Abstract
Purpose
To compare the clinical and radiological results of the anterior approach versus the posterior approach versus the anterior–posterior approach for the treatment of thoracolumbar burst fractures.
Methods
The network meta-analysis was performed in accordance with the PRISMA Statement. Electronic searches of PubMed and Embase were conducted up to June 22, 2023, for relevant randomized controlled trials. STATA13.0 was used to perform network meta-analysis. p < .05 was considered significant.
Results
Nine RCTs with a total of 550 patients receiving surgical treatment in at least two of the three approaches, including anterior, posterior and anterior–posterior approaches, were included. The surgical duration and intraoperative bleeding volume in the posterior approach were significantly lower than those in the anterior (SMD, −1.72; 95% CI, −2.82, −0.62) and anterior–posterior approaches (SMD, 3.33; 95% CI, 1.65, 5.00). The surgical duration in the anterior approach was significantly lower than that in the anterior–posterior approach (SMD, 1.61; 95% CI, 0.12, 3.10). The Cobb angle in the anterior–posterior approach was significantly lower than that in the anterior approach (MD, −4.83; 95% CI, −9.60, −0.05). The VAS score in the posterior approach was significantly higher than that in the anterior approach (MD, 0.85; 95% CI, 0.55, 1.16) and anterior–posterior approach (MD, −0.84; 95% CI, −1.12, −0.55). No significant difference was identified among the three surgical approaches in implant failure rate and infection rate.
Conclusion
All three approaches were safe approaches with advantages and disadvantages. The selection of surgical approaches for the treatment of thoracolumbar burst fractures may be individualized.
Introduction
The thoracolumbar vertebra was where two physiological arcs of the spine met. It is where the stress was concentrated. Therefore, thoracolumbar fractures account for 90% of spinal fractures [Citation1–3]. Nonoperative treatment has been reported to have good results for the treatment of thoracolumbar burst fractures without neurologic deficits [Citation4]. Unstable thoracolumbar burst fractures lead to spinal canal stenosis and spinal cord injury [Citation5]. Surgery should be performed to relieve the pressure on the spinal cord and restore stability of the spine. However, at present, the ideal surgical treatment for unstable thoracolumbar burst fractures remains controversial [Citation6]. The most suitable surgical approaches (anterior, posterior, or combined anterior–posterior) to treat unstable thoracolumbar burst fractures are still under debate [Citation7–9]. Posterior pedicle screw fixation was better in the correction of kyphosis deformity. However, it is also associated with a high implant failure rate and incomplete spinal cord decompression [Citation10–12]. In the meantime, anterior reconstruction with instrumentation was associated with better results in decompression of the spinal canal [Citation13]. Combined anterior–posterior surgery had the advantages of both anterior and posterior surgery. In this case, an anterior–posterior approach was advocated. However, it is also accompanied by a longer surgical duration and more intraoperative bleeding volume [Citation14]. These three approaches all have advantages and disadvantages. The aim of this network meta-analysis is to compare the clinical and radiological results of the anterior approach versus the posterior approach versus the anterior–posterior approach for the treatment of thoracolumbar burst fractures.
Materials and methods
Search strategy
The network meta-analysis was performed in accordance with the PRISMA Statement [Citation15]. We comprehensively searched PubMed and Embase up to June 22, 2023, for relevant randomized controlled trials. The keywords “thoracolumbar” OR “thoraco-lumbar” AND “fracture(s)” AND “random*” AND “anterior” or “posterior” or “anterior–posterior” or “anteroposterior” were used in the literature search in all fields, and no language restriction was applied. In the meantime, we reviewed reference lists of included articles for possibly relevant studies. Only published data were reviewed.
Inclusion and exclusion criteria
We systematically reviewed the relevant literature according to certain standards. Studies that met the following criteria were included: (1) patients clinically confirmed of fresh thoracolumbar burst fracture that needed surgical intervention; (2) studies comparing the clinical and radiological outcomes among at least two of the three surgical approaches (anterior approach, posterior approach and anterior–posterior approach) in treatment for thoracolumbar burst fracture; and (3) randomized or quasirandomized controlled trial. We excluded studies that met the following criteria: (1) papers that did not report at least two of the three relevant surgical approaches in the treatment of thoracolumbar burst fracture; (2) conference abstracts; (3) thoracolumbar burst fracture combined with dislocation; (4) systematic reviews or meta-analyses; (5) case reports; and (6) biomechanical studies.
Data extraction
Two authors independently reviewed the full texts of the included studies. The information we needed, including year of publication, basic characteristics of patients, Cobb angle, VAS score, surgical duration and intraoperative bleeding volume, infection rate, implant failure rate and follow-up period, were extracted in Excel.
Risk of bias assessment
The Detsky scale [Citation16] is a reliable and consistent method to assess the methodological quality of randomized controlled trials and was used to assess the risk of bias of eligible studies independently by two authors. It consists of five domains: outcome, eligibility, randomization, therapy, and statistical analysis. Scores of less than 10, more than 11 but less than 15, and more than 16 were considered low quality, moderate quality and high quality, respectively.
Statistical analysis
STATA13.0 (StataCorp, Texas) was used to perform this network meta-analysis. The Cobb angle and VAS score were analyzed using mean difference (MD) and 95% confidence intervals (CI). The standard mean difference (SMD) and 95% CI were used to assess the surgical duration and intraoperative bleeding volume. Dichotomous data, including infection rate and implant failure, were evaluated by relative risk (RR) and 95% CI. The small-study effects were evaluated using the ‘netfunnel’ procedure. The global, loop and local inconsistencies were estimated using ‘network meta i’, ‘ifplot’ and ‘node-splitting’ procedures, respectively. If there was no source of heterogeneity found, a fixed model was applied. Otherwise, a random model was performed. The network of comparisons was obtained by using the ‘network map’ procedure. The ‘sucra’ procedure was performed to rank the three surgical approaches through the value of the surface under the cumulative ranking curve (SUCRA). p < .05 was considered significant.
Result
Identification of relevant studies
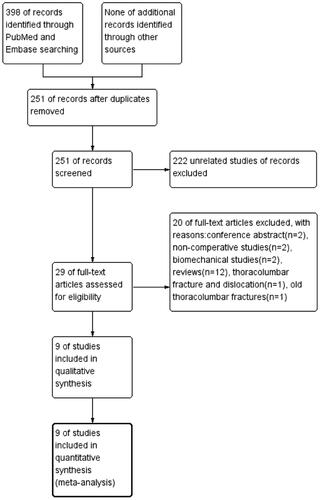
We identified 398 studies by searching PubMed and Embase. After removing duplicate studies, 251 articles were retrieved. There were 222 unrelated studies, 12 reviews, 2 conference abstracts, 2 biomechanical studies, and 2 noncomparative studies excluded. One RCT [Citation17] was excluded due to reporting old thoracolumbar fractures. One RCT [Citation18] was excluded, since the patients had not only simple fractures but also combined dislocations. Eventually, nine studies [Citation2,Citation19–26] were eligible for the network meta-analysis. A flow diagram of the literature review of relevant studies is shown in .
Characteristics of the included studies and risk of bias assessment
Nine RCTs with a total of 550 patients randomized to receive one of the three surgical approaches in treating thoracolumbar burst fractures were identified. The basic characteristics of the patients are presented in . There were 240 patients treated with the anterior approach, 208 patients with the posterior approach and 102 patients with the anterior–posterior approach. Each included study was evaluated according to the Detsky scale, which is shown in . The mean score (range from 8 to 20) of all included RCTs was 15.3. All comparisons within the network meta-analysis are shown in .
Figure 2. Network of the comparisons for the Bayesian network meta-analysis. Note: 1: anterior approach; 2: posterior approach; 3: anterior-posterior approach.
Table 1. Patient and study characteristics of the included studies in the network meta-analysis.
Table 2. Detsky quality Scores of the included randomized controlled trials.
Inconsistency test
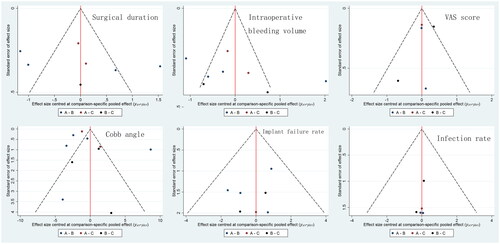
The comparison-adjusted funnel plot in shows that no significant asymmetry to the middle line was found, which indicates that no small-study effects were found in our network meta-analysis. indicates that the CIs of the included studies are compatible with 0.
Meta-analysis of outcomes
Surgical duration
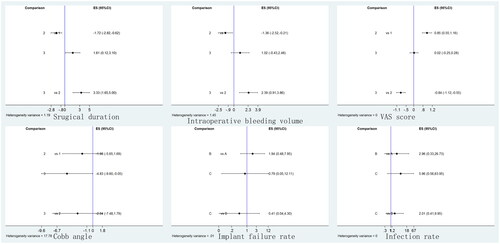
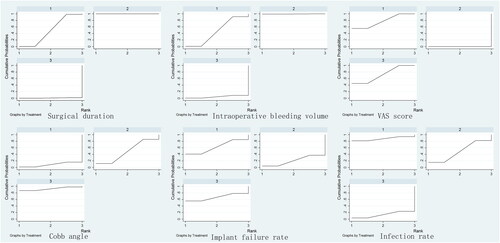
Five included RCTs reported surgical duration. A total of 174 patients, 113 patients and 81 patients were randomly assigned to the anterior approach group, posterior approach group and anterior–posterior group, respectively. The mean surgical duration was 168.2 min in the anterior approach group, 141.3 min in the posterior approach group and 172.4 min in the anterior–posterior approach group. The surgical duration in the posterior approach group was significantly lower than that in the anterior approach group (SMD, −1.72; 95% CI, −2.82, −0.62) and anterior–posterior approach group (SMD, 3.33; 95% CI, 1.65, 5.00). The surgical duration in the anterior approach group was significantly lower than that in the anterior–posterior approach group (SMD, 1.61; 95% CI, 0.12, 3.10). The forest plot is shown in . The SUCRA probabilities of the anterior approach (49.1%), posterior approach (100.0%) and anterior–posterior approach (0.9%) are presented in .
Intraoperative bleeding volume
The intraoperative bleeding volume was reported in six included RCTs. A total of 174 patients were randomly assigned to the anterior approach group, 126 patients to the posterior approach group and 95 patients to the anterior–posterior group. The intraoperative bleeding volumes were 586.0 ml, 382.5 ml, and 697.9 ml for the anterior approach, posterior approach and anterior–posterior approach, respectively. The intraoperative bleeding volume in the posterior approach group was significantly lower than that in the anterior approach group (SMD, −1.36; 95% CI, −2.52, −0.21) and anterior–posterior group (SMD, 2.39; 95% CI, 0.91, 3.86). No significant difference was found between the anterior approach group and the anterior–posterior approach group (SMD, 1.02; 95% CI, −0.43, 2.48). The forest plot is shown in . In addition, the SUCRA probabilities of the anterior approach, posterior approach and anterior–posterior approach, which are shown in , were 46.7%, 99.5% and 0.3.8%, respectively.
Cobb angle
The Cobb angle at the last follow-up was reported in all eight included RCTs. A total of 224 patients were randomly assigned to the anterior approach group, 186 patients to the posterior approach group and 102 patients to the anterior–posterior group. The Cobb angles were 13.95°, 13.06° and 5.22° for the anterior approach, posterior approach and anterior–posterior approach, respectively. The Cobb angle in the anterior–posterior approach group was significantly lower than that in the anterior approach group (MD, −4.83; 95% CI, −9.60, −0.05). No significant difference was identified between the anterior approach group and posterior approach group (MD, −1.98; 95% CI, −5.65, 1.69) or between the posterior approach group and anterior–posterior group (MD, −2.04; 95% CI, −7.48, 1.79) in Cobb angle at the last follow-up. The forest plot is shown in . In addition, the SUCRA probabilities of the anterior approach, posterior approach and anterior–posterior approach, which are shown in , were 8.6%, 48.5% and 92.9%, respectively.
VAS score
Only three included RCTs reported VAS scores at the last follow-up. A total of 42 patients, 54 patients and 35 patients were randomly assigned to the anterior approach group, posterior approach group and anterior–posterior group, respectively. The mean VAS scores in the anterior, posterior and anterior–posterior approach groups were 1.91, 2.44 and 1.36, respectively. No source of heterogeneity was identified. A fixed model was applied in the analysis. The VAS score in the posterior approach group was significantly higher than that in the anterior approach group (MD, 0.85; 95% CI, 0.55, 1.16) and anterior–posterior approach group (MD, −0.84; 95% CI, −1.12, −0.55). No significant difference was found in the VAS score between the anterior approach group and the anterior–posterior approach group (MD, 0.02; 95% CI, −0.25, 0.28). The forest plot is shown in . The SUCRA probabilities of the anterior approach (77.4%), posterior approach (0%) and anterior–posterior approach (72.6%) are presented in .
Implant failure rate
Five included RCTs reported the implant failure rate at the last follow-up. A total of 92 patients, 108 patients and 21 patients were randomly assigned to the anterior approach group, posterior approach group and anterior–posterior group, respectively. The mean implant failure rates in the anterior, posterior and anterior–posterior approach groups were 2.2%, 7.4% and 0%, respectively. No significant difference was found between the anterior approach group and posterior approach group (MD, 1.94; 95% CI, 0.48, 7.95), posterior approach group and anterior–posterior approach group (MD, 0.41; 95% CI, 0.04, 4.30), or anterior approach group and anterior–posterior approach group (MD, 0.79; 95% CI, 0.05, 12.11). The forest plot is shown in . The SUCRA probabilities of the anterior approach (64.1%), posterior approach (20.6%) and anterior–posterior approach (65.3%) are presented in .
Infection rate
Only three included RCTs reported the infection rate at the last follow-up. A total of 42 patients, 54 patients and 35 patients were randomly assigned to the anterior approach group, posterior approach group and anterior–posterior group, respectively. The mean infection rates in the anterior, posterior and anterior–posterior approach groups were 0%, 3.7% and 8.6%, respectively. No source of heterogeneity was identified. A fixed model was applied in the analysis. No significant difference was found in the infection rate between the anterior approach group and posterior approach group (MD, 2.96; 95% CI, 0.33, 26.73), anterior approach group and anterior–posterior approach group (MD, 5.96; 95% CI, 0.56, 63.95), and posterior approach group and anterior–posterior approach group (MD, 2.01; 95% CI, 0.41, 9.95). The forest plot is shown in . The SUCRA probabilities of the anterior approach (87.9%), posterior approach (48.3%) and anterior–posterior approach (13.8%) are presented in .
Discussion
In this network meta-analysis, we found that the anterior–posterior approach was more effective than the anterior approach and posterior approach in the correction of kyphosis. Although the difference between the anterior and posterior approaches was not significant, the SUCRA probabilities of the Cobb angle in the posterior group were higher than those in the anterior group. One of the goals of surgical treatment is correction of kyphotic deformity and stabilization of the spine. Kyphotic deformities can be effectively corrected by pedicle screw fixation and prebending rods [Citation27]. A retrospective study conducted by Tan et al. [Citation28] found that sagittal correction was better achieved with the posterior approach than with the anterior approach in the treatment of flexion-distraction thoracolumbar burst fractures at the six-month follow-up. An analysis reported by Safdari et al. [Citation29] of 273 patients with thoracolumbar fractures who underwent posterior surgery showed that posterior surgery was effective in improving kyphosis.
There was no significant difference in the comparisons of implant failure rate among all groups. The SUCRA probabilities of the implant failure rate, ranking from highest to lowest, were as follows: anterior–posterior approach, anterior approach and posterior approach. The greater the kyphosis was corrected, the greater the stress on the pedicle screw and rod. This could be the reason why posterior pedicle screw fixation is associated with a higher implant failure rate [Citation30].
Our network meta-analysis indicated that the rank of treatment regarding decreasing surgical duration and intraoperative bleeding volume was as follows: posterior approach, anterior approach and anterior–posterior approach. The anterior–posterior approach was associated with higher hospitalization costs and longer hospital stays [Citation24], which may increase the risk. The anterior approach was also associated with higher hospitalization costs than the posterior approach [Citation24]. Smits et al. [Citation31] recommended that an anterior–posterior approach with a cage should be performed for the treatment of highly unstable thoracolumbar burst fractures to prevent loss of sagittal correction. A short surgical duration and less intraoperative bleeding volume in the posterior approach should lead to a low infection rate. No difference was found in the infection rate among the three surgical approaches. However, the SUCRA probabilities of the infection rate, ranking from highest to lowest, were as follows: anterior approach, posterior approach and anterior–posterior approach. The increasing stress on the posterior pedicle screw and rod would lead to decreased stability. Eventually, poor healing of the surrounding soft tissue and muscles occurred.
In terms of the VAS score, the anterior and anterior–posterior approaches had better outcomes than the posterior approach, and the ranking of treatment was anterior approach, anterior–posterior approach and posterior approach. From the above, the anterior approach and anterior–posterior approach are both associated with better clinical results than the posterior approach. Posterior fixation has been a popular method to reduce fractured vertebral bodies and decompression of neurological structures by indirect methods in treating thoracolumbar burst fractures [Citation32]. The anterior approach can achieve direct and complete decompression of the spinal canal compared to the posterior approach, which offers a better chance of neurological improvement than the posterior approach [Citation20]. Other studies [Citation22] indicated that the posterior approach with indirect decompression was usually effective, and no difference was found between direct and indirect decompression in neurological improvement. However, Li et al. [Citation21] demonstrated that the ASIA light tactile score and motor score in the posterior approach group were significantly higher than those in the anterior approach group. That question remains controversial. More RCTs comparing the neurological improvement of different surgical approaches for the treatment of thoracolumbar burst fractures are needed. In addition, some studies [Citation22] have demonstrated that the operative risk of the anterior approach is relatively higher than that of the posterior approach, while another study [Citation25] found that the anterior approach was associated with fewer complications than the posterior approach.
There was a published network meta-analysis [Citation8] of similar topic of our study. But that meta-analysis may have some problems. All prospective, retrospective cohort studies and RCTs were also included in this study, which could result in a large bias. Only studies reported in English and Spanish were included. Some articles which meet the inclusion criteria was left out. There were also some cohort studies wrongly identified as RCTs in this study. What’s more, some studies were excluded because the reports could not be retrieved. Based on those conditions, the results and conclusion of that published network meta-analysis are not credible. We only included RCTs in our study. No language restriction was applied in our study. The results and conclusion of our study were different from that published study. We believe that our meta-analysis has more value in guiding clinical practice.
Strengths and weaknesses
There were some strengths in this network meta-analysis: (1) only randomized controlled trials were included in our network meta-analysis; (2) we used a combination of keywords in all fields in our search to minimize the possibilities of publication bias; (3) the article referred to the results of direct and indirect comparisons; (4) we performed Bayesian network meta-analysis to compare three surgical approaches for thoracolumbar burst fractures; and (5) we used posterior probabilities of outcomes and SUCRA to distinguish the differences among three surgical approaches. However, this network meta-analysis has several limitations that should be acknowledged: (1) only nine studies (a total of 550 patients) were included in our network meta-analysis; thus, the sample size was relatively small. (2) The follow-up period of some included studies was short. Thus, more RCTs with long-term follow-up are needed to demonstrate the conclusion of this meta-analysis.
Conclusion
All three approaches were safe approaches with advantages and disadvantages. The selection of surgical approaches for the treatment of thoracolumbar burst fractures may be individualized. A posterior approach may be performed for patients with severe kyphotic deformities. An anterior approach may be selected for patients with severe symptom. An anterior–posterior approach may be chosen for the treatment of patients with both severe kyphotic deformity and severe symptom.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine (Phila Pa 1976). 1983;8(8):1–9. doi:10.1097/00007632-198311000-00003.
- Esses SI, Botsford DJ, Kostuik JP. Evaluation of surgical treatment for burst fractures. Spine (Phila Pa 1976). 1990;15(7):667–673. doi:10.1097/00007632-199007000-00010.
- Kraemer WJ, Schemitsch EH, Lever J, McBroom RJ, McKee MD, Waddell JP. Functional outcome of thoracolumbar burst fractures without neurological deficit. J Orthop Trauma. 1996;10(8):541–544. doi:10.1097/00005131-199611000-00006.
- Tanasansomboon T, Kittipibul T, Limthongkul W, Yingsakmongkol W, Kotheeranurak V, Singhatanadgige W. Thoracolumbar burst fracture without neurological deficit: review of controversies and current evidence of treatment. World Neurosurg. 2022;162:29–35. doi:10.1016/j.wneu.2022.03.061.
- Suzuki T, Abe E, Miyakoshi N, et al. Anterior decompression and shortening reconstruction with a titanium mesh cage through a posterior approach alone for the treatment of lumbar burst fractures. Asian Spine J. 2012;6(2):123–130. doi:10.4184/asj.2012.6.2.123.
- Rosenthal BD, Boody BS, Jenkins TJ, Hsu WK, Patel AA, Savage JW. Thoracolumbar burst fractures. Clin Spine Surg. 2018;31(4):143–151. doi:10.1097/BSD.0000000000000634.
- Roblesgil-Medrano A, Tellez-Garcia E, Bueno-Gutierrez LC, et al. Thoracolumbar burst fractures: a systematic review and meta-analysis on the anterior and posterior approaches. Spine Surg Relat Res. 2022;6(2):99–108. doi:10.22603/ssrr.2021-0122.
- Hinojosa-Gonzalez DE, Estrada-Mendizabal RJ, Bueno-Gutierrez LC, et al. A network meta-analysis on the surgical management of thoracolumbar burst fractures: anterior, posterior, and combined. Spine Surg Relat Res. 2023;7(3):211–218. doi:10.22603/ssrr.2022-0196.
- Anderson PA, Raksin PB, Arnold PM, et al. Congress of neurological surgeons systematic review and evidence-based guidelines on the evaluation and treatment of patients with thoracolumbar spine trauma: surgical approaches. Neurosurgery. 2019;84(1):E56–E58. doi:10.1093/neuros/nyy363.
- Choovongkomol K, Piyapromdee U, Tanaviriyachai T, Jongkittanakul S, Sudprasert W. Incidence and associated factors for kyphosis progression in short-segment fixation thoracolumbar spine fractures. Int J Spine Surg. 2022;16(5):815–820. doi:10.14444/8343.
- Choudhury AM, Alam MS, Saha MK, Jonayed SA, Rasel MH, Akter K. Short segment pedicle screw fixation including fracture vertebrae for the management of unstable thoracolumbar burst fracture. Mymensingh Med J. 2021;30(2):485–492.
- Kapoen C, Liu Y, Bloemers FW, Deunk J. Pedicle screw fixation of thoracolumbar fractures: conventional short segment versus short segment with intermediate screws at the fracture level-a systematic review and meta-analysis. Eur Spine J. 2020;30(9):2728–2504. doi:10.1007/s00586-021-06937-7.
- Wang T, Wang Z, Ji P, Zhang J, Zhang C, Zhang L. The efficacy and safety of anterior versus posterior approach for the treatment of thoracolumbar burst fractures: a systematic review and meta-analysis. Ann Transl Med. 2022;10(6):309–309. doi:10.21037/atm-22-903.
- Hughes H, Carthy AM, Sheridan GA, Donnell JM, Doyle F, Butler J. Thoracolumbar burst fractures: a systematic review and meta-analysis comparing posterior-only instrumentation versus combined anterior-posterior instrumentation. Spine (Phila Pa 1976). 2021;46(15):E840–E849. doi:10.1097/BRS.0000000000003934.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71.
- Detsky AS, Naylor CD, O’Rourke K, McGeer AJ, L’Abbé KA. Affiliations expand incorporating variations in the quality of individual randomized trials into meta-analysis. J Clin Epidemiol. 1992;45(3):255–265. doi:10.1016/0895-4356(92)90085-2.
- Shi J, Bai L, Ding H, Zhao H, Wang Z. Comparative study of different operating methods in treating old thoracolumbar fractures with spinal cord injury. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2008;22(11):1327–1329. [In Chinese, English abstract]
- Hao D, Wang W, Duan K, et al. Two-year follow-up evaluation of surgical treatment for thoracolumbar fracture-dislocation. Spine (Phila Pa 1976). 2014;39(21):E1284–1290. doi:10.1097/BRS.0000000000000529.
- Gumussuyu G, Islam NC, Kose O, Gungor M, Ozcan H. Comparison of two segment combined instrumentation and fusion versus three segment posterior instrumentation in thoracolumbar burst fractures: a randomized clinical trial with 10 years of follow up. Turk Neurosurg. 2019;29(4):555–563. doi:10.5137/1019-5149.JTN.25025-18.3.
- Jiang Y, Wang F, Yu X, et al. A comparative study on functional recovery, complications, and changes in inflammatory factors in patients with thoracolumbar spinal fracture complicated with nerve injury treated by anterior and posterior decompression. Med Sci Monit. 2019;25:1164–1168. doi:10.12659/MSM.912332.
- Li J, Wang YS, Feng T, Wang B, Qiu JZ. Posterior laminectomy for thoracolumbar fracture and spinal cord compression: a follow-up on cobb’s angle and vertebral height. Chin J Tissue Eng Res. 2016;20(22):3249–3254. [In Chinese, English abstract]
- Lin B, Chen ZW, Guo ZM, Liu H, Yi ZK. Anterior approach versus posterior approach with subtotal corpectomy, decompression, and reconstruction of spine in the treatment of thoracolumbar burst fractures: a prospective randomized controlled study. J Spinal Disord Tech. 2012;25(6):309–317.
- Scholz M, Kandziora F, Tschauder T, Kremer M, Pingel A. Prospective randomized controlled comparison of posterior vs. posterior-anterior stabilization of thoracolumbar incomplete cranial burst fractures in neurological intact patients: the RASPUTHINE pilot study. Eur Spine J. 2018;27(12):3016–3024. doi:10.1007/s00586-017-5356-4.
- Wang J, Liu P. Analysis of surgical approaches for unstable thoracolumbar burst fracture: minimum of five year follow-up. J Pak Med Assoc. 2015;65(2):201–205.
- Wood KB, Bohn D, Mehbod A. Anterior versus posterior treatment of stable thoracolumbar burst fractures without neurologic deficit: a prospective, randomized study. J Spinal Disord Tech. 2005;18 Suppl:S15–S23. doi:10.1097/01.bsd.0000132287.65702.8a.
- Zhang B, Wang JC, Jiang YZ, Song QP, An Y. Effectiveness and postoperative rehabilitation of one-stage combined anterior-posterior surgery for severe thoracolumbar fractures with spinal cord injury. World J Clin Cases. 2022;10(18):6001–6008. doi:10.12998/wjcc.v10.i18.6001.
- Agrawal H, Sharma A, Singh V, Naseem A, Gaddikeri M, Amin A. Long-segment fixation versus short-segment fixation with instrumentation of index vertebra for thoracolumbar fractures. Surg Neurol Int. 2022;13:233. doi:10.25259/SNI_238_2022.
- Tan T, Huang MS, Mathew J, et al. Anterior versus posterior approach in the management of AO Type B1 & B2 traumatic thoracolumbar fractures: A level 1 trauma centre study. J Clin Neurosci. 2020;72:219–223. doi:10.1016/j.jocn.2019.11.039.
- Safdari M, Safdari Z, Pishjoo M, Seifirad S, Kheradmand D, Saghebdoust S. Radiological outcome of operative treatment with posterior approach in patients with thoracolumbar junction traumatic injuries: a single-center pilot study in a developing country. Surg Neurol Int. 2022;13:376. doi:10.25259/SNI_46_2022.
- Zhang H, Li T, Sun H, Zhang J, Hao D. Retrospective analysis of reasons and revision strategy for failed thoracolumbar fracture surgery by posterior approach: a series of 31 cases. Am J Transl Res. 2022;14(9):6323–6331.
- Smits AJ, Polack M, Deunk J, Bloemers FW. Combined anteroposterior fixation using a titanium cage versus solely posterior fixation for traumatic thoracolumbar fractures: a systematic review and meta-analysis. J Craniovertebr Junction Spine. 2017;8(3):168–178. doi:10.4103/jcvjs.JCVJS_8_17.
- Piccone L, Cipolloni V, Nasto LA, et al. Thoracolumbar burst fractures associated with incomplete neurological deficit in patients under the age of 40: Is the posterior approach enough? Surgical treatment and results in a case series of 10 patients with a minimum follow-up of 2 years. Injury. 2020;51(2):312–316. doi:10.1016/j.injury.2019.12.031.