levels
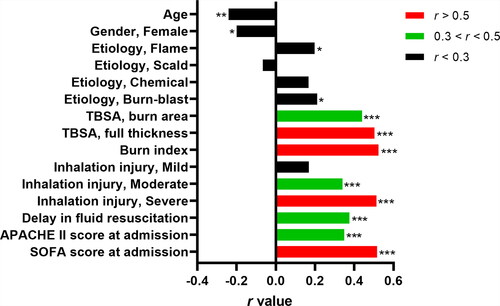
In this study, the peak value of PCT in the shock phase of 72 patients was higher than 2.00 ng/ml, but there were no obvious signs of infection. 68 patients with a peak PCT value higher than 2.00 ng/ml during the infection phase had different degrees of infection, of which 42 cases (61.8%) met the diagnostic criteria of burn sepsis. Spearman’s Rank correlation coefficient analysis showed that full thickness TBSA (r = 0.503; P < 0.001), burn index (r = 0.524; P < 0.001), severe inhalation injury (r = 0.513; P < 0.001), and SOFA score (r = 0.516; P < 0.001) was moderately correlated with PCT level in the shock phase of extensive burns, r > 0.5. In addition, PCT was also mildly correlated with age (r = −0.241; P < 0.01), burn area (r = 0.440; P < 0.001), moderate inhalation injury (r = 0.341; P < 0.001), delay in fluid resuscitation (r = 0.375; P < 0.001), APACHE II score (r = 0.349; P < 0.001), r > 0.3 ().
Figure 1. Correlation analysis of procalcitonin and other factors in the shock phase of extensive burns. TBSA: total body surface area; Burn index: full thickness TBSA + 1/2 partial thickness TBSA; APACHE II score: Acute Physiology and Chronic Health Evaluation II; SOFA Score: Sequential Organ Failure Assessment Score; r value: correlation coefficient. *p < 0.05, **p < 0.01, ***p < 0.001.
The predictive power of PCT
There was an association between the peak PCT in shock and infection phases after burns and the risk of poor prognosis. The mortality rate of patients rose with the increase of PCT, and the peak PCT during shock (r = 0.395; P < 0.001) and infection (r = 0.584; P < 0.001) phases were positively correlated with the mortality rate (). The peak PCT during shock [11.16 (3.57–22.95) versus 1.48 (0.64–4.32); P < 0.001] and infection [22.29 (9.34–64.23) versus 1.59 (0.77–4.43); P < 0.001] was significantly higher in the non-survivors than in the survivors ().
Figure 2. Procalcitonin levels between survivors and non-survivors during shock and infection phases. (a): shock phase; (b) infection phase. Mann-Whitney U test. Data are expressed as median (interquartile range). PCT: procalcitonin; PCT-SP: The maximum concentration of PCT during the shock phase after burn injury; PCT-IP: The maximum concentration of PCT during the infection phase after burn injury.
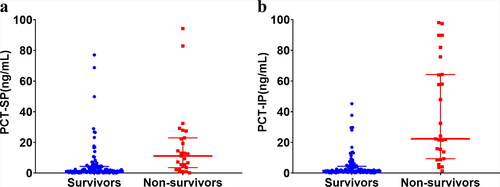
Table 2. Correlation analysis between PCT and mortality.
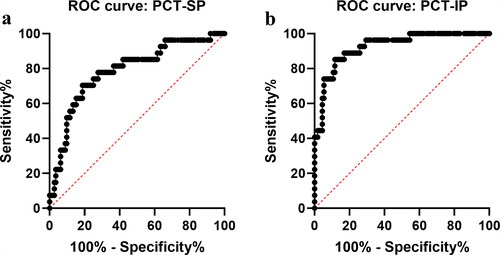
The ROC curves were used to further analyze the predictive power of PCT on death. The area under the curve (AUC) of peak PCT during shock and infection phases in predicting death was 0.788 (95%CI = 0.690–0.887; P < 0.001) and 0.926 (95%CI = 0.874–0.977; P < 0.001), and the optimal threshold was 5.4 ng/mL (sensitivity 70.4%, specificity 81.3%) and 8.5 ng/mL (sensitivity 85.2%, specificity 88.4%), respectively ().
Figure 3. Receiver operating characteristic (ROC) curves of procalcitonin during shock and infection phases for predicting death in severe burn patients. (a) shock phase; (b) infection phase. PCT: procalcitonin; PCT-SP: The maximum concentration of PCT during the shock phase after burn injury; PCT-IP: The maximum concentration of PCT during the infection phase after burn injury.
The included patients were sub-grouped according to the optimal threshold to compare and the differences in clinical outcomes between the subgroups (). Patients whose peak PCT during the infection phase was higher than the threshold had a higher incidence of sepsis, multiple organ failure, and death, as well as a higher number of operations during hospitalization and a longer stay in the ICU. Patients whose peak PCT during the shock phase was higher than the threshold were more likely to have a peak PCT that exceeded the threshold during the infection phase (58.5% vs. 12.2%). Kaplan-Meier survival analysis showed that the 120-day survival rates of PCT < 5.4 ng/mL and PCT ≥ 5.4 ng/mL were 92.9% and 51.2%, respectively, and the difference was statistically significant (Log Rank = 36.24, P < 0.001) ().
Figure 4. Kaplan-Meier survival analysis based on procalcitonin concentrations in the shock phase of extensive burns. PCT: procalcitonin; PCT-SP: The maximum concentration of PCT during the shock phase after burn injury.
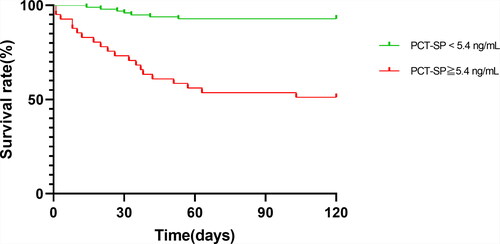
Table 3. Clinical outcomes.
Multiple logistic regression
Multiple logistic regression was employed to rectify demographic characteristics (age, gender, etiology of burn, inhalation injury, and delay in fluid resuscitation), traditional burn severity evaluation indicators (burn area, burn index), and disease severity score upon admission (APACHE II score, SOFA score). The results revealed that the peak PCT during shock greater than 5.4 ng/mL (OR = 5.33; 95%CI = 1.35–21.06; P < 0.001) and the peak PCT during the infection phase greater than 8.5 ng/mL (OR = 14.49; 95%CI = 2.75–76.31; P = 0.002) were a strong predictor of death in patients with severe burns, confirming that a significant increase in the PCT level was an important risk factor for predicting the outcome of death, and the combination of the two indicators represented a greater risk of death, about 55 times that of patients whose indicators were below this threshold ().
Table 4. Multiple logistic regression.
Typical cases
Case 1
Male, 27 years old, with flame burns (TBSA 90%, partial thickness 5%, full thickness 85%; burn index: 88) combined with moderate inhalation injury. The residual healthy skin was located on the thigh (4%), perineum (1%), waist (4%), and head (1%). The patient received fluid resuscitation (500 ml) during the transfer to our burn center. He received fluid resuscitation (500 ml) immediately after arriving at our burn center 1 hour after injury. The patient’s APACHE II score was 8 (age score: 0, CPS score: 0, APS score: 8) and SOFA score was 5 (respiratory score: 2, hepatic score: 1, cardiovascular score: 0, neural score: 1, renal score: 1, coagulation score: 0). The clinical parameters used for scoring are shown in . The urine was light brown and the urine volume was 60–90 mL/h. During the shock stage, the microbial cultures of the wound surface, sputum, and urine were all negative, and the peak PCT value was 1.14 ng/mL. He stayed at our burn ICU for 37 days, and no sepsis occurred during the disease. The peak PCT level during the infection period was 0.66 ng/mL.
Table 5. The clinical parameters of typical cases.
Case 2
Male, 34 years old, with flame burns (TBSA 90%, partial thickness 4%, full thickness 86%; burn index: 88) complicated with severe inhalation injury. The residual healthy skin was located in both feet (3%), perineum (1%), waist (3%), armpit (1%), and head (2%). The patient did not receive fluid resuscitation until the 6th to 8th hours after injury (1,000 ml). He was referred to our burn center 8 hours after injury and received rapid fluid resuscitation immediately after admission. The patient’s APACHE II score was 16 (age score: 0, CPS score: 0, APS score: 15) and SOFA score was 10 (respiratory score: 3, hepatic score: 2, cardiovascular score: 1, neural score: 1, renal score: 3, coagulation score: 0). The clinical parameters used for scoring are shown in . The urine output at 9, 10, 11, and 12 hours after injury was 10 ml, 35 ml, 25 ml, and 20 ml, and the color looked like soy sauce. The urine volume gradually recovered to 40–70 ml after 12 hours. During the shock stage, the microbial cultures of the wound surface, sputum, and urine were all negative, and the peak PCT value was 12.96 ng/mL. During the infection period, blood culture was positive for 5 times (8, 9, 11, 12, and 14 days after injury). When the patient was diagnosed with sepsis, the peak PCT was 64.23 ng/mL. Finally, he died of multiple organ failure 20 days after injury.
Case 3
Male, 32 years old, with flame burns (TBSA 96%, full thickness 96%; burn index: 96) complicated with severe inhalation injury. The residual healthy skin was located on the waist (1%), perineum (1%), and hips (2%). The patient did not receive fluid resuscitation until the 6th to 10th hours after injury (1,000 ml). He was referred to our burn center 10 hours after injury and received rapid fluid resuscitation immediately after admission. The patient’s APACHE II score was 22 (age score: 0, CPS score: 0, APS score: 22) and SOFA score was 14 (respiratory score: 3, hepatic score: 2, cardiovascular score: 1, neural score: 2, renal score: 3, coagulation score: 3). The clinical parameters used for scoring are shown in . The urine output at 11, 12, 13, and 14 hours after injury was 7 ml, 10 ml, 22 ml, and 19 ml, and the color looked like soy sauce. The urine volume gradually recovered to 50–80 ml after 14 hours. On admission, the patient underwent tracheotomy and ventilator-assisted breathing (SIMV mode), resulting in a blood oxygen saturation of 92–98%. During the shock stage, the microbial cultures of the wound surface, sputum, and urine were all negative, and the peak PCT value was 77.03 ng/mL. He stayed at our burn ICU for 142 days, during which severe gastrointestinal bleeding and systemic infection occurred. Blood culture was positive for 22 times. During the infection period, escharectomy and skin grafting operation was performed for 5 times, digital subtraction angiography (DSA) and artery embolization therapy was performed for 4 times and continuous renal replacement therapy (CRRT) was performed for 3 times. The peak PCT level during the infection period was 45.19 ng/mL. After active treatment, the symptoms were relieved. Finally, the patient was healed and discharged.
Discussion
In this study, we analyzed for the first time the relevant factors affecting the increase in serum PCT levels in the shock phase after burns in severely burned patients with a burn area greater than 50% TBSA and discussed its clinical value in the evaluation of burn severity. Then we further explored whether the significant increase in serum PCT levels during shock and infection phases could be used as an early warning indicator for death in patients with extensive burns.
In clinical practice, significant increases in serum PCT levels are common within 48 hours after extensive burns, also known as the shock phase. There are similar phenomena in traumatic patients. Studies have reported that PCT levels can increase significantly in the early stage of severe trauma, and maintain a high level 0 to 2 days after injury [Citation31]. In this study, more than half (51.8%) of patients showed a significant increase in PCT levels (≥2.00 ng/mL) during the shock phase. However, according to clinical manifestations and test results, these patients had no obvious signs of infection, suggesting that the increase in PCT levels early after injury may be related to the release of large amounts of DAMPs caused by tissue organ damage [Citation18].
The evaluation of burn severity is of great significance in guiding the formulation of clinical treatment protocols, such as the level of care, monitoring measures, degree of attention, whether to evacuate and prognostic evaluation. Burn area and wound depth are the most direct indicators to evaluate the severity of burns as well as an important basis for evaluating prognosis and formulating treatment plans. The burn index (BI) which considers the burn area and burn depth can better reflect the severity of skin damage [Citation21]. In this study, with the increase of BI, there were more cases where the PCT of burn patients increased significantly during the shock phase, and the greater the increase, the higher the overall average value. Skin injury is the driving factor for the development of severe burns. The larger and deeper the burn, the more severe shock, and more extensive organ damage. However, the injury factors of extensive burns are complex, such as age, inhalation injury, delayed resuscitation, stress response, etc., which also have a greater impact on the condition. BI only assesses the severity of burns from the anatomical level. It lacks physiological indicators that reflect the function of organs, nor can it reflect the impact of patients’ age and physiological conditions on the degree of injury. Therefore, it cannot reflect the overall severity of burns in patients with extensive burns. We found that although the PCT level in the shock phase was positively correlated with BI, they were not completely parallel. In patients with similar BI, the magnitude of the increase in PCT showed a great difference, indicating that the PCT level in the shock phase may reflect more information about the severity of burns in addition to the area and depth of burns.
Inhalation injury refers to the damage of the respiratory tract and even the lung parenchyma caused by heat or smoke, which contributes to a high incidence of multiple organ failure and mortality [Citation32, Citation33]. According to the literature, inhalation injury alone can increase the fatality rate of burn patients by about 20% [Citation34]. Burns combined with inhalation injuries aggravate the condition and increase the difficulty of treatment. Hensel et al. [Citation35] reported that serum PCT level of patients with noninfectious acute lung injury can increase significantly. According to Nylen et al. [Citation36], serum PCT level may be a valuable predictor of the severity of inhalation injury in burn patients. This study demonstrated that in patients with extensive burns combined with inhalation injury, the PCT level in the shock phase was positively correlated with the severity of inhalation injury, and the higher the degree of inhalation injury, the more significant the increase in serum PCT level, which was consistent with previous findings and further confirmed that the severity of inhalation injury was one of the influencing factors of the increase in PCT during shock.
Delayed resuscitation refers to fluid resuscitation that is provided when burn shock has occurred and continued for a period. When the ischemia and hypoxia of tissues and organs caused by burn shock reach a certain level, fluid resuscitation can aggravate the damage, and the more severe the tissue and organ ischemia and hypoxia, the longer the duration, and the more serious the reperfusion injury after fluid resuscitation [Citation37]. In this study, the longer the delay in fluid resuscitation for patients with extensive burns, the more obvious the increase in PCT during the shock phase, indicating that with the same burn area, the PCT level in the shock phase was correlated with the delay in fluid resuscitation, which could reflect the severity of the ischemia-hypoxic injury and reperfusion injury caused by delayed resuscitation.
According to our research, both APACHE II and SOFA scores were influencing factors for the increase of PCT level in the shock phase in patients with extensive burns. The higher the score, the greater the possibility that the PCT level in the shock phase would increase, and there was a significant correlation between the two, suggesting that PCT, to a certain extent, could reflect the stress level and the degree of organ damage in the early stage of severe burns. Our study displayed that the level of PCT in the shock phase could make up for burn area and depth in the evaluation of the condition, and simply and directly reflect more damage information behind the skin injury, such as inhalation injury, insufficient perfusion, stress response, and organ damage, etc., which represented the severity of burns comprehensively. In addition, the fatality rate of burn patients increased with the increase of PCT levels during shock. High PCT peaks often indicated poor prognosis and were an important risk factor for death in patients with extensive burns.
It is well known that the PCT level is an established diagnostic marker for severe or systemic bacterial. However, in the localized infections, such as skin and skin structure infections, bacteria may remain localized without proceeding to invasive and/or resulting in further systemic manifestations [Citation38]. The role of PCT serum levels in localized bacterial infections is weak. Unlike local skin infections, extensive burns can cause extensive damage to skin tissue. After the shock phase, the granulation barrier of the wound has not been formed, and the organ function of the systemic system has not fully recovered from the severe shock. In addition, there are a lot of necrotic tissues and exudates on the wound, which is suitable for bacterial reproduction and is prone to infection. Therefore, the course of the disease enters the infection phase. A. Lavrentieva et al. [Citation39] found that PCT could be used as a diagnostic tool for infectious complications during ICU hospitalization of burn patients, and the maximum value of PCT during the course of the disease was an independent predictor of death. In this study, we revealed that the high PCT peak during the infection phase often indicated the presence of severe systemic infection, high incidence of sepsis and multiple organ failure, and a greatly increased risk of death, which was consistent with the previously reported results.
The more severe the burn, the more difficult it is to repair the wound during the treatment process, and the more difficult it is to prevent complications such as infection. In this study, patients whose PCT peak was higher than the threshold during the shock phase were more likely to have a high level of PCT peak during the infection phase. This may be because patients with a high PCT peak during shock had relatively severe damage to their skin and organ functions, and were more likely to develop systemic infections, even sepsis, and multiple organ failure during the development of the disease.
It was thus recommended that PCT be monitored daily as a routine inspection item during the treatment of critical burns until the patient’s condition was stable. In the post-burn shock phase, PCT should be combined with traditional indicators such as burn area and depth to more comprehensively assess the severity of burns, thereby guiding the formulation of clinical treatment protocols. In the post-burn infection phase, close attention should be paid to the changes in PCT as an early warning indicator of severe infection. Once a high PCT peak appears, high vigilance is required, followed by immediate investigation on the cause behind, accelerated wound repair, targeted anti-infective treatment, and active correction of other complications to improve the success rate of treatment. At present, PCT is only a monitoring indicator in clinical practice, not an intervention object. Basic experiments have shown that neutralization of peripheral blood PCT can improve the mortality of animals with sepsis, which is also a direction worthy of further research in the future [Citation40].
This study has certain limitations. Firstly, this study was a single-center retrospective study. The data came from one single burn center, and the conclusions reached still required larger-scale multi-center prospective clinical trials to verify its reliability. Secondly, we evaluated the clinical significance of the peak of PCT during shock and infection in this study, and the value of dynamic changes of PCT levels in disease assessment and prognostic judgment needed to be further clarified. Thirdly, other confounding factors, such as drug use, acute kidney injury, the use of renal replacement therapy, liver injury, and surgical procedures may also have a potential impact on the PCT value, and these factors were not included for analysis in this study.
Conclusions
Serum PCT level can be used as an effective biomarker for the evaluation of the severity of the condition and the prognosis of patients with extensive burns. PCT in the early phase after severe burns is a comprehensive manifestation of the degree of body damage, which can make up for more damage information beyond the area and depth of the burn, and is expected to become a new indicator of burn severity. Elevated PCT levels in the post-burn shock phase indicate a serious condition, and complications such as severe infection and organ dysfunction are likely to occur in the later stage. Elevated PCT levels in the post-burn infection phase can be used as an early warning indicator for burns-related sepsis and possibly associated with patient mortality. It is thus necessary to identify the injury factors immediately and comprehensively during the early phase of extensive burn, especially delayed resuscitation and inhalation injury. At the same time, it is also important to accelerate wound repair, monitor changes in PCT on a daily basis, and keep an eye on serious infections.
Acknowledgments
The authors hereby acknowledge the help of our colleagues in the Laboratory Department for they provided us the necessary data and information.
Disclosure statement
The authors declare that they have no conflicts of interest.
Additional information
Funding
References
- Jeschke MG, van Baar ME, Choudhry MA, Chung KK, Gibran NS, Logsetty S. Burn injury. Nat Rev Dis Primers. 2020;6(1):11. doi:https://doi.org/10.1038/s41572-020-0145-5.
- Jeschke MG, Pinto R, Kraft R, et al. Morbidity and survival probability in burn patients in modern burn care. Crit Care Med. 2015;43:808–815. doi:https://doi.org/10.1097/ccm.0000000000000790.
- Yao Y, Liu Y, Zhou J, et al. The epidemiology of civilian inpatients’ burns in Chinese military hospitals, 2001-2007. Burns. 2011;37(6):1023–1032. doi:https://doi.org/10.1016/j.burns.2011.03.021.
- Cheng W, Shen C, Zhao D, et al. The epidemiology and prognosis of patients with massive burns: a multicenter study of 2483 cases. Burns. 2019;45(3):705–716. doi:https://doi.org/10.1016/j.burns.2018.08.008.
- Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993;341(8844):515–518. doi:https://doi.org/10.1016/0140-6736(93)90277-N.
- van Engelen TSR, Wiersinga WJ, Scicluna BP, van der Poll T. Biomarkers in Sepsis. Crit Care Clin. 2018;34(1):139–152. doi:https://doi.org/10.1016/j.ccc.2017.08.010.
- Tujula B, Hämäläinen S, Kokki H, Pulkki K, Kokki M. Review of clinical practice guidelines on the use of procalcitonin in infections. Infect Dis (Lond). 2020;52(4):227–234. doi:https://doi.org/10.1080/23744235.2019.1704860.
- Kouritas V, Zissis C, Bellenis I. Procalcitonin measurement in pleural fluid to predict infectious complications of the chest post lung resection. J Invest Surg. 2021;34(12):1317–1321. doi:https://doi.org/10.1080/08941939.2020.1801912.
- Hamade B, Huang DT. Procalcitonin: where are we now? Crit Care Clin. 2020;36(1):23–40. doi:https://doi.org/10.1016/j.ccc.2019.08.003.
- Annborn M, Dankiewicz J, Erlinge D, et al. Procalcitonin after cardiac arrest - an indicator of severity of illness, ischemia-reperfusion injury and outcome. Resuscitation. 2013;84(6):782–787. doi:https://doi.org/10.1016/j.resuscitation.2013.01.004.
- Hoshino K, Irie Y, Mizunuma M, Kawano K, Kitamura T, Ishikura H. Incidence of elevated procalcitonin and presepsin levels after severe trauma: a pilot cohort study. Anaesth Intensive Care. 2017;45(5):600–604. doi:https://doi.org/10.1177/0310057X1704500510.
- Meisner M, Tschaikowsky K, Hutzler A, Schick C, Schuttler J. Postoperative plasma concentrations of procalcitonin after different types of surgery. Intensive Care Med. 1998;24(7):680–684. doi:https://doi.org/10.1007/s001340050644.
- Muroi C, Lemb JB, Hugelshofer M, Seule M, Bellut D, Keller E. Early systemic procalcitonin levels in patients with aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2014;21(1):73–77. doi:https://doi.org/10.1007/s12028-013-9844-z.
- Sandkovsky U, Kalil AC, Florescu DF. The use and value of procalcitonin in solid organ transplantation. Clin Transplant. 2015;29(8):689–696. doi:https://doi.org/10.1111/ctr.12568.
- Sternby H, Hartman H, Johansen D, Thorlacius H, Regner S. Predictive capacity of biomarkers for severe acute pancreatitis. Eur Surg Res. 2016;56(3–4):154–163. doi:https://doi.org/10.1159/000444141.
- Tan J, Li N, Gong Y, Yuan L, Zhou J, Luo G. Procalcitonin kinetics early after severe burn injury and its value in diagnosis of sepsis. Burns. 2021;47(8):1802–1809. doi:https://doi.org/10.1016/j.burns.2021.02.024.
- Evavold CL, Kagan JC. How inflammasomes inform adaptive immunity. J Mol Biol. 2018;430(2):217–237. doi:https://doi.org/10.1016/j.jmb.2017.09.019.
- Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4(6):469–478. doi:https://doi.org/10.1038/nri1372.
- Committee I, Steering S, Advisory S. ISBI practice guidelines for burn care. Burns. 2016;42:953–1021. doi:https://doi.org/10.1016/j.burns.2016.05.013.
- Greenhalgh DG. Management of burns. N Engl J Med. 2019;380(24):2349–2359. doi:https://doi.org/10.1056/NEJMra1807442.
- Tagami T, Matsui H, Fushimi K, Yasunaga H. Validation of the prognostic burn index: a nationwide retrospective study. Burns. 2015;41(6):1169–1175. doi:https://doi.org/10.1016/j.burns.2015.02.017.
- Greenhalgh DG, Saffle JR, Holmes J, American Burn Association Consensus Conference on Burn Sepsis and Infection Group, et al. American Burn Association consensus conference to define sepsis and infection in burns. J Burn Care Res. 2007;28(6):776–790. doi:https://doi.org/10.1097/BCR.0b013e3181599bc9.
- Müller B, Becker KL, Schächinger H, et al. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Crit Care Med. 2000;28:977–983. doi:https://doi.org/10.1097/00003246-200004000-00011.
- Schuetz P, Beishuizen A, Broyles M, et al. Procalcitonin (PCT)-guided antibiotic stewardship: an international experts consensus on optimized clinical use. Clin Chem Lab Med. 2019;57(9):1308–1318. doi:https://doi.org/10.1515/cclm-2018-1181.
- Mann EA, Baun MM, Meininger JC, Wade CE. Comparison of mortality associated with sepsis in the burn, trauma, and general intensive care unit patient: a systematic review of the literature. Shock. 2012;37(1):4–16. doi:https://doi.org/10.1097/SHK.0b013e318237d6bf.
- Albright JM, Davis CS, Bird MD, et al. The acute pulmonary inflammatory response to the graded severity of smoke inhalation injury. Crit Care Med. 2012;40:1113–1121. doi:https://doi.org/10.1097/CCM.0b013e3182374a67.
- Xu L, Jin J, Wu G, et al. Elevated serum procalcitonin early after extensive burn: influencing factors and clinical significance. Burns. 2021;47(6):1399–1407. doi:https://doi.org/10.1016/j.burns.2020.12.010.
- LeGall JR, Loirat P, Alpérovitch A. APACHE II-a severity of disease classification system. Crit Care Med. 1986;14(8):754–755. doi:https://doi.org/10.1097/00003246-198608000-00027.
- Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. doi:https://doi.org/10.1007/BF01709751.
- Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Critical Care Med. 1995;23(10):1638–1652. doi:https://doi.org/10.1097/00003246-199510000-00007.
- Castelli GP, Pognani C, Cita M, Paladini R. Procalcitonin as a prognostic and diagnostic tool for septic complications after major trauma. Crit Care Med. 2009;37:1845–1849. doi:https://doi.org/10.1097/CCM.0b013e31819ffd5b.
- Brusselaers N, Hoste EA, Monstrey S, et al. Outcome and changes over time in survival following severe burns from 1985 to 2004. Intensive Care Med. 2005;31(12):1648–1653. doi:https://doi.org/10.1007/s00134-005-2819-6.
- Jones SW, Williams FN, Cairns BA, Cartotto R. Inhalation injury: pathophysiology, diagnosis, and treatment. Clin Plast Surg. 2017;44(3):505–511. doi:https://doi.org/10.1016/j.cps.2017.02.009.
- Shirani KZ, Pruitt BA, Jr, Mason AD, Jr. The influence of inhalation injury and pneumonia on burn mortality. Ann Surg. 1987;205(1):82–87. doi:https://doi.org/10.1097/00000658-198701000-00015.
- Hensel M, Volk T, Döcke WD, et al. Hyperprocalcitonemia in patients with noninfectious SIRS and pulmonary dysfunction associated with cardiopulmonary bypass. Anesthesiology. 1998;89(1):93–104. doi:https://doi.org/10.1097/00000542-199807000-00016.
- Nylen ES, O’Neill W, Jordan MH, et al. Serum procalcitonin as an index of inhalation injury in burns. Horm Metab Res. 1992;24(9):439–443. doi:https://doi.org/10.1055/s-2007-1003354.
- Wu MY, Yiang GT, Liao WT, et al. Current mechanistic concepts in ischemia and reperfusion injury. Cell Physiol Biochem. 2018;46(4):1650–1667. doi:https://doi.org/10.1159/000489241.
- Saeed K, Ahmad N, Dryden M. The value of procalcitonin measurement in localized skin and skin structure infection, diabetic foot infections, septic arthritis and osteomyelitis. Expert Rev Mol Diagn. 2014;14(1):47–54. doi:https://doi.org/10.1586/14737159.2014.864238.
- Lavrentieva A, Papadopoulou S, Kioumis J, Kaimakamis E, Bitzani M. PCT as a diagnostic and prognostic tool in burn patients. Whether time course has a role in monitoring sepsis treatment. Burns. 2012;38(3):356–363. doi:https://doi.org/10.1016/j.burns.2011.08.021.
- Tavares E, Miñano FJ. Immunoneutralization of the aminoprocalcitonin peptide of procalcitonin protects rats from lethal endotoxaemia: neuroendocrine and systemic studies. Clin Sci (Lond). 2010;119(12):519–534. doi:https://doi.org/10.1042/cs20100007.

