Abstract
Background: Opinions differ on the relationship between tar level and risk of smoking-related disease. However, except for lung cancer, few reviews have evaluated the epidemiological evidence. Here the relationship of tar level to risk of the four main smoking-related diseases is considered.
Methods: Papers comparing risk of lung cancer, COPD, heart disease or stroke in smokers of lower and higher tar yield cigarettes were identified from reviews and searches, relative risk estimates being extracted comparing the lowest and highest tar groups. Meta-analyses investigated heterogeneity by various study characteristics.
Results: Twenty-six studies were identified, nine of prospective design and 17 case–control. Two studies grouped cigarettes by nicotine rather than tar. Seventeen studies gave results for lung cancer, 16 for heart disease, five for stroke and four for COPD. Preferring relative risks adjusted for daily amount smoked, where adjusted and unadjusted estimates were available, combined estimates for lowest versus highest tar (or nicotine) groups were 0.78 (95% confidence interval 0.70–0.88) for lung cancer, 0.86 (0.81–0.91) for heart disease, 0.77 (0.62–0.95) for stroke and 0.81 (0.65–1.02) for COPD. Lower risks were generally evident in subgroups by publication period, gender, study design, location and extent of confounder adjustment. Estimates were similar preferring data unadjusted for amount smoked or excluding nicotine-based estimates.
Conclusions: Despite evidence that smokers substantially compensate for reduced cigarette yields, the results clearly show lower risks in lower tar smokers. Limitations of the evidence are discussed, but seem unlikely to affect this conclusion.
Introduction
Over the last 50 years or so, tar levels of cigarettes smoked have reduced substantially (Forey et al., Citation2006–2016; Lee, Citation2001; US Surgeon General, Citation2014). However, few reviews have considered whether the health effects of smoking are lower in smokers of lower yield cigarettes. Most attention has been given to lung cancer where opinions vary on whether smoking lower, compared to higher tar, cigarettes is associated with a reduction in risk (Kabat, Citation2003; Lee & Sanders, Citation2004), little or no change in risk (Alberg & Samet, Citation2003; Stratton et al., Citation2001; Thun & Burns, Citation2001) or even an adverse effect on specific histological types (Burns et al., Citation2011; US Surgeon General, Citation2014). The effect of tar level on other major smoking-related diseases has rarely been discussed, and a recent major report discussing 50 years of progress in studying the health consequences of smoking (US Surgeon General, Citation2014) did not even consider whether tar reduction had affected the risk of either respiratory or cardiovascular disease.
In this review, I summarize the evidence relating tar level of cigarettes smoked to the four major smoking-related diseases – lung cancer, heart disease, stroke and chronic obstructive pulmonary disease (COPD). As, by now, virtually all cigarettes smoked have filters, I do not consider comparison of risk in smokers of filtered and non-filtered (plain) cigarettes. For reviews of the evidence on filter/plain differences for lung cancer, the reader is referred to some earlier reviews (Kabat, Citation2003; Lee, Citation2001; Lee & Sanders, Citation2004). I restrict attention to findings from epidemiological case–control and prospective studies, deriving relative risks (RRs) and 95% confidence intervals (CIs) associated with the comparison of smokers of low and high tar cigarettes. As “deliveries of nicotine and tar are generally correlated” (Benowitz, Citation1989) and as there has been a “concomitant shift towards lowered levels of tar and nicotine” (Alberg & Samet, Citation2003) I also consider evidence from studies classifying smokers by nicotine rather than tar level. It should be noted that this review does not compare risk in smokers of low and high tar (or nicotine) cigarettes with that in those who have never smoked.
Methods
Papers comparing the risk of lung cancer, COPD, heart disease or stroke in smokers of lower and higher tar (or nicotine) yield cigarettes were identified from earlier reviews (Kabat, Citation2003; Lee et al., Citation2012; Lee & Sanders, Citation2004; National Cancer Institute, Citation2001; US Surgeon General, Citation2014), from Medline searches, and from reference lists in identified publications. As in previous reviews, the definitions of the outcomes studied were as follows: lung cancer – overall, including at least squamous cell carcinoma and adenocarcinoma (Lee et al., Citation2012), COPD – overall but not chronic bronchitis or emphysema specifically (Forey et al., Citation2011), heart disease – including ischemic heart disease, coronary heart disease or acute myocardial infarction, but not overall cardiovascular disease (Lee et al., Citation2017a), and stroke – overall, but not specific types (Lee et al., Citation2017a). Overlaps between studies were noted.
For each study, estimates of the RR and 95% CI were obtained for the lowest versus highest tar level for which results were reported. Where results by tar level were not available, comparisons of risk for the lowest versus highest nicotine yield or extremes of a combined tar and nicotine grouping were used instead. In a few studies RRs estimated for a given decrease in tar yield were also included. Studies relating risk only to a cumulative tar index (Meyers et al., Citation2017) were not included as they do not allow separation of effects of tar level and amount smoked. Where possible, estimates obtained were gender specific, as is usual in studies of smoking and disease.
For lung cancer and heart disease, I attempted to obtain, if possible, two different RR estimates together with their 95%CI. One of these estimates was adjusted for the daily number of cigarettes smoked, and one was unadjusted. Both estimates were adjusted for the maximum number of other potential confounding variables for which results were reported. For COPD and stroke, only estimates that were adjusted for number of cigarettes smoked were obtained.
Many estimates were taken directly from the source paper. Others were derived by inverting high versus low estimates to become low versus high estimates, or by various other procedures, similar to those used elsewhere (Lee et al., Citation2012) and described more fully in the . Excel spreadsheets are also available on request giving details of the calculations made.
Table 1. Studies of tar yield and major smoking-related diseases.
Fixed and random effects meta-analyses were conducted using standard methods (Fleiss & Gross, Citation1991) though, as fixed effect estimates were quite similar, only random effects results are reported here. For lung cancer and heart disease, initial analyses included results from all the studies, giving summary results based separately on the estimates unadjusted and adjusted for amount smoked. The remaining analyses omit certain studies where overlaps had been identified in order to avoid double counting. For the reduced set of studies, results are shown based firstly on estimates that are unadjusted for amount smoked, secondly on estimates adjusted for amount smoked, thirdly preferring estimates unadjusted for amount smoked where there was a choice, and finally preferring estimates adjusted for amount smoked. For the final set of estimates heterogeneity was investigated by comparing the estimated relative risk by levels of specific factors (period of publication, gender, study design, number of confounding variables considered and continent). Results were also presented excluding estimates not specifically based on tar groupings.
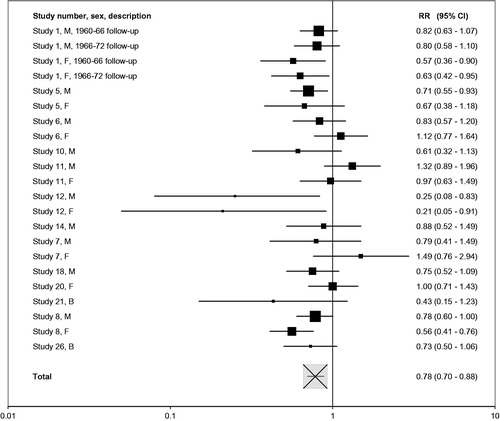
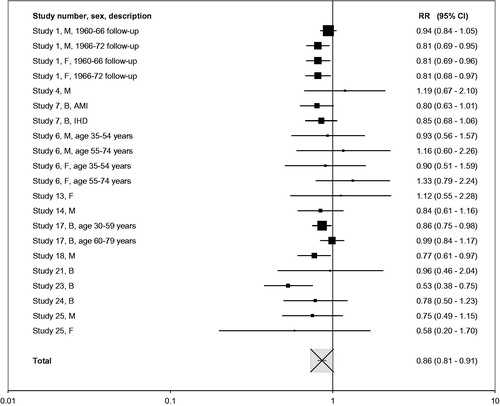
Results of some meta-analyses are given in Figures as forest plots. Each result is presented as a square centered at the RR value and a horizontal line representing the width of the CI. This width is shown on a logarithmic scale. Within each Figure the area of each square is approximately proportional to the meta-analysis weight of the result (the inverse of its variance). The overall meta-analysis result is represented as a white square with a black cross, scaled as for the individual study results.
Unless stated otherwise, p < .05 is taken as statistically significant.
Results
Studies identified
Twenty-six relevant studies were identified, which are subsequently referred to by study number. shows the relevant references, the location and which of the four diseases they provide data for. The final study, recently published, is of particular interest in that it is the only one studying risk in ultra-low tar level cigarettes. Fuller details of the studies are given in .
Of the 26 studies, 15 were conducted in Europe and 11 in North America (all in the USA and one additionally in Canada). Nine were prospective studies and 17 case–control studies. Half of the studies were first reported in or before 1990. Seventeen studies reported results for lung cancer, 16 for heart disease, five for stroke and four for COPD. Three studies used surrogate measures for tar; nicotine in two studies of heart disease, and a combined tar and nicotine index in a study of all four diseases.
Meta-analysis results
Individual effect estimates for lung cancer are presented in , followed in by the detailed meta-analysis results. Similarly, gives individual estimates and meta-analysis results for heart disease. Given the more limited data for these diseases, (stroke) and (COPD) present both the individual and combined effect estimates. Further details, shown in , give, for each disease separately, fuller details of the comparisons made, the factors adjusted for, and the derivation of estimates. The results for the four diseases are considered separately in the paragraphs that follow.
Table 2. Relative risks of lung cancer in lower compared to higher tar cigarette smokers.
Table 3. Meta-analyses for lung cancer.
Table 4. Relative risk of heart disease in lower compared to higher tar cigarette smokers.
Table 5. Meta-analyses for heart disease.
Table 6. Relative risks of stroke in lower compared to higher tar cigarette smokers.
Table 7. Relative risks of COPD in lower compared to higher tar cigarette smokers.
Lung cancer
Of the 45 estimates shown in , 40 show a lower risk in the lower tar group (16 statistically significant), one shows the same risk in the two groups, and four show a higher risk (none significant). Not surprisingly, therefore, the meta-analysis results shown in generally show a significantly lower risk in the low tar groups. Adjustment for amount smoked somewhat reduced the combined relative risk estimate. Using estimates adjusted for amount smoked where available, and avoiding double-counting by excluding results from studies 2, 3, 9 and 16, the overall relative risk for low versus high tar is 0.78 (95% CI 0.70–0.88), with modest heterogeneity (p = .04), the results being illustrated further in . The lower risk in lower tar smokers is evident regardless of period of publication, gender, study design, number of confounders or continent, with no clear evidence of heterogeneity by level of these factors. Omitting results from study 1, which classified individuals based on a combined index of tar and nicotine, little affected the combined relative risk estimate.
Heart disease
Of the 34 estimates shown in , 26 show a lower risk in the lower tar group (eight significant), one shows the same risk in the two groups, and seven show a higher risk (none significant). In line with this, the meta-analysis results shown in generally show a significantly lower risk in the lower tar group. Estimates are similar, whether or not the estimates are adjusted for amount smoked, and whether the studies based on nicotine are included or not. A combined estimate using adjusted estimates where available, and omitting results from studies 15, 19 and 22 to avoid double counting, is 0.86 (95% CI 0.81–0.91) with no significant heterogeneity (see also ). Again there is evidence of a lower risk in lower tar smokers regardless of time, gender, study design, extent of adjustment for confounding variables or continent. There is some indication that the differential in risk is more marked in studies published this century. The estimate was little changed if estimates based on nicotine or a combined tar and nicotine index are excluded, or if only estimates based specifically on nicotine are excluded.
Stroke
The seven available estimates given in show, overall, a modest reduction in risk for lower tar smoking, with a combined estimate of 0.77 (95% CI 0.62–0.95) and no substantial heterogeneity (p = .07).
COPD
As shown in , only six estimates are available. Here the relative risk is 0.81, equivalent to a risk reduction of 19%, which is similar in magnitude to that seen for lung cancer (22%), heart disease (14%) and stroke (23%). However, unlike for the other diseases, the reduction is not statistically significant, the 95% CI of the relative risk being 0.65–1.02.
Discussion
For the four smoking-related diseases considered the data summarized are consistent with smokers of lower tar yield cigarettes having a lower risk than smokers of higher tar yield cigarettes. Using estimates adjusted for amount smoked where possible, and avoiding overlaps, the risk in the lowest tar group is estimated to be 22% lower for lung cancer and 14% for heart disease than that in the highest tar group. There is little evidence of heterogeneity between the individual estimates. Estimates indicating a lower risk considerably outnumbered those indicating a higher increase. The number of studies providing estimates was much smaller for stroke and for COPD, though again the combined estimates indicated a similarly lower risk for low tar smokers; by 23% for stroke and by 19% for COPD. With the exception of COPD all the overall relative risk estimates were statistically significant, those for lung cancer and heart disease very highly so.
Although risks in smokers of low tar cigarettes are reduced compared to those in smokers of high tar cigarettes, it is clear that they are much higher than in those who have never smoked, the estimated reductions in risk for low versus high tar smokers being much less than the increase in risk associated with cigarette smoking (US Surgeon General, Citation2014).
The conclusions reached are not dissimilar from those in a review by Kabat (Citation2003) who suggested that “smokers of lower tar cigarettes have a lower risk (by 20–30%) compared to smokers of higher tar cigarettes” and pointed to a tendency for risks also to be lower for heart disease and COPD. The estimate reported here of a 22% reduction for lung cancer is also not dissimilar to the estimates of 27% and 30% that I have reported earlier based on fewer studies (Lee et al., Citation2012; Lee & Sanders, Citation2004). While some authors (Alberg & Samet, Citation2003; Thun & Burns, Citation2001; US Surgeon General, Citation2014) have concluded that lower tar cigarettes provide little or no health advantage, or may even have an adverse effect, these opinions are not based on meta-analyses. Instead, they derive from a combination of concerns about the propensity for bias in the epidemiology, evidence that smokers of low tar/nicotine cigarettes may “compensate” in order to obtain their required nicotine dose, and the fact that, over a period when tar levels were decreasing, overall lung cancer risk rose in the US and the relative frequency of lung adenocarcinoma to squamous cell carcinoma rose in many countries. Sources of bias and compensation are discussed separately below. Detailed consideration of the data on lung cancer rate trends is beyond the scope of this paper, although the interested reader might wish to refer to publications of mine suggesting alternative explanations for the trends (Lee, Citation2016; Lee & Gosney, Citation2016; Lee & Sanders, Citation2004).
A limitation of the evidence is that subdivision into low and high tar level groups is based on the brands on the market at a particular time. Thus, while early studies included high tar groups with average tar yields of around 30 mg, they included low tar groups with average yields that were higher than for any cigarettes currently on the market. No studies are available which, say, directly compared smokers who, in their whole life, smoked, either 30 + mg tar cigarettes or <10 mg tar cigarettes, and indeed such a study would be impossible to design as tar reduction has been ongoing for more than 50 years.
The magnitude of the reduction seems relatively small, given that reductions over time in the average tar levels of cigarettes smoked have been substantial. There are two main reasons for this.
One is that, as noted above, comparisons of tar level within any given study do not reflect the full range of tar yields which have been available over the years. Indeed study 26 was specifically set up to investigate risk associated with smoking cigarettes with yields of ≤3 mg/cig, as risk of these so called “ultra-low” tar cigarettes, which have become increasingly popular in the last 30 years, had never previously been investigated.
The other reason lies in what is termed “compensation”, with smokers of products which have reduced yields (as measured by machines under standard smoking conditions) modifying how they smoke their cigarettes so that the reduction in yields is not reflected in the actual dose received by the smoker. While some authorities (National Cancer Institute, Citation2001) believe that compensation is complete enough for smokers of reduced tar cigarettes not to benefit at all from the nominal reduction in yield, a recent detailed review (Scherer & Lee, Citation2014) which I collaborated in revealed that though compensation is substantial, it is clearly not complete. Based on 19 brand-switching studies, in which nicotine uptake had been measured, a compensation index of 0.744 (95% CI 0.682–0.806) was estimated, where 1 indicates complete and 0 no compensation. A value of 0.744 is consistent with a 50% reduction in nominal yield being associated with a 17% reduction in uptake. Thus, even if tar yield is markedly reduced, one would not expect a substantial reduction in dose to the smoker, and consequently in risk of smoking-related disease.
The question of whether or not one should adjust RR estimates for daily amount smoked is an interesting one. If smokers switching to a reduced yield product increase their daily cigarette consumption as a form of compensation, then adjustment for amount smoked in analysis can be regarded as a form of “over-adjustment”, and would bias the RR estimate. However, if smokers choosing a lower yield product tend to be less “addicted” smokers and smoke fewer cigarettes a day than those choosing a higher yield product, then failure to adjust for amount smoked could lead to confounding. In principle, the approach to solve this dilemma is to compare smokers switching or not switching to a reduced yield product, and adjust for average daily cigarette consumption before the switch. However, such data are generally not available, and in the absence of this, I presented results for lung cancer and heart disease, both unadjusted and adjusted for daily amount smoked. In practice, the evidence for a modest reduction in risk was clear whether or not such adjustment was made. The estimated risk reductions by 22% for lung cancer and 14% for heart disease referred to above were derived preferring adjusted estimates where there was a choice. Had unadjusted estimates been preferred these reductions would be little changed, becoming 26% for lung cancer and 15% for heart disease. For stroke and COPD, I only presented adjusted results, mainly because, for four of the studies (1, 6, 7 and 18) which contributed almost all the estimates, only adjusted results were available.
Arguments about the appropriateness or not of adjusting for amount smoked also apply to other aspects of smoking which might be affected by the tar level of the brand smoked. This would particularly apply to adjustment for inhalation, given the evidence discussed above relating to “compensation”. However, only two studies (2 and 16) have presented results adjusted for inhalation as self-reported, and in both cases a reduction in risk in smokers of lower tar cigarettes was evident, whether or not adjustment for inhalation was made. Adjustment for age of starting to smoke is less problematic, the decision to start smoking being unlikely to depend materially on the tar level of the brand smoked. In general, adjustment for aspects of smoking, whether this is amount smoked, inhalation, age of starting to smoke or duration of smoking does not affect the conclusion of a lower risk of the diseases studied in smokers of lower tar cigarettes.
A limitation of the meta-analysis lies in the variation in the comparisons made in the different studies. The fact that two studies of heart disease compared smokers of cigarettes with a low and high yield of nicotine, and a further study considered yields of both tar and nicotine does not seem important as exclusion of results from these studies hardly affected the overall estimates. In any case, as noted in the introduction, tar and nicotine levels are correlated. More important is the variation in average yield levels between low and high groups, which one might expect to be reflected in differing RRs, if tar level is relevant to risk. Ideally one would wish to express all the comparisons in terms of a “per x mg difference”, where x is constant over study, but the data do not allow this, very few studies giving average yields of cigarettes smoked in the groups being compared. That said, the fact that the great majority of the RRs were below 1, many being significant, and that no significant increases were seen, gives confidence in the main conclusion of this review, namely, that there is a moderate but clear reduction in risk associated with smoking lower tar cigarettes. While the conclusion of a risk reduction could have been reached simply from the preponderance of RRs below 1, the meta-analyses, despite the limitations noted, give an indication of the magnitude of the risk reduction, and allow one to test for consistency of the reduction over subgroups.
A further limitation of the evidence is that subdivision into low and high tar level groups in the studies considered is often based on the brand smoked at a single time point, or over a limited period of the smokers’ lifetime. Even where lifetime histories are recorded, errors in recall may limit the ability to detect changes in risk associated with tar reduction.
Those who choose to use lower tar cigarettes (from the cigarettes on the market at any given time) may have other characteristics indicative of lower risk than those who choose to use higher tar cigarettes. For example, they may be more educated, or less employed in “risky” occupations. The extent to which risk factors other than smoking was taken into account in the studies considered was quite limited for lung cancer where, of the 17 studies considered (), only seven took into account education/occupation/social class, only two adjusted for diet and only one adjusted for a family history of lung cancer. However, the failure to consider well known risk factors may not be serious, since adjustment generally has little effect on relative risks comparing current and never smokers (Lee et al., Citation2012). For heart disease, adjustment for other risk factors was more extensive, with , between seven and 11 of the 16 studies considered adjusting for education/occupation/social class, history of heart disease, blood pressure, exercise/physical activity, obesity/BMI, alcohol, diet and cholesterol. Given that the advantage to lower tar smokers was no less for estimates adjusted for five or more potential confounding variables than for those adjusted for less than five, it seems unlikely that the advantage can be explained as being due to incomplete confounder adjustment.
Our analyses were limited, as explained, to the four main smoking-related diseases, without any consideration of other diseases, or sub-categories of the diseases that were considered. It has been argued (US Surgeon General, Citation2014) that tar reduction has led to an increase in the relative frequency of adenocarcinoma to squamous carcinoma of the lung, due to increased inhalation and deposit of particulate matter in the peripheral areas of the lung. While there is an unfortunate lack of data relating tar level (rather than filter/plain use) to histological type of lung cancer it is clear that this claim has little foundation for a number of reasons. Apart from the lack of evidence of an increased risk of adenocarcinoma in filter compared to plain cigarette smokers (Brooks et al., Citation2005; Lee et al., Citation2012; Lee & Sanders, Citation2004; Marugame et al., Citation2004; Papadopoulos et al., Citation2011), it is also clear that there has been a rise in relative frequency of adenocarcinoma to squamous carcinoma in non-smokers (Lee et al., Citation2016) and persuasive evidence that changes in methods for classification of histological type have contributed to the observed increase in relative frequency (Lee & Gosney, Citation2016).
Conclusion
Smokers of lower tar cigarettes have a clearly demonstrable lower risk of the four main smoking-related diseases than do smokers of higher tar cigarettes.
Acknowledgements
I thank Barbara Forey for her help in checking the data extraction and meta-analyses, and commenting on the paper and Yvonne Cooper and Diana Morris for assistance in literature searching and in preparing the manuscript.
Disclosure statement
Peter Lee, director of P.N. Lee Statistics and Computing Ltd. is a long-term consultant to various tobacco companies and organizations.
Additional information
Funding
References
- Alberg AJ, Samet JM. (2003). Epidemiology of lung cancer. Chest 123:21S–49S.
- Alderson MR, Lee PN, Wang R. (1985). Risks of lung cancer, chronic bronchitis, ischaemic heart disease, and stroke in relation to type of cigarette smoked. J Epidemiol Community Health 39:286–93.
- Alderson MR, Lee PN, Wang R. (1986). Risk of lung cancer, chronic bronchitis, ischaemic heart disease, and stroke in relation to type of cigarette smoked, passive smoking and other factors. Sutton, Surrey: P. N. Lee Statistics and Computing Ltd. Available from: www.pnlee.co.uk/Reports.htm [Download ALDERS1986].
- Benhamou S, Benhamou E, Auquier A, Flamant R. (1994). Differential effects of tar content, type of tobacco and use of a filter on lung cancer risk in male cigarette smokers. Int J Epidemiol 23:437–43.
- Benowitz NL. (1989). Dosimetric studies of compensatory cigarette smoking. In: Proceedings from Symposium 'Nicotine, smoking, and the low tar programme', 18–20 November 1986, London, Wald, N., Froggatt, P. (eds.) Oxford, New York, Tokyo: Oxford University Press, 133–50.
- Bosetti C, Negri E, Tavani A, et al. (1999). Smoking and acute myocardial infarction among women and men: a case-control study in Italy. Prev Med 29:343–8.
- Brooks DR, Austin JHM, Heelan RT, et al. (2005). Influence of type of cigarette on peripheral versus central lung cancer. Cancer Epidemiol Biomarkers Prev 14:576–81.
- Burns DM, Anderson CM, Gray N. (2011). Do changes in cigarette design influence the rise in adenocarcinoma of the lung? Cancer Causes Control 22:13–22.
- Fleiss JL, Gross AJ. (1991). Meta-analysis in epidemiology, with special reference to studies of the association between exposure to environmental tobacco smoke and lung cancer: a critique. J Clin Epidemiol 44:127–39.
- Forey B, Hamling J, Hamling J, et al. (Eds.). (2006–2016). International Smoking Statistics. A collection of worldwide historical data. Web edition. Sutton, Surrey: P N Lee Statistics and Computing Ltd. Available from: www.pnlee.co.uk/iss.htm.
- Forey BA, Thornton AJ, Lee PN. (2011). Systematic review with meta-analysis of the epidemiological evidence relating smoking to COPD, chronic bronchitis and emphysema. BMC Pulm Med 11:36.
- Gallus S, Randi G, Negri E, et al. (2007). Tar yield and risk of acute myocardial infarction: pooled analysis from three case-control studies. Eur J Cardiovasc Prev Rehabil 14:299–303.
- Garfinkel L, Stellman SD. (1988). Smoking and lung cancer in women: findings in a prospective study. Cancer Res 48:6951–5.
- Gillis CR, Hole DJ, Boyle P. (1988). Cigarette smoking and male lung cancer in an area of very high incidence. I. Report of a case-control study in the West of Scotland. J Epidemiol Community Health 42:38–43.
- Hamling J, Lee P, Weitkunat R, Ambühl M. (2008). Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med 27:954–70.
- Hammond EC, Garfinkel L, Seidman H, Lew EA. (1976). “Tar” and nicotine content of cigarette smoke in relation to death rates. Environ Res 12:263–74.
- Harris JE, Thun MJ, Mondul AM, Calle EE. (2004). Cigarette tar yields in relation to mortality from lung cancer in the cancer prevention study II prospective cohort, 1982-8. BMJ 328:72–6.
- Higenbottam T, Shipley MJ, Rose G. (1982). Cigarettes, lung cancer, and coronary heart disease: the effects of inhalation and tar yield. J Epidemiol Community Health 36:113–7.
- Kabat GC. (2003). Fifty years' experience of reduced-tar cigarettes: what do we know about their health effects? Inhal Toxicol 15:1059–102.
- Kaufman DW, Helmrich SP, Rosenberg L, et al. (1983). Nicotine and carbon monoxide content of cigarette smoke and the risk of myocardial infarction in young men. N Engl J Med 308:409–13.
- Kaufman DW, Palmer JR, Rosenberg L, et al. (1989). Tar content of cigarettes in relation to lung cancer. Am J Epidemiol 129:703–11.
- Kuller LH, Ockene JK, Meilahn E, et al. (1991). Cigarette smoking and mortality. MRFIT Research Group. Prev Med 20:638–54.
- Lee PN. (2001). Lung cancer and type of cigarette smoked. Inhal Toxicol 13:951–76.
- Lee PN. (2016). Possible explanations for the observed rise in the incidence of lung adenocarcinoma relative to that of lung squamous cell carcinoma. J Adenocarcinoma 1:1–3.
- Lee PN, Forey BA, Coombs KJ. (2012). Systematic review with meta-analysis of the epidemiological evidence in the 1900s relating smoking to lung cancer. BMC Cancer 12:385.
- Lee PN, Forey BA, Coombs KJ, et al. (2016). Time trends in never smokers in the relative frequency of the different histological types of lung cancer, in particular adenocarcinoma. Regul Toxicol Pharmacol 74:12–22.
- Lee PN, Fry JS, Forey BA. (2014). Estimating the decline in excess risk of chronic obstructive pulmonary disease following quitting smoking – a systematic review based on the negative exponential model. Regul Toxicol Pharmacol 68:231–9.
- Lee PN, Fry JS, Hamling JF, et al. (2017a). Estimating the effect of differing assumptions on the population health impact of introducing a Reduced Risk Tobacco Product in the USA. Regul Toxicol Pharmacol 88:192–213.
- Lee PN, Fry JS, Hamling JS. (2017b). Investigation into the risk of ultra-low tar cigarettes and lung cancer. Regul Toxicol Pharmacol 89:112–7.
- Lee PN, Garfinkel L. (1981). Mortality and type of cigarette smoked. J Epidemiol Community Health 35:16–22.
- Lee PN, Gosney JR. (2016). The effect of time changes in diagnosing lung cancer type on its recorded distribution, with particular reference to adenocarcinoma. Regul Toxicol Pharmacol 81:322–33.
- Lee PN, Sanders E. (2004). Does increased cigarette consumption nullify any reduction in lung cancer risk associated with low-tar filter cigarettes? Inhal Toxicol 16:817–33.
- Lubin JH, Blot WJ. (1984). Assessment of lung cancer risk factors by histologic category. J Natl Cancer Inst 73:383–9.
- Lubin JH, Blot WJ, Berrino F, et al. (1984). Patterns of lung cancer risk according to type of cigarette smoked. Int J Cancer 33:569–76.
- Marugame T, et al. (2004). Filter cigarette smoking and lung cancer risk; a hospital-based case-control study in Japan. Br J Cancer 90:646–51.
- Meyers TJ, Chang SC, Chang PY, et al. (2017). Case-control study of cumulative cigarette tar exposure and lung and upper aerodigestive tract cancers. Int J Cancer 140:2040–50.
- National Cancer Institute, Shopland DR, Burns DM, Benowitz NL (Eds.), 2001. Risks associated with smoking cigarettes with low machine-measured yields of tar and nicotine. Bethesda, MD: US Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Smoking and Tobacco Control. Monograph No. 13. NIH Pub. No. 02-5047. Available from: http://cancercontrol.cancer.gov/tcrb/monographs/13/index.html.
- Negri E, Franzosi MG, La Vecchia C, et al. (1993). Tar yield of cigarettes and risk of acute myocardial infarction. GISSI-EFRIM Investigators. BMJ 306:1567–70.
- Ockene JK, Kuller LH, Svendsen KH, Meilahn E. (1990). The relationship of smoking cessation to coronary heart disease and lung cancer in the Multiple Risk Factor Intervention Trial (MRFIT). Am J Public Health 80:954–8.
- Palmer JR, Rosenberg L, Shapiro S. (1989). “Low yield” cigarettes and the risk of nonfatal myocardial infarction in women. N Engl J Med 320:1569–73.
- Papadopoulos A, Guida F, Cénée S, et al. (2011). Cigarette smoking and lung cancer in women: results of the French ICARE case-control study. Lung Cancer 74:369–77.
- Parish S, Collins R, Peto R, et al. (1995). Cigarette smoking, tar yields, and non-fatal myocardial infarction: 14,000 cases and 32,000 controls in the United Kingdom. The International Studies of Infarct Survival (ISIS) Collaborators. BMJ 311:471–7.
- Petitti DB, Friedman GD. (1985a). Cardiovascular and other diseases in smokers of low yield cigarettes. J Chronic Dis 38:581–8.
- Petitti DB, Friedman GD. (1985b). Respiratory morbidity in smokers of low- and high-yield cigarettes. Prev Med 14:217–25.
- Sauer WH, Berlin JA, Strom BL, et al. (2002). Cigarette yield and the risk of myocardial infarction in smokers. Arch Intern Med 162:300–6.
- Scherer G, Lee PN. (2014). Smoking behaviour and compensation: a review of the literature with meta-analysis. Regul Toxicol Pharmacol 70:615–28.
- Sidney S, Tekawa IS, Friedman GD. (1993). A prospective study of cigarette tar yield and lung cancer. Cancer Causes Control 4:3–10.
- Speizer FE, Colditz GA, Hunter DJ, et al. (1999). Prospective study of smoking, antioxidant intake, and lung cancer in middle-aged women (USA). Cancer Causes Control 10:475–82.
- Stratton K, Shetty P, Wallace R, Bondurant S (Eds.). (2001). Clearing the smoke. Assessing the science base for tobacco harm reduction. Washington, DC: National Academy Press.
- Tang J-L, Morris JK, Wald NJ, et al. (1995). Mortality in relation to tar yield of cigarettes: a prospective study of four cohorts. BMJ 311:1530–3.
- Tavani A, Bertuzzi M, Negri E, et al. (2001). Alcohol, smoking, coffee and risk of non-fatal acute myocardial infarction in Italy. Eur J Epidemiol 17:1131–7.
- Thun MJ, Burns DM. (2001). Health impact of “reduced yield” cigarettes: a critical assessment of the epidemiological evidence. Tob Control 10(Suppl I):i4–i11.
- US Surgeon General. (2014). The health consequences of smoking – 50 years of progress: a report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. Available from: http://www.surgeongeneral.gov/library/reports/index.html.
- Vutuc C, Kunze M. (1982). Lung cancer risk in women in relation to tar yields of cigarettes. Prev Med 11:713–6.
- Vutuc C, Kunze M. (1983). Tar yields of cigarettes and male lung cancer risk. J Natl Cancer Inst 71:435–7.
- Wilcox HB, Schoenberg JB, Mason TJ, et al. (1988). Smoking and lung cancer: risk as a function of cigarette tar content. Prev Med 17:263–72.
- Woodward M. (2001). Is compulsory restriction of tar yield of cigarettes a worthwhile public health policy? Am J Prev Med 21:284–90.
- Woodward M, Moohan M, Tunstall-Pedoe H. (1999). Self-reported smoking, cigarette yields and inhalation biochemistry related to the incidence of coronary heart disease: results from the Scottish heart health study. J Epidemiol Biostat 4:285–95.
- Wynder EL, Kabat GC. (1988). The effect of low-yield cigarette smoking on lung cancer risk. Cancer 62:1223–30.
- Zhang Q-L, Baumert J, Ladwig K-H, et al. (2011). Association of daily tar and nicotine intake with incident myocardial infarction: results from the population-based MONICA/KORA Augsburg Cohort Study 1984-2002. BMC Public Health 11:273.
Appendix
Table A1. Further details of the studies.
Table A2. Further details associated with (lung cancer).
Table A3. Further details associated with (heart disease).
Table A4. Further details associated with (stroke).
Table A5. Further details associated with (COPD).