Abstract
Purpose
Limited attention was paid to focus on rectal melanomas (RM). This study aimed to evaluate the survival rate and prognostic factors of RM.
Methods
The data for patients with RM from Surveillance, Epidemiology, and End Results (SEER) database were used to analyze tumor survival. Kaplan-Meier method and log-rank test were employed to estimate cancer-specific survival (CSS) and overall survival (OS). A nomogram was established based on the risk factors of survival by the forest plot for multivariate Cox regression analysis. Receiver operating characteristic (ROC) and calibration curve were conducted for validation.
Results
A total of 187 patients with RM were selected to perform survival analyses. The median survival time of OS was 12 months (range: 0-146 months), and the median survival time of CSS was 12 months (range: 0-74 months). Patients’ age, tumor size, stage, the number of nodes examined, surgery, and radiation were identified as prognostic indicators for CSS by the forest plot for multivariate Cox regression analysis. The nomogram was validated as a reliable model for CSS.
Conclusion
Clinicopathologic relevance with tumor prognosis was confirmed in this study. Our nomogram can provide a relatively accurate prediction of the survival rate of patients with RM.
Primary malignant melanomas are a distinct form of tumor that originates from melanocytes [Citation1]. Among these, gastrointestinal mucosal melanoma is a rare variant that can be found in the esophagus, stomach, colon, rectum, and anus [Citation2]. Primary anorectal melanomas are one of the most prevalent types of mucosal melanoma, characterized by a particularly poor prognosis [Citation3]. The United States has observed an incidence rate of 0.343 per 1 million population for anorectal melanomas, and this incidence has been steadily increasing over the years from 1973 to 2011, as reported by the Surveillance, Epidemiology, and End Results (SEER) program [Citation4].
The prevalence of rectal melanomas (RM) is comparatively lower than that of anal melanomas, however, there has been a consistent increase in the incidence of RM between 1973 and 2011 [Citation4]. Patients with RM often exhibit symptoms such as bleeding, abdominal pain, and tenesmus before receiving a diagnosis. Furthermore, patients with RM are frequently diagnosed at an advanced stage [Citation5, Citation6]. A poor prognosis was observed with a median disease-free of 27 months and median overall survival of 22 months in Tchelebi L’s study [Citation5]. However, due to the scarcity of RM cases and limited clinical data, current research is confined to a small number of case reports [Citation7, Citation8]. Consequently, further comprehensive investigations on the prevalence and prognosis of RM are imperative.
A population‑based study was conducted with the aim of analyzing the survival rates of RM in our study based on data extracted from the SEER database. Additionally, we created and verified a new nomogram to forecast the survival rates of RM.
Methods
Patients and search strategy
According to the SEER Research Plus Data, 17 Registries, Nov 2021 Sub, we extracted data regarding patients diagnosed with RM between the years 2000 and 2019. The primary site of the rectum is identified by the C20.9 code. All tumors with histologic codes 8720–8790 were classified as melanomas based on the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3). Our study specifically included rectal tumors with positive histology, while patients with unknown surgical records were excluded from the dataset ().
The following clinicopathological variables were examined: age (<60, > =60), sex (male, female), race (white, black, and other), tumor size (<30, 30-60, and >60), stage (distant, localized, regional, and unknown), regional nodes examined (<10, 10-30, and >30), positive regional nodes (<20, >20), regional nodes dissection (Yes, No/Unknown), radiation, chemotherapy, and surgery.
Statistical analyses
Statistical analyses were conducted using R version 4.2.2. Descriptive statistics were employed to analyze the clinicopathological variables of the patients. The primary endpoints of the study were overall survival (OS) and cancer-specific survival (CSS). OS refers to the period between diagnosis and death from any cause, while CSS refers to the time between diagnosis and death from RM. Kaplan-Meier analysis and log-rank test were employed to construct survival curves and assess discrepancies in OS and CSS among the different subgroups.
Among patients with RM, all patients were divided into a training group and a validation group by a 7:3 ratio. A forest plot was utilized to perform multivariate Cox regression analysis in the training group, aiming to determine the relationship between survival and clinicopathological variables. The clinicopathological variables were compared between the training and validation groups using Pearson two-sided Chi-squared tests. Based on the independent risk factors for survival, a novel nomogram was developed in the training group to predict the probability of 1-, 2-, and 3-year CSS. In addition, calibration curves, receiver operating characteristic (ROC) curves, and the area under the curve (AUC) were utilized to assess the feasibility of the nomogram in both the training and validation groups.
Results
Patient characteristics
A total of 187 patients with RM met the inclusion criteria between 2000 and 2019. Out of these patients, 17 patients suffered death due to any cause and 170 patients suffered death due to RM. The pertinent characteristics of these patients can be found in . The majority of patients included in the study were elderly (>60 years) and identified as White. Most patients have less than 10 regional lymph nodes and more than 20 regional positive lymph nodes. Only 19.8% of patients have explicitly undergone regional lymph node resection. In terms of treatment options, surgical intervention was performed on 60.4% of patients. Additionally, 56 patients underwent radiotherapy, and 53 patients received chemotherapy.
Table 1. Characteristics of patients with rectal melanoma diagnosed from 2000 to 2019 obtained from the SEER database.
Survival of patients with RM
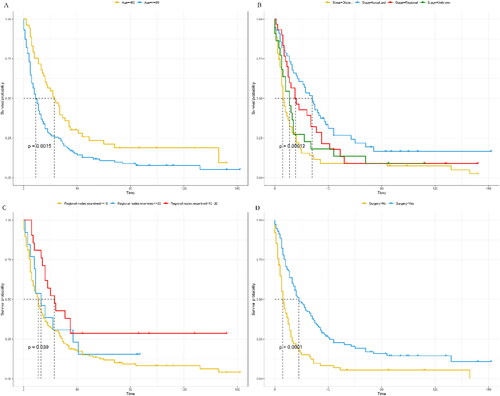
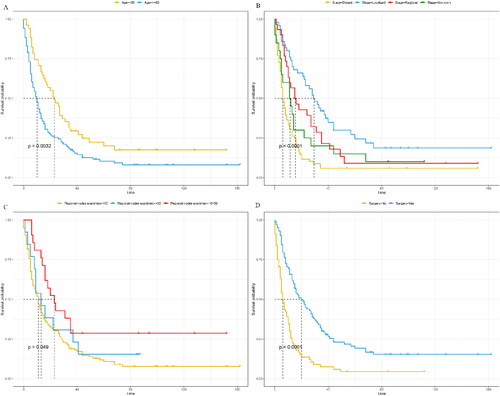
The median survival time for overall survival (OS) was 12 months, ranging from 0 to 146 months. Similarly, the median survival time for cancer-specific survival (CSS) was also 12 months, with a range of 0 to 74 months. The one-year and two-year OS rates were 49.0% (42.3%-56.7%) and 31.6% (25.6%-39.1%), respectively. For CSS, the one-year and two-year rates were 49.8% (42.8%-57.9%) and 31.8% (25.5%-39.7%), respectively. To analyze the Kaplan-Meier curves, patient age, tumor stage, the number of positive lymph nodes, and surgery were taken into consideration. As shown in and , elderly patients were observed to have worse CSS and OS than young patients (<60 years). Localized tumors and the number of positive regional nodes were associated with worse OS and CSS (p < 0.05). In addition, patients who underwent surgery had better OS (median survival rate: 6 months for non-surgery and 18 months for surgery) and CSS (median survival rate: 6 months for non-surgery and 20 months for surgery).
Construction of the nomogram
Among the 170 patients who experienced death as a result of RM, 52 were assigned to the validation group while 118 were assigned to the training group. There were no significant disparities observed in terms of clinicopathological variables between the training and validation groups (p > 0.05) (). By examining the forest plot for multivariate Cox regression analysis, patients’ age, tumor size, stage, the number of nodes examined, surgery, and radiation were incorporated into the development of a nomogram. This nomogram serves the purpose of predicting the precise 1-, 2-, and 3-year CSS rates within the validation group ( and ). Each clinicopathological variable was assigned a specific score, which can be summed to calculate the overall score for prognosticating the survival rate at 1-, 2-, and 3-year CSS.
Figure 4. Forest plot for multivariable Cox regression analysis of the cancer-specific survival (CSS) in patients with rectal melanomas (training group).
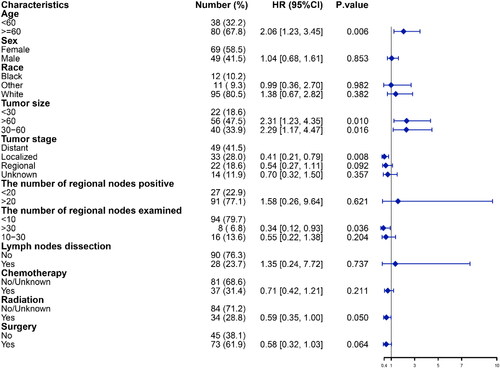
Figure 5. A prognostic nomogram predicting the cancer-specific survival (CSS) of patients with rectal melanomas for the 12, 24, and 36 months (training group).
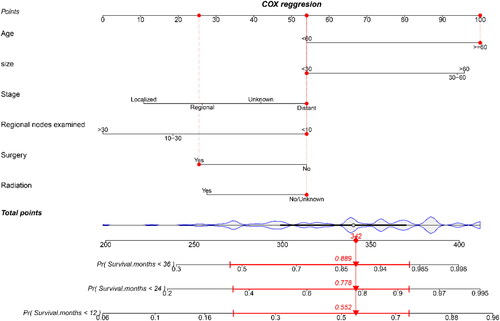
Table 2. Baseline clinical characteristics of patients diagnosed with rectal melanoma in training and validation groups.
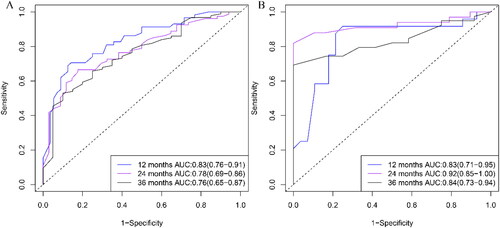
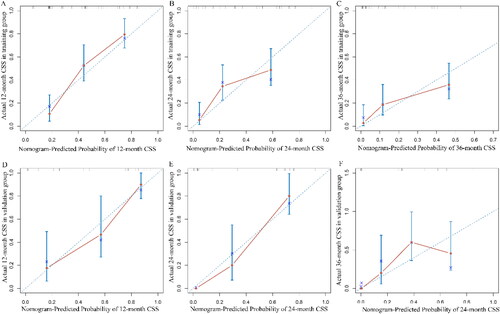
The accuracy of the nomogram model in the validation and training groups was evaluated using ROC curves (). In the training group, the AUC values were 0.83 (95%CI: 0.76-0.91), 0.78 (95%CI: 0.69-0.86), and 0.76 (95%CI: 0.65-0.87), respectively. In the validation group, the AUC values were 0.83 (95%CI: 0.71-0.95), 0.92 (95%CI: 0.85-1.00), and 0.84 (95%CI: 0.73-0.94), respectively. Satisfactory AUC values were observed between the two groups. The calibration curves further showed high consistency between the nomogram-predicted and actual CSS at 1-, 2-, and 3-year ().
Discussion
Owing to the limited number of studies on RM and their focus primarily on the combination of rectal and anal melanoma[Citation9–11], there is a lack of comprehensive understanding of clinicopathological variables, survival, and prognosis of RM. To address this, a population-based study was conducted using data from the SEER Database. The study identified several factors, including age, tumor size, stage, lymph node examination, radiation, and surgery, that significantly impact the survival of RM. Additionally, a reliable nomogram model for 1-, 2-, and 3-year survival of RM was constructed and validated through multivariate Cox regression analysis.
In accordance with prior research, there exists a correlation between patient age and prognosis, with older individuals exhibiting a poorer prognosis[Citation12, Citation13]. In terms of the CSS, patients below the age of 60 demonstrated a median survival time of 23 months (95%CI: 16-34 months), while those aged 60 and above had a median survival time of 10 months (95%CI: 7-13 months). Chen H et al. conducted a retrospective assessment of individuals diagnosed with mucosal melanoma, concluding that age had a negative impact on survival prognosis [Citation14].
The prognosis of patients is associated with the size and stage of the tumor. Patients with larger tumors and advanced stages are more likely to have a poor prognosis. A study has revealed that tumor size is an important factor in predicting survival[Citation15]. However, there is still debate regarding whether tumor size independently affects patient survival. Some studies found no correlation between tumor size and prognosis[Citation16, Citation17], which contradicts our study. In a retrospective study involving 444 patients with mucosal melanoma conducted by Heppt MV, the advanced tumor stage was identified as an independent risk factor for prognosis[Citation18]. In our study, we found that the median survival time for tumors with distant stage was 6 months (95%CI: 5-9 months), while tumors with localized stage had a median survival time of 29.5 months (95%CI: 22-44 months). It was observed that the tumor with a distant stage served as a negative factor influencing patients’ survival. Furthermore, our findings were consistent with previous research, indicating that patients with localized disease had a median OS of 29 months and a 5-year survival rate of 25.6%. Better prognosis was observed in patients with localized disease than those of the regional (median OS 21 months, 5-year rate 16.1%; p = 0.013) and distant tumor (median OS 7 months, 5-year rate 8.9%; p < 0.001) [Citation19].
The impact of regional lymph node involvement on the prognosis of RM remains uncertain. Ren M et al. conducted a clinicopathological study that revealed a significant correlation between lymphatic metastasis and survival in their univariate analysis [Citation20]. The rate of lymph node metastasis emerged as an important predictive factor, but Ciarrocchi A's study concluded that lymphadenectomy does not improve survival[Citation16]. In our study, neither the number of positive regional nodes nor lymphadenectomy was found to be associated with the CSS of the tumor. However, tumors with more than 30 regional lymph nodes had a better prognosis compared to tumors with less than 10 regional lymph nodes. Therefore, the impact of lymph node involvement on the prognosis of patients with RM still needs to be focused on.
Regarding the management of RM, the prognosis of patients can be enhanced through surgical intervention and radiotherapy, whereas the efficacy of chemotherapy is limited. Surgery was the most commonly recommended approach for patients with RM, either alone or in conjunction with radiation therapy, chemotherapy, or immunotherapy[Citation13]. Surgical treatment was recommended for patients with RM in Lei X’s study, although extensive surgery and appendectomy did not significantly affect CSS or OS[Citation10]. Furthermore, irrespective of the extent of resection, comparable survival rates were observed post-surgery in both groups[Citation21]. Emile SH conducted an analysis of the National Cancer Database, which included 641 patients. The study revealed that surgical excision independently predicted improved OS (HR = 0.266, 95%CI: 0.089-0.789, p = 0.017)[Citation22]. Melanoma was previously believed to be resistant to radiotherapy[Citation23]. Limited research has been conducted to investigate the influence of radiotherapy on the prognosis of anorectal melanoma in a population-based study. In a comprehensive examination by Mitra D, 108 patients with anorectal melanoma underwent sphincter-sparing local excision followed by adjuvant radiation therapy, which resulted in the noteworthy achievement of local control and freedom from ostomy[Citation24]. However, further clinical evidence is required to better understand the effects of surgery, radiotherapy, and chemotherapy on the prognosis of rectal melanoma.
This study is subject to several limitations. Firstly, the extraction of data from the SEER database is inherently limited, which subsequently impacts the accuracy of the patient’s survival rate. Certain clinical variables related to tumor survival, such as tumor markers (CA199 and CEA), immunotherapy, specific details about radiotherapy and chemotherapy, and postoperative complications, cannot be obtained from the SEER database. Secondly, despite the large population-based cohort from which the data was extracted, the sample size of RM remains relatively small. Lastly, it is inevitable that selection bias will arise, as this study is a retrospective analysis founded on the SEER database.
Conclusion
To summarize, RM is a less common form of rectal cancer that carries a poorer prognosis. Independent risk factors impacting survival include the age of patients, tumor size, stage, the number of examined lymph nodes, radiation therapy, and surgical interventions. Furthermore, a novel nomogram was developed and validated as a reliable model to help clinicians evaluate the prognosis of patients with RM.
Author contributions
Fan Zhang, Shan Tong and Runda Wu were responsible for the design of the study, statistical analysis, and drafting and revising of the manuscript; Yao Peng, Boqi Xu, and Zhongqi Mao provided critical comments and review of the manuscript; Fan Zhang, Shan Tong, Zhongqi Mao, and Runda Wu supervised the study and revised the manuscript. All authors contributed to the article and approved the submitted version.
Consent for publication
All patients consented to the publication of the results of this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Availability of data and materials
Publicly available datasets were analyzed in this study. This data can be found here: Surveillance, Epidemiology, and End Results (SEER) database (https://seer.cancer.gov/).
References
- Carbó-Bagué A, Rubió-Casadevall J, Puigdemont M, et al. Epidemiology and molecular profile of mucosal melanoma: A population-based study in Southern Europe. Cancers (Basel). 2022;14(3):1. doi:10.3390/cancers14030780.
- Du Y, Chang X, Li X, Xing S. Incidence and survival of patients with primary gastrointestinal melanoma: a population-based study. Int J Colorectal Dis. 2023;38(1):87. doi:10.1007/s00384-023-04385-x.
- Bleicher J, Cohan JN, Huang LC, et al. Trends in the management of anorectal melanoma: A multi-institutional retrospective study and review of the world literature. World J Gastroenterol. 2021;27(3):267–8. doi:10.3748/wjg.v27.i3.267.
- Chen H, Cai Y, Liu Y, et al. Incidence, surgical treatment, and prognosis of anorectal melanoma from 1973 to 2011: A population-based SEER analysis. Medicine (Baltimore). 2016;95(7):e2770. doi:10.1097/md.0000000000002770.
- Tchelebi L, Guirguis A, Ashamalla H. Rectal melanoma: epidemiology, prognosis, and role of adjuvant radiation therapy. J Cancer Res Clin Oncol. 2016;142(12):2569–2575. doi:10.1007/s00432-016-2245-x.
- Mastoraki A, Schizas D, Ntella V, et al. Clinical evidence, diagnostic approach and challenging therapeutic modalities for malignant melanoma of the anorectum. ANZ J Surg. 2021;91(3):276–281. doi:10.1111/ans.16497.
- Guan X, Ning J. A rare case of primary anorectal malignant melanoma. Asian J Surg. 2023;46(7):2746–2747. doi:10.1016/j.asjsur.2023.01.018.
- Khan S, Neupane A, Gautam S, Sapkota S. Primary anorectal melanoma: A case report. JNMA J Nepal Med Assoc. 2023;61(261):469–471. doi:10.31729/jnma.8150.
- Dimitriou F, Namikawa K, Reijers I, et al. Single-agent anti-PD-1 or combined with ipilimumab in patients with mucosal melanoma: an international, retrospective, cohort study. Ann Oncol. 2022;33(9):968–980. doi:10.1016/j.annonc.2022.06.004.
- Lei X, Qingqing L, Weijie Y, et al. Effect of surgical treatment for anorectal melanoma: a propensity score-matched analysis of the Surveillance, Epidemiology, and End Results programme data. BMJ Open. 2022;12(4):e053339. doi:10.1136/bmjopen-2021-053339.
- Mitra D. Locoregional-directed therapy is still a cornerstone of anorectal melanoma management. Int J Radiat Oncol Biol Phys. 2022;112(5):1075. doi:10.1016/j.ijrobp.2021.12.170.
- Sarac E, Amaral T, Keim U, et al. Prognostic factors in 161 patients with mucosal melanoma: a study of German Central Malignant Melanoma Registry. J Eur Acad Dermatol Venereol. 2020;34(9):2021–2025. doi:10.1111/jdv.16306.
- Taylor J, Stem M, Yu D, et al. Treatment strategies and survival trends for anorectal melanoma: Is it time for a change? World J Surg. 2019;43(7):1809–1819. doi:10.1007/s00268-019-04960-w.
- Ercelep O, Topcu T, Bayoglu I, et al. Retrospective multicenter evaluation of patients diagnosed with mucosal melanoma: a study of Anatolian Society of Medical Oncology. Tumour Biol. 2016;37(9):12033–12038. doi:10.1007/s13277-016-5076-0.
- Wang M, Zhang Z, Zhu J, et al. Tumour diameter is a predictor of mesorectal and mesenteric lymph node metastases in anorectal melanoma. Colorectal Dis. 2013;15(9):1086–1092. doi:10.1111/codi.12250.
- Ciarrocchi A, Pietroletti R, Carlei F, Amicucci G. Extensive surgery and lymphadenectomy do not improve survival in primary melanoma of the anorectum: results from analysis of a large database (SEER). Colorectal Dis. 2017;19(2):158–164. doi:10.1111/codi.13412.
- Chae WY, Lee JL, Cho DH, et al. Preliminary suggestion about staging of anorectal malignant melanoma may be used to predict prognosis. Cancer Res Treat. 2016;48(1):240–249. doi:10.4143/crt.2014.305.
- Heppt M, Roesch A, Weide B, et al. Prognostic factors and treatment outcomes in 444 patients with mucosal melanoma. Eur J Cancer. 2017;81:36–44. doi:10.1016/j.ejca.2017.05.014.
- Liu C, Tang C, Zhang J, Zhu P. Extensive resection improves overall and disease-specific survival in localized anorectal melanoma: A SEER-based study. Front Surg. 2022;9:997169. doi:10.3389/fsurg.2022.997169.
- Ren M, Lu Y, Lv J, et al. Prognostic factors in primary anorectal melanoma: a clinicopathological study of 60 cases in China. Hum Pathol. 2018;79:77–85. doi:10.1016/j.humpath.2018.05.004.
- Jutten E, Kruijff S, Francken A, van Westreenen H, Wevers K. Survival following surgical treatment for anorectal melanoma seems similar for local excision and extensive resection regardless of nodal involvement. Surg Oncol. 2021;37:101558. doi:10.1016/j.suronc.2021.101558.
- Emile S, Horesh N, Freund M, et al. Treatment and outcome trends and predictors of overall survival of rectal melanoma: Analysis of the National Cancer Database. Eur J Surg Oncol. 2023;49(7):1275–1282. doi:10.1016/j.ejso.2023.01.021.
- Oxenberg J, Kane J. The role of radiation therapy in melanoma. Surg Clin North Am. 2014;94(5):1031–1047, viii. doi:10.1016/j.suc.2014.07.006.
- Mitra D, Rao P, Nagarajan P, et al. Outcomes after sphincter-sparing local therapy for anorectal melanoma: 1989 to 2020. Pract Radiat Oncol. 2022;12(5):437–445. doi:10.1016/j.prro.2022.02.012.