Abstract
This literature review on refractory ceramic fibers (RCF) summarizes relevant information on manufacturing, processing, applications, occupational exposure, toxicology and epidemiology studies. Rodent toxicology studies conducted in the 1980s showed that RCF caused fibrosis, lung cancer and mesothelioma. Interpretation of these studies was difficult for various reasons (e.g. overload in chronic inhalation bioassays), but spurred the development of a comprehensive product stewardship program under EPA and later OSHA oversight. Epidemiology studies (both morbidity and mortality) were undertaken to learn more about possible health effects resulting from occupational exposure. No chronic animal bioassay studies on RCF have been conducted since the 1980s. The results of the ongoing epidemiology studies confirm that occupational exposure to RCF is associated with the development of pleural plaques and minor decrements in lung function, but no interstitial fibrosis or incremental lung cancer. Evidence supporting a finding that urinary tumors are associated with RCF exposure remains, but is weaker. One reported, but unconfirmed, mesothelioma was found in an individual with prior occupational asbestos exposure. An elevated SMR for leukemia was found, but was absent in the highly exposed group and has not been observed in studies of other mineral fibers. The industry will continue the product stewardship program including the mortality study.
Introduction
The most recent comprehensive review of refractory ceramic fibers (RCF) toxicology, epidemiology and exposure was published in this journal in 2010 (Utell & Maxim, Citation2010). Although no new chronic animal bioassays were conducted since the earlier publication, there are more recent data on occupational exposures and updates from the ongoing University of Cincinnati (UC) and other epidemiology studies that are noteworthy. Additionally, several other studies have been published that are relevant to assessing possible health risks of occupational exposure to RCF.
Hoffmann et al. (Citation2017) suggests that the procedure for selection of papers to be included in a review article be made explicit. The papers and reports included in this article were identified based on the specific knowledge of the authors (useful for reports) and a sequence of Internet searches using PubMed and Google Scholar. For papers dealing specifically with refractory ceramic fiber, search terms included; ceramic fiber, synthetic vitreous fiber, man-made mineral fiber, Alumino Silicate wool, health effects, pleural plaques, spirometry, epidemiology, toxicology, lung cancer, mesothelioma, exposure, recommended exposure guideline, recommended exposure limit, occupational exposure limit, durability, dissolution rates, biopersistence and product stewardship. The focus of this article is on developments since 2010, but earlier years were included in the searches to discover any articles that might have been missed in the 2010 review.
Brief background
Composition and manufacture
RCF (CAS no. 142844-00-6), first invented in the 1940s, are amorphous fibers that belong to a class of materials termed synthetic vitreous fibers (SVFs), also called synthetic mineral fibers (SMFs), man-made mineral fibers (MMMFs) or man-made vitreous fibers (MMVFs), which also includes alkaline earth silicate (AES) wool, glass wool, rock (stone) wool, slag wool and special-purpose glass fibers. RCF are produced by melting (at ∼1925 °C) a mixture of alumina (Al2O3) and silica (SiO2) in approximately equal proportions. Other inorganic oxides, such as Zr2O3, Cr2O3, B2O3 and TiO2 are sometimes added to alter the properties (e.g. durability or maximum end-use temperature) of the resulting product (AFSSET, Citation2007; NIOSH, Citation2006). RCF can also be made by melting blends of calcined kaolin, alumina and silica. The molten material is made into fibers by either a blowing or a spinning process. Typical compositions of RCF and other SVFs are given in several sources (AFSSET, Citation2007; ATSDR, Citation2004; IARC, Citation2002; National Research Council, Citation2000; NIOSH, Citation2006). As manufactured, RCF are in the form of bulk fibers. Subsequent processing steps are used to convert the RCF into other physical forms such as a blanket, modules (folded blanket with hardware for rapid installation), paper, felt, board, vacuum formed shapes, textiles and as a putty or paste.
Physical properties
RCF have several desirable physical properties as a high-temperature insulating material. These include low thermal conductivity, low heat storage (low volumetric heat capacity), high thermal shock resistance, lightweight, good corrosion resistance and ease of installation (ERM, Citation1995). Depending upon the formulation, the maximum use temperature can be as high as 1430 °C (ERM, Citation1995; NIOSH, Citation2006; TIMA, Citation1993). For this reason, RCF (and certain other fibers) are also termed high-temperature insulating wools (HTIWs). These properties make RCF useful as a lightweight high-temperature insulation material.
RCF applications are principally industrial, where it is used as a furnace lining in the chemical, fertilizer, petrochemical, steel, glass, ceramic, cement, foundry, and forging industries (Mast et al., Citation2000a,Citationb; Utell & Maxim, Citation2010). RCF is also used as fire protection for buildings and industrial process equipment, as aircraft/aerospace heat shields, and in automotive uses, such as catalytic converters, metal reinforcements, heat shields, brake pads, and airbags (Everest Consulting Associates, Citation1996; ERM, Citation1995 [focus on European applications]; Maxim et al., Citation1997). IARC (Citation2002) noted that RCF production was approximately 1–2% of all mineral fibers as of that date.
Our current understanding of fiber toxicology (the so-called 3D paradigm of dose, dimension and durability; see Bernstein, Citation2007; Greim et al., Citation2014; Hesterberg & Hart Citation2001; Lippmann, Citation2014) indicates that fibers with greatest toxicological potential are those that are:
Respirable, that has lengths l > 5 μm, diameters d ≤ 3 μm and aspect ratios l/d ≥3 (WHO) or ≥5 (NIOSH 7400 B),
Sufficiently long (>20 μm) to impede clearance by alveolar macrophages and
Biopersistent (not rapidly cleared from the lung by dissolution or breakage).
Refer Greim et al. (Citation2014), Mast et al. (Citation2000a,Citationb) and Maxim et al. (Citation2000) for length-diameter distributions of RCF in occupational exposures.
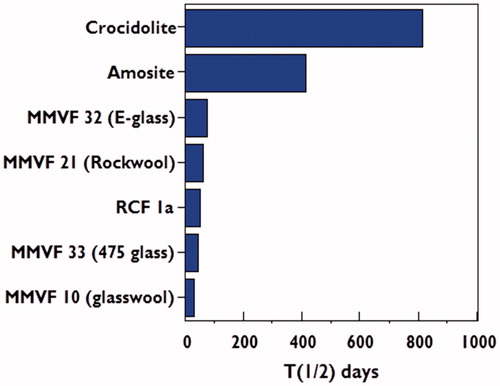
shows weighted half-times (days) of long (>20 μm) fibers from short-term inhalation studies for two types of amphibole asbestos (crocidolite and amosite) and several SVFs (including RCF) as measured by Hesterberg et al. (Citation1998). RCF 1, one of three most common forms in the US and one of the fibers tested in the animal bioassays, was specially prepared to be rat respirable. However, later analysis (Maxim et al., Citation1997) showed that the particle to fiber ratio of RCF 1 was not representative of that found in workplace samples. RCF 1a was prepared from RCF 1 to match the particle to fiber ratio found in workplace samples. Additional information on RCF 1a has been published (Bellmann et al., Citation2001; Brown et al., Citation2000; Mast et al., Citation2000a,Citationb]). As can be seen, RCF are more durable and biopersistent than several other SVFs, although very much less biopersistent than amphibole asbestos (see data from various sources summarized in Brown et al., Citation2005; Greim et al., Citation2014; Matsuzaki et al., Citation2015; Maxim et al., Citation2006). The averagely weighted half-time for RCF in rodents from several studies is approximately 50 days. There are no half-time data in humans, however, Lockey et al. (Citation2012) noted that RCF can persist in human lung tissue for as much as 20 years.
Figure 1. Weighted half-times (days) in the pulmonary region for amphibole asbestos and selected SVFs (Greim et al., Citation2014; Hesterberg et al., Citation1998; Maxim et al., Citation2006).
Animal tests and hazard classification
The earliest animal study, published in 1956 (Gross et al., Citation1956) on male rats exposed by intratracheal injection, concluded that RCF was no more hazardous than a “nuisance dust”, but later studies conducted in the 1980s (Davis et al., Citation1984; Smith et al., Citation1987) and 1990s called this conclusion into question. Chronic nose-only studies on rats and hamsters conducted at the Research and Consulting Company (RCC) (then located in Geneva, Switzerland) indicated that nose-only inhalation exposure to high concentrations of RCF, resulted in fibrosis and tumors (for reviews and references see Greim et al., Citation2014; Mast et al., Citation1995a,Citationb; McConnell et al., Citation1995; Mast et al., Citation2000a; NIOSH, Citation2006; Utell & Maxim, Citation2010). In 1988, an IARC Working Group reviewed the available evidence for RCF and placed RCF in Group 2B (possibly carcinogenic to humans). This classification was reaffirmed by a subsequent Working Group meeting in 2001 (IARC, Citation2002), which concluded that there was sufficient evidence in experimental animals but inadequate evidence in humans for the carcinogenicity of refractory ceramic fibers. Later analyzes, however, indicated that the RCC animal studies were compromised by overload, a factor that has made it difficult to derive accurate estimates of risk by extrapolation of animal study results (Brown et al., Citation2005; Drummond et al., Citation2016; Greim et al., Citation2014; Maxim et al., Citation2003; NIOSH, Citation2006; Utell & Maxim, Citation2010). Notwithstanding limitations of the animal studies, these results prompted concern that exposure to RCF might lead to similar health effects to those for various types of asbestos.
Harrison et al. (Citation2015) provide a discussion of regulatory approaches in both the US and Europe. Useful discussions of RCF carcinogen classification and setting occupational exposure limits (OELs) in Europe can also be found in DECOS (Citation2011), Greim et al. (Citation2014) and SCOEL (2011). SCOEL recommended an OEL of 0.3 fibers/ml based on a calculated no-effect level for impact on lung function. OELs vary by country in Europe from 0.1 f/ml to 2.0 f/ml (Refer Table 3 of Harrison et al., Citation2015).
Product stewardship program
Beginning in the late 1980s, producers of RCF developed a unified and comprehensive industry-wide product stewardship program (PSP) to assess and control possible risks associated with the production and use of RCF-containing products. The PSP was developed and administered through a series of organizations including (in chronological order) the Thermal Insulation Manufacturers Association (TIMA), the Refractory Ceramic Fibers Coalition (RCFC) and the HTIW Coalition in the US and through similar organizations, including ECFIA in Europe and the Refractory Ceramic Fiber Association (RCFA) in Japan. This comprehensive multi-faceted program includes health research (epidemiology studies and medical surveillance), workplace monitoring, exposure assessment, development of workplace controls to reduce exposure in both production plants and end-user facilities, product research (to develop less biopersistent fibers), special studies (emissions, energy savings, waste generation rates and possible substitutes) and a communications outreach component [see Maxim et al. (Citation2008) for more detail]. The voluntary PSP in the US has been conducted under regulatory agency oversight (from 1993 to 1998 by USEPA and from 2002 onwards by OSHA). Annual reports are written to OSHA summarizing PSP progress, exposure measurements and recent developments in toxicology and epidemiology. The most recent annual report to OSHA (Everest Consulting Associates, Citation2017) provides additional detail on the product stewardship program.
Epidemiology studies prior to 2010
The results of animal studies on the toxicity of RCF raised questions about possible adverse respiratory effects of exposure to these fibers in humans. These have been addressed in a series of epidemiology studies. Relevant summaries of epidemiology studies prior to 2010 are given in IARC (Citation2002) and Utell & Maxim (Citation2010). Additional comments are provided below.
One of the early epidemiology studies was reported by Chiazze et al. (Citation1997). Costa & Orriols (Citation2012) summarized the results of this study as follows:
Chiazze et al. (Citation1997) carried out a case–control study in men with LC [lung cancer] from a cohort of 2933 workers at a continuous fiberglass filament factory [in Anderson, South Carolina]. They assessed the exposure to fiberglass, asbestos and RCF, among others. The risk for LC was lower in those exposed to RCF compared with the controls. [Material in square brackets added for clarity.]
Carel et al. (Citation2007) reported on a multicenter case–control study in six Central and East European countries and the United Kingdom. Occupational and demographic data were collected from 2205 newly diagnosed male lung cancer cases and 2305 controls. The study did not find a carcinogenic effect of exposure to MMVF or RCF; however, the power of this study was low. The study found a significant association between asbestos exposure and lung cancer in the United Kingdom but failed to observe a similar association with asbestos exposure among subjects in other European countries.
Marchand et al. (Citation2000) reported results of a case–control study of the relationship between occupational exposure to asbestos and man-made vitreous fibers (including RCF) and laryngeal and hypopharyngeal cancer. This study involved 315 incident cases of laryngeal cancer, 206 cases of hypopharyngeal cancer and 305 hospital-based controls with other types of cancer, all recruited in 15 hospitals in six French cities. These investigators concluded that exposure to asbestos resulted in a significant increase in the risk of hypopharyngeal cancer (OR =1.80, 95% CI: 1.08–2.99) and a non-significant increase in the risk of laryngeal cancer (OR =1.24, 95% CI: 0.83–1.90). Exposure to mineral wools was of borderline significance for the risk of hypopharyngeal cancer (OR =1.55, 95% CI: 0.99–2.41), and not significantly associated with the risk of laryngeal cancer (OR =1.33, 95% CI: 0.91–1.95). No significant results were observed for the other MMVFs including RCF.
The most comprehensive series of epidemiology studies on occupational RCF exposure was sponsored by the industry as part of the PSP and initiated at the UC in the US and at the Institute of Occupational Medicine (IOM) in Europe. The earlier results of morbidity (US and Europe) and mortality (US) studies have been described/reviewed in several publications (Burge et al., Citation1995; Cowie et al., Citation2001; LeMasters et al., Citation1998, Citation2003; Lockey et al., Citation1996, Citation1998, Citation2002; McKay et al., Citation2011; Trethowan et al., Citation1995; Utell & Maxim, Citation2010). Elements of these studies were also included in a study of Australian ceramic fiber production workers (Rogers et al., Citation1997). As of 2010, these studies indicated that occupational exposure to RCF resulted in:
Respiratory symptoms (e.g. dyspnea, wheezing, chronic cough, chronic phlegm) “similar to those reported in other dust-exposed populations” (LeMasters et al., Citation1998);
A statistically, but not clinically, a significant decrease in certain measures of respiratory function in one cross-sectional study (LeMasters et al., Citation1998) for certain subgroups (e.g. male current or former smokers);
However, further longitudinal study (Lockey et al., Citation1998) revealed no excess decline in lung function;
A statistically significant increase in the prevalence of pleural plaques (Lockey et al., Citation1996, Citation2002), but no evidence of parenchymal disease; and
No increased deaths from lung cancer or, at that time, any cases of mesothelioma (LeMasters et al., Citation2003). The mortality study also found an unexpected, but statistically significant, association with cancers of the urinary organs (more below).
To summarize, the available epidemiological data from several studies as of 2010 indicated that symptoms were similar to other dust-exposed populations; there was evidence of minor decreases in certain measures of lung function and a dose-related increase in pleural plaques, but no interstitial fibrosis or elevated lung malignancy rates.
More recent results
Exposures
Exposure monitoring and using engineering controls and improved workplace practices for exposure reduction have been key elements of the PSP. Exposures are monitored (based on a stratified random sampling plan) at plants/facilities operated both by RCF producers and customers. Exposures to RCF at manufacturing locations can occur during fiber production, processing or converting (e.g. vacuum forming) or manufacture or assembly of other specific product forms (e.g. modules), packaging and warehousing. At customer facilities, exposures can result from many of the same activities (except fiber manufacture) and additionally when installing, repairing or removing after-service furnace linings.
Exposures vary by the type of work performed (the exposed population is divided into eight functional job categories [FJCs]) and other factors (e.g. the form of the product, type of engineering controls and industrial segment). Data on exposures by functional job category in the US and Europe for various time periods are provided in several publications (Burley et al., Citation1997; Everest Consulting Associates, Citation2017; Maxim et al., Citation1994, Citation1997, Citation1998, Citation2000, Citation2008; Rogers et al., Citation1997).
A useful summary statistic is the weighted average fiber concentration (fibers per milliliter, f/ml or fibers per cubic centimeter f/cc), calculated by taking the weighted average (weighted by the estimated number of workers in each FJC) of the measured 8-h time-weighted average (TWA) fiber concentrations.
shows the time series of a weighted average of measured fiber concentrations for RCF producers and customers in the US over the period from 1990 through 2016. (Similar data are collected for European producers and customers.) Respirators are worn for some jobs, but the data plotted in do not include any correction for the assigned protection factors of the respirators. There are some data to indicate that fiber concentrations were even higher in the years before 1990. Shown also in (dashed lines) are the recommended exposure guidelines (REGs) over this same time period. (The REGs were established on the basis of prudence and demonstrated feasibility rather than an explicit risk calculation.) The current REG, 0.5 f/ml, is numerically identical to the REL established by NIOSH. NIOSH (Citation2006) wrote a criteria document containing a detailed discussion of the rationale for setting this REL. As can be seen:
Figure 2. Time trends in weighted average TWA fiber concentrations at RCF manufacturers and customers (Everest Consulting Associates, Citation2017).
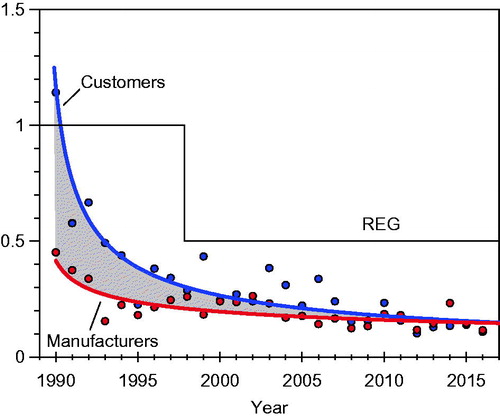
Exposures at both RCF manufacturing plants and customer facilities have decreased substantially over this period. Weighted average exposures are now substantially lower than the US industry REG of 0.5 f/ml.
The gap between exposures at customer and manufacturer facilities (shown by the shaded area in ) has decreased over time to the point that weighted average exposures are nearly the same for both groups.
The rate of decrease in weighted average fiber concentrations has slowed in recent years, suggesting that the limits of present control technologies are being reached.
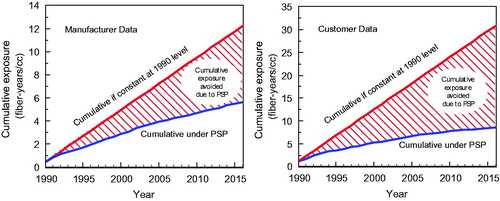
Notwithstanding the flattening of the exposure trends in more recent years, there has been significant progress in reducing exposures, which has materially reduced the cumulative exposure of workers (fiber-years/ml) employed in this industry compared to what would have occurred without the exposure reductions resulting from the PSP. shows for both manufacturer and customer measurements (see the shaded areas) that the cumulative exposure of either the manufacturer or customer cohorts beginning in 1990 when the first integrated industry-wide stewardship program was developed would have been substantially larger but for the exposure reductions brought about by the product stewardship program. The general shapes of these curves will remain the same even if there are no further exposure reductions.
Figure 3. Graphs demonstrate cumulative exposure avoided by implementing the PSP program versus continued uncontrolled exposure at 1990 levels for manufacturer plants (left) and customers (right). Source: Everest Consulting Associates (Citation2017).
Other exposure studies
Zhu et al. (Citation2015a) explored the relationship between mass dust and fiber concentrations in a plant producing RCF. The authors found that there was a broad correlation between these two measures, but the relationship was not sufficiently accurate to enable reliable predictions. This finding is consistent with earlier studies conducted by RCF producers in the US and Europe. Simply stated, dust concentrations are not an adequate surrogate for fiber concentrations.
Zhu et al. (Citation2015d) presented data on fiber and total dust concentrations for various production jobs in a factory in China. Gravimetric dust concentrations ranged from 0.63 to 16 mg/m3 and for fiber concentrations from 0.01 to 1.04 f/ml. Mean values for dust and fiber concentrations were 2.56 mg/m3 and 0.19 f/ml, respectively. This study adopted “reference values” of 5 mg/m3 and 0.5 f/ml for total dust and fiber concentrations, respectively. (The 0.5 f/ml reference value for fiber concentrations matches the industry REG and the NIOSH REL for RCF included in the HTIW Coalition PSP.) The authors also conclude that measurement of both total dust and fiber concentrations better reflects occupational exposures. The range and average fiber concentrations are broadly consistent with measurements made as part of the industry PSP, but differences in protocol preclude exact comparisons.
Epidemiology studies since 2010
Several relevant papers describing the results of epidemiology studies have been published since 2010. These are summarized below.
Wild et al. (Citation2012) published a case–control study among males aged 40–79, including confirmed primary lung cancer cases from all hospitals of a study region consisting of four administrative districts in the Northern part of the French Lorraine region near the German and Luxembourgian borders. Cumulative occupational exposure indices for various materials, including RCF and other MMMFs, were obtained from the questionnaires. Attributable fractions were computed from multiple unconditional logistic regression models. A total of 246 cases and 531 controls were included. The odds ratios (ORs) adjusted on cumulative smoking and family history of lung cancer increased significantly with the cumulative exposure indices to asbestos, polycyclic aromatic hydrocarbons and crystalline silica, and with exposure to diesel motor exhaust. The authors also noted:
Neither RCF, MMMF, iron mining, stainless steel welding nor any other occupational exposure had any significant relation with lung cancer.
These results are consistent with the UC findings.
Lacourt et al. (Citation2014) published a large case–control study aimed to test the hypothesis that there was an increased risk of pleural mesothelioma resulting from co-exposure to asbestos and RCF compared to asbestos exposure alone. Males were selected from a French case–control study conducted in 1987–1993 and from the French National Mesothelioma Surveillance Program in 1998–2006. Two population controls were frequency matched to each case by year of birth. Complete job histories were collected and occupational asbestos and RCF exposures were assessed using job exposure matrices. The dose–response relationships for asbestos exposure were estimated from an unconditional logistic regression model in subjects exposed to asbestos only and the second group of subjects exposed to both asbestos and RCF. The authors concluded that there is an increased risk of pleural mesothelioma resulting from co-exposure of asbestos and refractory ceramic fibers compared to asbestos exposure alone. The authors were somewhat guarded in noting:
Further investigations are needed to confirm and understand the mechanisms of the effect [sic] modification of asbestos exposure in the presence of co-exposure to RCF.
This finding was surprising because other epidemiology studies have not disclosed any significant increase in the incidence of mesothelioma among cohorts exposed to RCF alone. In addition, the Carel et al. (Citation2007) study (though limited in sample size) reported results that were inconsistent with the findings of Lacourt. Specifically, Carel et al. (Citation2007) wrote:
We did not find a carcinogenic effect of exposure to MMVF or for ceramic fibers. Also, no synergistic effect—that is departure from a multiplicative joint effect—of simultaneous exposure to asbestos and MMVF could be shown. [Emphasis added.]
The methodology of the Lacourt et al. (Citation2014) analysis is similar to an earlier (Lacourt et al., Citation2013) study by many of the same authors that found a similar synergistic response between asbestos and mineral wool exposure. Carlsten & Georas (Citation2014) reviewed the earlier Lacourt et al. (Citation2013) paper and noted:
Lacourt and colleagues reported a case–control study investigating associations between pleural mesothelioma and occupational exposure to asbestos, mineral wool, and silica. A total of 1199 cases and 2379 control subjects were identified from different sources in France from 1987 to 1996 and 1998 to 2006. Although occupational exposure to asbestos is a well-known risk factor for pleural mesothelioma, the risks associated with mineral wool (which is used in insulation) and silica dust are less clear. The main finding was that occupational exposure to mineral wool was associated with a significantly increased OR for mesothelioma when adjusting for asbestos exposure (e.g. OR =2.5 for highest exposure group). No such associations were found for silica exposure in adjusted analyses, although a significantly higher OR was observed for silica exposure in the unadjusted subgroup analysis. These seemingly contradictory results could be the result of misclassification, the inability to independently separate exposures to the different compounds, or other factors, which were discussed in subsequent correspondence.
In the end, Carlsten and Georas concluded:
Taken together, these data suggest that more research is needed to understand how mineral wool and other fibers interact to increase risk of mesothelioma over time.
Boffetta et al. (Citation2014) reviewed the evidence on occupational exposure to SVFs (including RCF) and also analyzed an earlier (2013) Lacourt et al.’s study on co-exposure to asbestos and mineral wool. They showed that the conclusion reached by Lacourt and colleagues was sensitive to possible misclassification of asbestos exposure. They found that even a very small misclassification of asbestos exposure (95% sensitivity and specificity) offset the risk from SVF exposure and wrote:
The fact that the elevated OR found in some of the case–control studies are reduced after adjusting for asbestos exposure and that they are not confirmed in cohort studies of production workers detracts from the credibility of a causal relationship between SVF exposure and mesothelioma risk. The combined evidence from epidemiologic studies leads to the conclusion that exposure to SVF is not likely to increase the risk of mesothelioma.
Boffetta et al. (Citation2014) concluded:
The epidemiological literature reviewed here provides support for the notion that SVF do not cause mesothelioma and is in line with these toxicological arguments that SVF are not biopersistent. The evidence from epidemiology and toxicology is therefore consistent with the hypothesis that non-biopersistent SVF pose no risk of mesothelioma to humans.
Thus, the Lacourt findings for both mineral wool and RCF must be viewed with some caution.
Gérazime et al. (Citation2015) published another case–control (retrospective) study of male workers. The authors used data from ICARE, a population based case–control study conducted in France. Detailed lifetime tobacco and alcohol consumption, as well as complete occupational history, were collected. Analyses were restricted to men and included 1867 cases of head and neck squamous cell carcinomas, 2276 lung cancer cases and 2780 controls. Occupational exposure to RCF was assessed through a specific job-exposure matrix. The authors estimated odds ratios (ORs) and 95% confidence intervals (CIs), adjusted for age, residence area, tobacco smoking, alcohol consumption and cumulative exposure to asbestos with logistic models. The authors concluded that “although a moderate increase in risk cannot be excluded”, occupational exposure to RCF was not significantly associated with risk of head and neck cancer (OR = 0.83; 95% CI = 0.64–1.10) or lung cancer (OR = 0.91; 95% CI = 0.72–1.16). Results of this study (no statistically significant excess in head and neck or lung cancer) are consistent with the UC results.
UC mortality results since 2010
The UC mortality study is ongoing. The mortality study includes current and former male and female employees hired from 1952 through 1999 at two RCF manufacturing plants, one in New York and the other in Indiana who had at least 1-year RCF employment (see as LeMasters et al. [Citation2003] for details). The latest published results of the prospective cohort study included all deaths as of 12/31/2015 (LeMasters et al. Citation2017). Participants (N = 1119) in the mortality study include current and former male and female workers at the New York (N = 818) and Indiana (N = 301) plants. The majority of workers are Caucasian (91%), male (84%) and current or former smokers (54%) and approximately half had self-reported prior asbestos exposure. The median age at death or time of follow up was 63.1 years, with a mean time since first exposure (latency) of 32.9 years and mean cumulative fiber exposure (CFE) of 46.1 fiber-months/cc At date of death or 12/31/2015, 87.3% had >20 years and 60.6% had >30 years time since first exposure. Exposure analyses enabled the identification of the most highly exposed subgroup in the cohort, workers (N = 285) with more than 45 fiber-months/cc RCF exposure.
shows standardized mortality rates (SMRs) and two-sided 95% confidence intervals for deaths from malignant neoplasms (MNs) and selected other causes for both the entire cohort and most exposed group of RCF exposed workers in the latest update to the UC mortality study.
Table 1. SMRs and confidence intervals for full cohort (N = 1119) and most exposed group, abbreviated version, SMRs significantly elevated or reduced shown in boldface, excerpted from LeMasters et al. (Citation2017).
The pattern of SMRs is broadly similar to those reported earlier, but there are some differences related to kidney and bladder cancers and leukemia. Additionally, there was one reported, but unconfirmed mesothelioma in a worker with prior occupational exposure to asbestos – these points are discussed below.
LeMasters et al. (Citation2017) also conducted a cancer incidence study. Since 2003 these investigators collected lung and urinary cancer incidence data using questionnaires for 1011 participants. There were four participants reporting respiratory cancers with 9.9 expected (Standardized Incidence Ratio (SIR) = 0.40) and two reporting urinary cancer with 6.4 expected (SIR = 0.31).
Kidney and bladder cancers
New data on the prevalence of urinary cancers that indicate elevated SMRs for these diseases remain, but [according to LeMasters et al. (Citation2017)] might have been caused by other factors rather than RCF exposure. Known risk factors for these cancers, including cigarette smoking, obesity, certain drugs, medical conditions and treatments and exposures to many other toxicants are summarized in (kidney cancer) and A2 (bladder cancer) located in the Appendix. Most of these were not controlled for in the UC study. In reviewing the latest results, the UC investigators wrote:
…the strongest risk factor for bladder and renal cancers is cigarette smoking (McLaughlin et al., Citation2006; Zeegers et al., Citation2000); half of the bladder and renal cancer deaths in the current study were known smokers. In addition, three of the four bladder cancer deaths were millwrights with potential occupational exposures associated with bladder cancer, including exposure to polycyclic aromatic hydrocarbons (PAH) (Behrman, Citation2003; Kogevinas et al., Citation2003; Reulen et al., Citation2008; Spinelli, Citation1989).
In assessing the plausibility of a possible relationship between RCF exposure and urinary cancers, it is reasonable to examine whether exposure to other fibers or to asbestos has been linked to urinary cancers. SVFs are the logical fibers for comparison (see below), but one article (Lauriola et al., Citation2012) concluded that asbestos exposure was associated with a statistically significant increase in SMR for renal neoplasia. However, two meta-analyses have failed to demonstrate a significant association between kidney cancer and asbestos exposure (Goodman et al., Citation1999; Sali & Boffetta, Citation2000). In fact, if the observed and expected renal cancers in the Lauriola et al., study are pooled with those from the Sali and Boffetta paper, then the resulting SMR is not statistically significant.
Additionally, Kannio et al. (Citation1996) reported the following data regarding bladder cancer and asbestos exposure among patients with bladder cancer admitted for diagnostic or surveillance purposes to the Surgery Clinic, Turku University Central Hospital (Turko, Finland) between October and December 1988:
Of the 28 primary bladder cancer cases in group 1, 17 (61%) had been exposed to asbestos at work. Of these, 16 (94%) were considered to have been definitely exposed. A crude OR of 2.8 (95% Cl = 0.9–8.4) was calculated for definite exposure to asbestos against no asbestos exposure and 2.4 (95% CI = 0.8–7.0) for the pooled category of definite to possible exposure against no asbestos exposure.
Although the odds ratios were elevated, the 95% CIs included 1.0 and, therefore, the elevations are not statistically significant.
Karami et al. (Citation2011) investigated whether asbestos, as well as 20 other occupational dust exposures, were associated with renal cell carcinoma (RCC) risk in a large European, multi-center, hospital-based renal case–control study. The study included respondents from four Central and Eastern European countries (Moscow, Russia; Bucharest, Romania; Lodz, Poland; and Prague, Olomouc, Ceske-Budejovice and Brno, Czech Republic) between 1999 and 2003. In total, 1097 histologically confirmed RCC cases and 1476 controls were included in the study. These investigators found that among participants ever exposed to dust, significant associations were observed for glass fibers (OR: 2.1; 95% CI: 1.1–3.9), mineral wool fibers (OR: 2.5; 95% CI: 1.2–5.1) and brick dust (OR: 1.5; 95% CI: 1.0–2.4). Significant trends were also observed with exposure duration and cumulative exposure. No association between RCC risk and asbestos exposure was observed. (RCF was not included because of the small sample size.) The authors were careful to note the limitations of their study, but if the findings are correct, their results would provide a rationale for the contention that exposure to other SVFs, and potentially RCF, might be a risk factor for kidney or bladder cancer as reported in the UC studies. Among the reasons, why Karami et al. (Citation2011) were cautious in claiming that exposure to SVFs might be a risk factor was the fact that their finding was not consistent with results from other epidemiology studies – a fact that prompted the authors to write (at various points in their article) the following cautionary remarks:
The lack of supporting evidence from cohort studies, therefore, reduces the plausibility of an association between RCC risk and exposure to both glass and mineral wool fibers.
Thus, it is unclear whether our findings of an association with glass and mineral wool fibers are real.
Our observed associations also require replication before meaningful inferences can be concluded.
summarizes the reported links between exposure to SVFs and renal or bladder disease from cohort or case–control studies [most of which were not discussed or cited in the Karami et al. (Citation2011) article] that underscore some of the limitations of this study. With very few exceptions, these study results do not support the contention that SVF exposure (at least glass wool or mineral wool exposure) is a risk factor in the malignant or non-malignant bladder or kidney disease. Although some of the studies summarized in involved small sample sizes, there is more compelling evidence against this relationship in the various large well designed and executed cohort studies of Marsh and colleagues (Marsh et al., Citation1990, Citation2001a,Citationb; Stone et al., Citation2004).
Table 2. Studies addressing links between exposure to man-made mineral fibers and either non-malignant or malignant kidney or bladder disease.
Leukemia
The latest UC mortality study results () indicated an excess of leukemia deaths in the full cohort (four acute myeloid, two acute unspecified and two chronic lymphocytic). However, there was no significant excess of leukemia (all types) in the group with the highest exposures. The fact that there is no apparent exposure–response suggests that the observed increase in leukemia deaths in the cohort is related to other risk factors, rather than RCF exposure. (Appendix) summarizes the literature on causes, contributing factors or risk factors for various types of leukemia. (As these cases were all in adults, this table does not address factors for childhood leukemia.) Included in this table are occupational, lifestyle-related, environmental, medical procedures, genetic disorders and family history risk factors for various subtypes of leukemia (most of which were not measured or controlled for in the UC study). Among the more likely explanations for this finding are occupational exposures to certain chemicals and lifestyle-related risk factors, such as smoking and obesity.
Although occupational exposures to certain chemicals have been attributed to leukemia, exposure to fibers has not been shown to be a risk factor for leukemia (see below) and such an effect is arguably implausible, given what is known about fiber toxicology and epidemiology. Occupational exposure to various fibers has been included in several studies of adverse health effects, but leukemia has not been the endpoint of concern because of the assumption that fiber exposures are more likely to be associated with malignant or non-malignant lung-related diseases. Nonetheless, several studies on health effects of exposure to other fibers (PVA, rock/slag wool, glass fibers and asbestos) present data relevant to other malignant diseases, including leukemia. Shown below is a short summary of these data by type of fiber.
Polyvinyl alcohol (PVA): Morinaga et al. (Citation1999) studied a cohort of male workers in a Japanese factory producing PVA. This small study (38 deaths among 454 PVA fiber exposed workers and 210 deaths among 2441 non-exposed workers) calculated SMRs and confidence intervals for several malignant diseases, including leukemia (ICD8 204–209). The calculated SMR and 95% confidence interval for leukemia among exposed workers were SMR = 0 and (95% CI; 0–5.52), respectively, whereas the non-exposed group actually had a higher SMR; SMR = 1.53 (95% CI; 0.49–3.57).
Fiberglass (FG): Marsh et al. (Citation2001a) performed the most comprehensive study on FG workers in the US, including 32,110 persons at risk and 935,581 person-years at risk. As with other fiber studies, the focus of this study was on malignant and non-malignant lung diseases. Nonetheless, the study reported observed fatalities (199) for “all lymphatic and hematopoietic tissue diseases (ICD 200–209)”, including leukemia. The SMRs were not elevated when compared to either US or corresponding local county rates. For example, the SMR and corresponding confidence interval compared to US national rates were SMR = 0.92 and (95% CI; 0.8–1.06), respectively.
Rock-slag wool (RSW): Marsh et al. (Citation1996) published results of a large epidemiological study of workers exposed to rock-slag wool (RSW) in the US. Although the focus of this study was on respiratory cancers, data were available on lymphopoietic cancers (ICD8 200–209), including leukemia. Two cohorts were included and SMRs were not significantly elevated for either cohort.
Man-made mineral fibers (MMMFs) (Countries other than the US): Several authors have reported results for various MMMFs, including fiberglass, rock and slag wool in countries other than the US.
Claude & Frentzel-Beyme (Citation1984) studied a cohort of workers in a German rock-wool factory; the exposed and reference cohorts included male employees, consisting of 2096 males in the former and 1778 males in the latter group. The calculated risk ratio for “leukemia and lymphatic system (200–207)” was 0.57 (95% CI; 0.1 – 3.37).
Simonato et al. (Citation1986) reported results of a study of 24,609 workers in 13 European factories (Denmark, Finland, Germany, Italy, Norway, Sweden and the UK) that produced continuous filament, glass wool, rock wool and slag wool. Based on 17 leukemia deaths (both sexes) the calculated SMR and confidence interval were 0.80 and (95% CI; 0.47–1.28), respectively.
Plato et al. (Citation1995) reported a follow-up study of 3539 workers (74, 043 person-years) in three Swedish plants producing fiberglass or rock wool. The calculated SMR and 95% confidence interval for deaths from leukemia were 0.89 and (95% CI; 0.33 – 1.93), respectively.
Boffetta et al. (Citation1999) published the latest update to the European studies of MMMFs among 3,685 rock-slag wool (RSW) and 2,611 glass wool (GW) production workers employed for 21 years in factories in Denmark, Finland, Norway or Sweden, and the standardized incidence ratios (SIRs) were calculated on the basis of national incidence rates. For the entire cohort (male and female, RSW and GW), the calculated SIR and 95% CI for leukemia were 0.92 and (95% CI: 0.53–1.50), respectively.
Adegoke et al. (Citation2003) reported a population-based case–control study of 486 leukemia subjects and 502 healthy controls residing in Shanghai from 1987 to 1989. Adjusted odds ratios (OR) were calculated for the self-reported association between occupational factors and leukemia risk. Among the various chemicals included in the study was a substance described as “synthetic fiber dust” (otherwise undefined). The calculated odds ratio (OR) and 95% confidence interval for leukemia (all forms) among those exposed to “synthetic fiber dust” were 2.0 and ((95% CI: 1.2–3.5), respectively. The relevance of this study is unclear because no definition or characterization of “synthetic fiber dust” is given.
Asbestos: Asbestos (at least certain forms) has been proven to cause (among others) various malignant and non-malignant lung diseases. A few studies have been published that provide evidence regarding leukemia.
Selikoff (Citation1990) provided data on causes of death among 17,800 asbestos insulation workers in the US and Canada, from 1 January 1967 to 31 December 1986 – observed deaths from all causes totaled 4951 based on best evidence. The SMR and 95% confidence interval based on 33 observed deaths (best evidence) for leukemia were 1.148 and (Fisher exact test 95% CI; 0.79–1.61), respectively, which Selikoff included in a table labeled “cancers not found with increased incidence”.
Kishimoto et al. (Citation1988) & Chinushi et al. (Citation1990) published case reports on a total of three persons admitted to Kure Kyosai Hospital, Kure, Japan with leukemia had evidence of asbestos exposure. In a later publication, Kishimoto (Citation1992) noted that of 10 cases of leukemia, five showed evidence of asbestos exposure.
Goodman et al. (Citation1999) performed a meta-analysis of eight studies covering asbestos exposure and leukemia. The calculated meta-SMR and 95% confidence interval were 0.95 and (0.80, 1.13), respectively.
Seidler et al. (Citation2010) reported results of a large multicenter case–control study in Germany and Italy that examined the relationship between asbestos exposure and various diseases. Among various diseases, there were a total of 149 cases of chronic lymphocytic leukemia (CLL). Adjusted odds ratios (ORs) and confidence intervals were calculated as a function of fiber-years of asbestos exposure. All ORs (in each cumulative exposure category) were close to 1, there was no dose–response, and all confidence intervals included 1.0. The authors concluded: “We observed no statistically significant association between cumulative asbestos exposure and the risk of any lymphoma subtype”.
LeMasters et al. (Citation2017) commented on the leukemia results in the UC study as follows:
The elevated leukemia SMR in the total cohort, but not in the high exposed group, was a new and unexpected finding. These eight cases had job tasks that included machine operator (4), millwright (2), welder (1) and maintenance (1). Exposures associated with leukemia and, in particular, acute myeloid leukemia (50% of cases in the current study) include ionizing radiation and benzene (Polychronakis et al., Citation2013; Tsai et al., Citation2014).
On a weight of evidence basis, we conclude that these studies fail to implicate exposure to any of several types of fibers as a cause or contributing factor for leukemia. Because of this and the absence of a dose–response [one of the Hill (Citation1965) criteria], it is likely that the observed leukemia deaths are caused by some factor other than exposure to RCF or any of these other fibers, leaving occupational exposure to other materials or lifestyle factors as the likely explanation for the findings. Nonetheless, leukemia will continue to be included in the ongoing mortality study.
Mesothelioma
As noted above there was one reported, but unconfirmed, death from mesothelioma in the RCF-exposed cohort.
Although the calculated SMR was not significant, it is appropriate to examine this case further. The worker in question had self-reported occupational asbestos exposure working as a vehicle mechanic for 6.5 years. Numerous studies have shown that vehicle mechanics are exposed to asbestos-containing materials, which can result in adverse health effects, including lung cancer and mesothelioma (Castleman et al., Citation1975; Egilman & Billings, Citation2005; Egilman & Longo, Citation2012; Finkelstein, Citation2008; Freeman & Kohles, Citation2012; Hansen, Citation1989; Huncharek, Citation1990, Citation1992; Imbernon et al., Citation2005; Kakooei et al., Citation2011; Lemen, Citation2004; Michaels & Monforton, Citation2007; Roggli et al., Citation2002; Welch, Citation2007). For example, in the analysis of 1445 cases of mesothelioma studied by Roggli et al. (Citation2002), the automotive sector ranked eighth in terms of the number of mesothelioma cases among 12 industrial sectors evaluated. Epidemiology studies of occupationally exposed cohorts are less easy to interpret, with some studies or analyzes concluding that automobile mechanics have an elevated risk. Nonetheless, other studies and meta-analyzes indicate that mesothelioma risks are not significantly elevated (Aguilar-Madrid et al., Citation2010; Butnor et al., Citation2003; Dotson, Citation2006; Finley et al., Citation2012; Garabrant et al., Citation2016; Goodman et al., Citation2004; Hessel et al., Citation2004; Laden et al., Citation2004; Paustenbach & White, Citation2012; Peto et al., Citation2009; Rake et al., Citation2009; Richter et al., Citation2009; Teschke et al., Citation1997; Wong Citation2001, Citation2006). This certainly does not mean that the incremental risks are zero. As noted by Lemen (Citation2004):
The results of the exposure studies, experimental studies, case reports, and findings from the equivocal epidemiological studies by no means exonerate the brake mechanic from being susceptible to a causal relationship between asbestos exposure and mesothelioma.
Several articles have been published that address the relationship between mesothelioma and low levels of asbestos exposure (Iwatsubo et al., Citation1998; Rödelsperger et al., Citation2001). Moreover, the results of Roggli et al. (Citation2002) clearly show that vehicle mechanics have developed mesothelioma.
Of greater probable relevance, in our judgment, is the history of other jobs held by this worker. LeMasters et al. (Citation2017) report:
Other prior jobs this individual reported that have historically been associated with potential asbestos exposure included machinist apprentice overhauling railroad steam engines (Schenker et al., Citation1986; Roggli et al., Citation2002) in the early 1950s (only exposure he reported was cutting oil), aviation electrician from 1952 to 1956 in the US Navy (reported exposure was to aviation fuel) and working as a technician in fuel systems (reported exposures were fuel and cleaning solvents) for South Bend Bendix Aviation from the mid-1950s to early 1970s. Bendix Corporation manufactured asbestos containing friction brake products.
Shown below are additional materials that address possible asbestos exposure in these jobs.
Asbestos exposure and railroads: Numerous epidemiological studies have been published of workers in the US and various countries in Europe and Asia showing that those workers employed performing various jobs in the railroad industry were exposed to asbestos and experienced a statistically significant excess of various asbestos-related diseases, including mesothelioma (Battista et al., Citation1999; Garshick et al., Citation1987; Gasparrini et al., Citation2008; Gerosa et al., Citation2000; Hosoda et al., Citation2008; Hjortsberg et al., Citation1988; Huncharek, Citation1987; Malker et al., Citation1985; Maltoni et al., Citation1995; Mancuso, Citation1983, Citation1988, Citation1991; McDonald & McDonald, Citation1989; Ohlson et al., Citation1984; Oliver et al., Citation1985; Plato et al., Citation2016; Roelofs et al., Citation2013; Roggli et al., Citation2002; Rolland et al., Citation2010; Schenker et al., Citation1986; Tessari et al., Citation2004). According to Roggli et al. (Citation2002) of this paper, railroad workers ranked seventh in the top 12 industry types based on their large (1445 cases) sample of mesothelioma deaths.
Asbestos exposure and US Navy: Until efforts to use substitute materials for asbestos on US Navy ships (and ships from other nations) and enhanced asbestos control measures accelerated in the 1970s (Anonymous, Citation1979; Franke & Paustenbach, Citation2011; Harries, Citation1968; Mangold et al., Citation1970; Rushworth, Citation2005; Strand et al., Citation2010; Twight Citation1991; Winer & Holtgren, Citation1976), both amosite and chrysotile asbestos was used extensively on US Navy ships. Nearly all Navy personnel aboard ships had some asbestos exposure, even in berthing quarters and mess halls, particularly prior to 1980. Numerous studies have evaluated the health risks to seamen and those employed in shipyards (Bianchi et al., Citation2001; Greenberg, Citation1991; Kurumatani et al., Citation1999; Roggli et al., Citation2002; Selikoff & Hammond, Citation1978; Selikoff et al., Citation1990). With respect to mesothelioma specifically Roggli et al. (Citation2002) noted that the numbers of mesothelioma cases were greatest in the shipbuilding and US. Navy sectors among his sample of 1445 mesothelioma cases [see illustration from of Roggli et al. (Citation2002)]. Similar results were reported in the Trieste-Monfalcone area, Italy (Bianchi & Bianchi, Citation2009; Bianchi et al., Citation2001) and the United Kingdom (Peto et al., Citation2009).
Bendix Aviation: This plant made both automobile and aircraft brake shoes and is listed on several websites as a location where asbestos exposure occurred.
One of the difficulties of epidemiological studies of fibers is that some members of the cohort may have also had prior asbestos exposure, as was the case with studies of other SVFs including RSW (Marsh et al., Citation2001b; Boffetta et al., Citation1997, Citation2014) and glass wool.
Pulmonary function studies
LeMasters et al. (Citation2017) also conducted pulmonary function studies on RCF-exposed workers. Among those with localized pleural thickening (LPT), percent predicted FVC and FEV1 differed from those without LPT by <3%, a value judged clinically insignificant.
Zhu et al. (Citation2015b) compared various measures of pulmonary function (FVC, FEV1 and FEV1/FVC) for 265 manufacturing and processing workers exposed to RCF with a group of 273 workers in a Chinese facility exposed to noise only. The exposed group was subdivided into subgroups based on gravimetric measurements of total dust and fibers. This study reported, inter alia, that the exposed group had statistically significantly lower mean values of FVC, FEV1 and FEV1/FVC (clinical significance unstated). The study reported that pulmonary effects were positively correlated with the fiber concentration (to a degree greater than total dust). This study did not consider the effects of initial weight or weight gain among the subjects. Unlike McKay et al. (Citation2011), these investigators did not report any longitudinal analysis. McKay et al. (Citation2011) offered the following comments on their longitudinal analysis of the US cohort:
In conclusion, no consistent longitudinal decline in FVC or FEV1 with increasing RCF exposure category was observed, although cross-sectional changes were observed for subjects in the highest exposure category. Critical to this analysis was the recognition that lung function declines with age are non-linear and accelerate in older age groups who also have the longest duration of exposure. To our knowledge, this specific analysis strategy has not been published for evaluating longitudinal change in lung function among adults, although a similar methodology has been used in children.
It is possible that Zhu and colleagues would have reached similar conclusions had they conducted a longitudinal analysis.
Pleural plaques
As noted above, the X-ray studies conducted by the UC researchers have shown a significant relationship between RCF exposure and the development of pleural plaques. The overall rate of pleural changes reported by LeMasters et al. (Citation2017) in their most recent publication was 6.1%, which increased across exposure categories reaching, in the highest CFE category, 21.4% (adjusted odds ratio (aOR) = 6.9, 95% CI 3.6–13.4) and 13.0% (OR= 9.1, 95% CI 2.5–33.6) for all subjects and for those with no potential asbestos exposure, respectively. The small increase in pleural plaques is not surprising as the presence of plaques is likely to be related to increasing latency. Prevalence among recent hires (>1985) was similar to the background. Interstitial changes were not elevated. And, as noted above, localized pleural thickening was found to be associated with small decreases in spirometry.
The finding that pleural plaques are related to RCF exposure is of potential concern because pleural plaques are widely considered to be a marker of exposure to fibers – particularly, but not exclusively, asbestos [see Clarke et al. [Citation2006] for a list of other reported cases of pleural plaques]. Because occupational asbestos exposure has been shown to lead to a variety of adverse health outcomes, including asbestosis, lung cancer and mesothelioma in exposed cohorts, it is plausible to believe that there would be a correlation between the prevalence of pleural plaques and these asbestos-related diseases. Useful perspectives on this possibility are provided by Utell & Maxim (Citation2010).
Three more recent papers have been published on the relevance of pleural plaques:
Moolgavkar et al. (Citation2014) published a detailed review and critique of U.S. EPA’s risk assessments for asbestos. EPA regarded pleural plaques an “adverse condition” and developed a reference concentration (RfC) based on this endpoint. They concluded:
Taken together, the totality of evidence suggests strongly that pleural plaques are markers of asbestos exposure with little or no clinical significance. Thus, pleural plaques do not appear to satisfy EPA’s own definition of an adverse condition.
Kerper et al. (Citation2015) published a systematic review of the relation between pleural plaques and lung function and concluded that the weight of evidence indicates that pleural plaques do not impact lung function and that the observed associations are most likely due to unidentified abnormalities or other factors. This result is also consistent with UC findings on the RCF-exposed cohort.
Maxim et al. (Citation2015) reviewed the extensive literature relating the linkage between pleural plaques and lung impairments/diseases and concluded that the available evidence supports the contention that, absent any other pleural disease, the presence of pleural plaques does not result in respiratory symptoms or clinically significant impacts on lung function. Pleural plaques are not premalignant, that is, they do not progress to malignant tumors (lung cancer or mesothelioma). For certain types of asbestos, the development of pleural plaques is statistically correlated with malignant disease, but the evidence is consistent with the hypothesis that pleural plaques (without other pleural diseases) are a marker of exposure, rather than an independent risk factor (see also IIAC, Citation2008).
Dermal studies
Zhu et al. (Citation2015c) also examined the effects of exposure to RCF (fibers) and dust on workers’ skin by comparing effects on 281 RCF-exposed manufacturing and processing workers with a control group of 274 workers in a Chinese facility. The study found that skin itching symptoms and contact dermatitis were significantly correlated with total gravimetric dust measurements, but not with fiber concentrations. This finding is consistent with other studies of skin irritation related to exposure to SVFs. Some (but not all) persons exposed to various SVFs, including glass fibers (ATSDR, Citation2004; Jolanki et al., Citation2002; Possick et al., Citation1970; Sertoli et al., Citation1992; Tarvainen et al., Citation1994), mineral wool (Petersen & Sabroe, Citation1991), rockwool (Kieć-Świerczyńska & Szymczk, Citation1995) and RCF (Kieć-Świerczyńska & Wojtczak, Citation2000) as well as PCWs develop adverse skin reactions (sometimes termed “fiberglass itch” or more properly irritant contact dermatitis [ICD]) including folliculitis (a common skin condition in which hair follicles become inflamed), irritant symptoms including itching without rash on arms, face or neck, and burning of eyes. With most SVFs and PCWs, this is believed to be a physical (mechanical rather than chemical) phenomenon, although some binders or coatings are believed to be sensitizing agents that also cause dermatitis. Though perhaps uncomfortable, this disease is not life-threatening and most irritation is described as “mild”. Unlike most respiratory illnesses resulting from exposure to respirable fibers (which may have a latency – the time from first exposure until the disease manifests itself – which can be 15–50 years), dermatitis arises in some subjects exposed for a short time (Sertoli et al., Citation1992). Thus, although dermatitis is typically a less severe illness than many respiratory illnesses, it is “obvious” and appears promptly, so it is likely to be recognized.
Numerous authors have studied the effects of dermal exposure to various SVFs and dermatitis. provides a summary of some of the relevant literature including review articles, focus groups, patch or rubbing tests, case reports and epidemiological studies.
Overall, these and other studies conclude that irritant contact dermatitis is associated with exposure to various SVFs/PCWs with diameters that are larger (∼5 μm or larger) than those of respirable fibers and typically becomes less pronounced with continued exposure (ATSDR, Citation2004; Possick et al., Citation1970; Sertoli et al., Citation1992). As noted by Sertoli et al. (Citation1992):
The pathogenic effect of fiberglass on the skin is directly proportional to the diameter and inversely proportional to the length. Fiberglass is harmful only if its diameter is greater than 4.5 µm or, according to other authors, 5 µm.
There is very little evidence of irritant contact dermatitis being caused by or associated with exposure to SVFs with diameters in the respirable range. But, because fibers with larger diameters weigh proportionally more than thinner fibers, it is reasonable that a gravimetric measure would be superior to fiber number concentrations as an indicator of the potential for ICD as reported by Zhu et al. (Citation2015c).
Other analyses
Several other potentially relevant works (shown below in chronological order) have been published since 2010.
The Scientific Committee on Occupational Exposure Limits (SCOEL, Citation2011) published a review of RCF toxicology and epidemiology and developed a no observed adverse effect level (for possible impacts on pulmonary function) of 0.3 f/ml. The SCOEL report acknowledged the lack of evidence of carcinogenicity from epidemiology studies, but based on the results of the animal studies concluded:
From the available information it is concluded that the genotoxic effects observed in the different studies are secondary so that RCF are classified as SCOEL Carcinogen group C carcinogens: Genotoxic carcinogens for which a practical threshold is supported
Some German scientists concluded based on IP injection study results that RCF was as potent as amphibole asbestos in causing lung diseases. Walker et al. (Citation2012a,Citationb) tested the hypothesis that RCF was as potent as amphibole asbestos in causing lung cancer or mesothelioma. These investigators found that a cohort occupationally exposed to asbestos at identical levels to the RCF exposed cohort would have experienced significantly greater mortality than actually observed. Otherwise, there is no new risk analyses published since the Utell & Maxim (Citation2010) review article. Earlier risk estimates based on extrapolation from animal studies span a very wide range (NIOSH, Citation2006; Maxim et al., Citation2003).
Costa & Orriols (Citation2012) published a review article dealing with MMVF (including RCF) and the respiratory tract. Among other topics, this review commented on the finding that there was no parenchymal lung disease reported in the UC and Cowie et al., (Citation2001) RCF studies as follows:
In spite of this, in most of the cohort studies in MMMF factory workers, exposure levels were estimated to be low; therefore, it is considered that the epidemiological studies may not have detected cases of pulmonary fibrosis for this reason.
Whether or not this conjecture is correct, it underscores the prudence of continuing efforts to maintain or reduce occupational exposures, a major objective of the industry PSP.
As noted above, the industry PSP also includes a product research component, focused on the development of possible substitutes for present high-temperature insulation materials with less potential for toxic effects. This effort led to the development of a new class of materials, termed alkaline earth silicate (AES) wools. AES wools are less biopersistent fibers capable of substituting for RCF in some (but not all) applications. Brown & Harrison (Citation2012) published an article describing these materials and the history of their development.
Greim et al. (Citation2014) explored analogies (e.g. fiber dimensions, breakage mechanism and measured biopersistence in animal inhalation studies) between RCF and conventional mineral wool and concluded that the risks associated with occupational exposure are likely to be comparable for these two fibers. This is of interest because large and robust epidemiological studies of occupational exposure to mineral wool found that risks of lung cancer and mesothelioma are not elevated. The evidence was sufficiently compelling that IARC downgraded the carcinogen classification for glass and mineral wool from 2B to 3.
Boffetta et al. (Citation2014) (see above) completed a systematic review of occupational exposure to SVFs and mesothelioma. This comprehensive review paper examined the epidemiological (case–control and cohort) and toxicological evidence for a linkage between occupational exposure to SVFs (including RCF) and mesothelioma. These investigators concluded that the combined evidence from epidemiology and toxicology “provide little evidence that exposure to SVF increases the risk of mesothelioma”.
The HTIW Coalition and ECFIA continue to publish outreach materials in English and several other languages (as part of the product stewardship program) designed to inform workers about good working practices for handling RCF and other high temperature insulation wools (see e.g. http://www.ecfia.eu/files/ecfia-cartoon-redesign-english-v1_0-2014-04.pdf and http://guidance.ecfia.eu/index.htm) as do other agencies (HSE, Citation2008; NIOSH, Citation2006).
Matsuzaki et al. (Citation2015) published a review article on biological effects of RCF exposure. Among their concluding remarks they wrote:
Thus, given the biological effects of RCF previously reported in the literature, it is necessary to consider further monitoring in addition to the development of measures to prevent adverse health effects caused by exposure to RFCs.
Harrison et al. (Citation2015) wrote a recent review of regulatory risk assessment approaches for synthetic mineral fibers (including RCF). They pointed out that occupational exposure limits (OELs) for RCF developed by regulatory and advisory bodies in various countries varied by more than an order of magnitude and argued that:
The resulting differences in established OELs prevent consistent and appropriate risk management of SMF worldwide, and that development of a transparent and harmonised approach to fibre risk assessment and limit-setting is required.
Andujar et al. (Citation2016) published a 5-year update on the relationships between malignant pleural mesotheliomas and exposure to asbestos and other elongated mineral particles. The article reviewed earlier studies published by some of the same authors, which (as noted above) reported an apparently synergistic relation between exposure to asbestos and certain mineral fibers, including mineral wool and RCF. This article recapitulated the findings and some of the limitations of these earlier studies and (in a more cautious vein) noted:
While the issue of the simultaneous effect of asbestos and other mineral particles needs to be further explored in reality, the hypothesis of a synergistic joint effect needs to be considered.
Drummond et al. (Citation2016) published a review article that examined the relationship between the results of intra-pleural and intraperitoneal studies with those from inhalation and intratracheal tests for the assessment of pulmonary responses to inhalable dust and fibers. They concluded:
For some of the fibrous material reviewed, there is conformity between the results of intraperitoneal and inhalation tests such that they are either consistently positive or consistently negative. For the remaining fibrous materials reviewed, intraperitoneal and inhalation tests give different results, with positive results in the intraperitoneal test not being reflected by positive inhalation results. It is suggested that the intraperitoneal test can be used to exonerate a dust or fiber (because if negative in the intraperitoneal test it is extremely unlikely to be positive in either inhalation or intratracheal tests) but should not be used to positively determine that a dust or fiber is carcinogenic by inhalation. We would argue against the use of intraperitoneal tests for human health risk assessment except perhaps for the purpose of exoneration of a material from classification as a carcinogen.
This article considered the relationship between IP and inhalation results for several fibers, including RCF. For RCF, the authors noted that the RCC animal inhalation studies that resulted in fibrosis and tumors might have been compromised by the presence of non-representative amounts of particulate material, a point made in several earlier articles (Brown et al., Citation2005).
Concluding comments
Several useful and informative publications have appeared since the Utell & Maxim (Citation2010) review. Based on these and the earlier studies, we conclude:
Correctly interpreting the animal bioassay data is difficult because of uncertainties in correcting for or otherwise accounting for the possible effects of lung overload. Published risk estimates based on extrapolation of animal data span a wide range of unknown accuracy.
The results of epidemiology studies (particularly the UC study) continue to support the conclusion that present RCF occupational exposures are unlikely to result in material decrements in lung function, interstitial fibrosis, incremental lung cancer or mesothelioma in the exposed population. The latest UC results confirm an elevated urinary cancer SMR in the occupationally exposed cohort, which UC investigators thought might be accounted for by smoking or other occupational exposures. The finding of an elevated leukemia SMR (without evidence of an exposure–response relation) is probably unrelated to RCF exposure and more likely related to other occupational exposures or lifestyle factors. The finding of an unconfirmed death from mesothelioma is probably the result of prior self-reported asbestos exposure and a work history of jobs for which occupational asbestos exposure is likely. Nonetheless, continued follow-up of the RCF-exposed cohort is important to learn more about possible linkages between RCF exposure and kidney and/or bladder disease, leukemia, and maintaining vigilance for mesothelioma given its long latency and relative infrequency in the population.
Product stewardship efforts have been successful in substantially reducing occupational exposures to RCF (and other fibers) and will be continued at both production and end-user facilities. Workers who joined the cohort in recent years have substantially lower exposures compared to those who were initially exposed during the 1980s or earlier.
The medical surveillance component of the stewardship program may provide indications of morbidity in the cohort not captured in the earlier UC morbidity studies or the ongoing UC mortality study.
The ongoing mortality study provides useful data and, as the cohort ages and the time since first exposure of the worker’s increases should yield yet more powerful data on long-latency outcomes.
Finally, even though epidemiological findings indicate that present RCF occupational exposures are unlikely to result in adverse health outcomes, it is prudent to continue efforts to reduce RCF exposures and to develop efficient high temperature insulating wools with yet lower biopersistence and greater diameters to reduce the respirable fraction.
Acknowledgements
We would like to acknowledge the helpful contributions of Mr Ronald Niebo, Everest Consulting Associates and useful comments by referees and Prof. Dr Med. Helmut Greim, Director of the Institute of Toxicology and Environmental Hygiene, Technische Universität München.
Disclosure statement
The authors performed this work as independent consultants. Both authors serve as consultants on a scientific review board for Unifrax, a major producer of HTIWs and have performed studies for the HTIW Coalition. Neither author has appeared in any legal or regulatory proceeding relative to RCF. The findings and conclusions are those of the authors alone and do not necessarily represent the views of the sponsor.
This research was sponsored by the HTIW Coalition (see http://www.htiwcoalition.org/for a list of members and associate members), an organization that focuses on health and safety matters for producers of RCF and other high-temperature insulating wools (HTIWs) in the US.
References
- Adams RM. (1990). Dermatitis due to fibrous glass. In: Adams RM (ed.) Occupational skin disease. 2nd ed. Philadelphia: WB Saunders Company, 16–7.
- Adegoke OJ, Blair A, Shu XO, et al. (2003). Occupational history and exposure and the risk of adult leukemia in Shanghai. Ann Epidemiol 13:485–94.
- Agence Française de Sécurité Sanitaire de L’environment et du Travail (AFSSET). (2007). Les fibres minérales artificielles, évaluation de l’exposition de la population générale et des travailleurs. Saisine 2004/012 Rapport Final Relatif aux Fibres. April 2007. Paris: AFFSET, 290 p. Available from: https://www.anses.fr/fr/system/files/AIR2004et0012Ra.pdf [last accessed 19 Apr 2017].
- Agency for Toxic Substances and Disease Registry (ATSDR). 2004. Toxicological Profile for Synthetic Vitreous Fibers. September 2004. Atlanta, GA: ATSDR. 332 p. Available from: https://www.atsdr.cdc.gov/ToxProfiles/tp161.pdf [last accessed 19 Apr 2017].
- Aguilar-Madrid G, Robles-Pérez E, Juárez-Pérez CA, et al. (2010). Case-control study of pleural mesothelioma in workers with social security in Mexico. Am J Ind Med 53:241–51.
- Aidan JC, Priddee NR, McAleer JJ. (2013). Chemotherapy causes cancer! A case report of therapy related acute myeloid leukaemia in early stage breast cancer. Ulster Med J 82:97–9.
- Anderson AS, Key TJ, Norat T, et al. (2015). European code against cancer 4th edition: obesity, body fatness and cancer. Cancer Epidemiol 39(Suppl 1):S34–S45.
- Andujar P, Lacourt A, Brochard P, et al. (2016). Five years update on relationships between malignant pleural mesothelioma and exposure to asbestos and other elongated mineral particles. J Toxicol Environ Health Part B 19:151–72.
- Anonymous. (1979). Asbestos, the insulation that lingers. All Hands, Magazine of the U.S. Navy, No. 755:6–11. Available from: http://www.navy.mil/ah_online/archpdf/ah197912.pdf [last accessed 10 July 2017].
- Band PR, Le ND, Fang R, et al. (1996). Cohort study of Air Canada pilots: mortality, cancer incidence, and leukemia risk. Am J Epidemiol 143:137–43.
- Baris D, Karagas MR, Verrill C, et al. (2009). A case-control study of smoking and bladder cancer risk: emergent patterns over time. J Natl Cancer Inst 101:1553–61.
- Battista G, Belli S, Combat P, et al. (1999). Mortality due to asbestos-related causes among railway carriage construction and repair workers. Occup Med 49:536–9.
- Behrman AJ. (2003). Welders and Joiners. In: Greenberg MI, editor. Occupational, industrial, and environmental toxicology. 2nd ed. Philadelphia, PA: Mosby; p. 398–405.
- Bellmann B, Muhle H, Creutzenberg O, et al. (2001). Effects of nonfibrous particles on ceramic fiber (RCF1) toxicity in rats. Inhal Toxicol 13:101–25.
- Bernstein DM. (2007). Synthetic vitreous fibers: a review toxicology, epidemiology and regulations. Crit Rev Toxicol 37:839–86.
- Bianchi C, Bianchi T. (2009). Malignant pleural mesothelioma in Italy. Indian J Occup Environ Med 13:80–3.
- Bianchi C, Brollo A, Ramani L, et al. (2001). Asbestos exposure in malignant mesothelioma of the pleura: a survey of 557 cases. Ind Health 39:161–7.
- Björnberg A. (1985). Glass fiber dermatitis. Am J Ind Med 8:395–400.
- Boffetta P, Saracci R, Andersen A, et al. (1997). Cancer mortality among man-made vitreous fiber production workers. Epidemiology 8:259–68.
- Boffetta P, Andersen A, Hansen J, et al. (1999). Cancer incidence among European man-made vitreous fiber production workers. Scand J Work Environ Health 25:222–6.
- Boffetta P, Donaldson K, Moolgavkar S, Mandel JS. (2014). A systematic review of occupational exposure to synthetic vitreous fibers and mesothelioma. Crit Rev Toxicol 44:436–49.
- Brown LM, Blair A, Gibson R, et al. (1990). Pesticide exposures and other agricultural risk factors for leukemia among men in Iowa and Minnesota. Cancer Res 50:6585–91.
- Brown RC, Sébastien P, Bellmann B, Muhle H. (2000). Particle contamination in experimental fiber preparations. Inhal Toxicol 12:99–107.
- Brown RC, Bellmann B, Muhle H, et al. (2005). Survey of the biological effects of refractory ceramic fibres: overload and its possible consequences. Ann Occup Hyg 49:295–307.
- Brown RC, Harrison PTC. (2012). Alkaline earth silicate wools – a new generation of high temperature insulation. Regul Toxicol Pharm 64:296–304.
- Brown T, Slack R, Rushton L, British Occupational Cancer Burden Study Group. (2012). Occupational cancer in Britain. Urinary tract cancers: bladder and kidney. Br J Cancer 107(S1):S76–S84.
- Brownson RC, Chang JC, Davis JR. (1987). Occupation, smoking, and alcohol in the epidemiology of bladder cancer. Am J Public Health 77:1298–300.
- Brownson RC, Novotny TE, Perry MC. (1993). Cigarette smoking and adult leukemia. A meta-analysis. Arch Intern Med 153:469–75.
- Burge PS, Calvert IA, Trethowan WN, Harrington JM. (1995). Are the respiratory health effects found in manufacturers of ceramic fibers due to the dust rather than the exposure to fibers?. Occup Environ Med 52:105–9.
- Burger M, Catto JWF, Dalbagni G, et al. (2013). Epidemiology and risk factors of urothelial bladder cancer. Eur Urol 63:234–41.
- Burley CG, Brown RC, Maxim LD. (1997). Refractory ceramic fibres: the measurement and control of exposure. Ann Occup Hyg 41, Supplement 1:267–72.
- Butnor KJ, Sporn TA, Roggli VL. (2003). Exposure to brake dust and malignant mesothelioma: a study of 10 cases with mineral fiber analyses. Ann Occup Hyg 47:325–30.
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. (2003). Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med 348:1625–38.
- Carel R, Olsson AC, Zaridze D, et al. (2007). Occupational exposure to asbestos and man‐made vitreous fibres and risk of lung cancer: a multicentre case‐control study in Europe. Occup Environ Med 64:502–8.
- Carlsten C, Georas SN. (2014). Update in Environmental and Occupational Lung Diseases 2013. Am J Respir Crit Care Med 89:1037–43.
- Castleman B, Camarota LA, Fritsch AJ, et al. (1975). The hazards of asbestos for brake mechanics. Public Health Rep 90:254–6.
- Cassidy A, Wang W, Wu X, Lin J. (2009). Risk of urinary bladder cancer: a case-control analysis of industry and occupation. BMC Cancer 9:443–51.
- Castillo JJ, Reagan JL, Ingham RR, et al. (2012). Obesity but not overweight increases the incidence and mortality of leukemia in adults: a meta-analysis of prospective cohort studies. Leuk Res 36:868–75.
- Chang C-H, Wang C-M, Ho C-K, et al. (1996). Fiberglass dermatitis: a case report. Kaohsiung J Med Sci 12:491–4.
- Chiazze L, Watkins DK, Fryar C. (1997). Historical cohort mortality study of a continuous filament fiberglass manufacturing plant. I. White Men. J Occup Environ Med 39:432–41.
- Chiazze L, Watkins DK, Fryar C, et al. (1999). Mortality from nephritis and nephrosis in the fiberglass manufacturing industry. Occup Environ Med 56:164–6.
- Chinushi M, Koyama S, Takahashi M, et al. (1990). The occurrence of leukemia in a patient with pulmonary asbestosis. Jpn J Med 29:607–10.
- Chiu WA, Jinot J, Scott CS, et al. (2013). Human health effects of trichloroethylene: key findings and scientific issues. Environ Health Perspect 121:303–11.
- Chow W-H, Devesa SS. (2008). Contemporary epidemiology of renal cell cancer. Cancer J 14:288–301.
- Chow W-H, Dong LM, Devesa SS. (2010). Epidemiology and risk factors for kidney cancer. Nat Rev. Urology 7:245–57.
- Chow W-H, Gridley G, Fraumeni JF, Järvholm B. (2000). Obesity, hypertension, and the risk of kidney cancer in men. N Engl J Med 343:1305–11.
- Clapp RW, Jacobs MM, Loechler EL. (2008). Environmental and occupational causes of cancer: new evidence 2005-2007. Rev Environ Health 23:1–37.
- Clarke CC, Mowat FS, Kelsh MA, Roberts MA. (2006). Pleural plaques: a review of diagnostic issues and possible nonasbestos factors. Arch Environ Occup Health 61:183–92.
- Claude J, Frentzel-Beyme R. (1984). A mortality study of workers employed in a German rock wool factory. Scand J Work, Environ Health 10:151–7.
- Collins JJ, Lineker GA. (2004). A review and meta-analysis of formaldehyde exposure and leukemia. Regul Toxicol Pharmacol 40:81–91.
- Costantini AS, Benvenuti A, Vineis P, et al. (2008). Risk of leukemia and multiple myeloma associated with exposure to benzene and other organic solvents: evidence from the Italian Multicenter Case-control study. Am J Ind Med 51:803–11.
- Costa R, Orriols R. (2012). Man-made mineral fibers and the respiratory tract. Arch Bronconeumol 48:460–8.
- Cowie HA, Wild P, Beck J, et al. (2001). An epidemiological study of the respiratory health of workers in the European refractory ceramic fiber (RCF) industry. Occup Environ Med 58:800–10.
- Curtis RE, Boice JD, Jr, Stovall M, et al. (1992). Risk of leukemia after chemotherapy and radiation treatment for breast cancer. New Engl J Med 326:1745–51.
- Danaei G, Vander Hoorn S, Lopez AD, et al. (2005). Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet 366:1784–93.
- Davis JMG, Addison J, Bolton RE, et al. (1984). The pathogenic effects of fibrous ceramic aluminium silicate glass administered to rats by inhalation or peritoneal injection. In Biological Effects of Man-made Mineral Fibres. Proceedings of a Symposium 1982. Copenhagen: World Health Organisation, 303–22.
- DECOS. (2011). Refractory Ceramic Fibres; Evaluation of the Carcinogenicity and Genotoxicity. Dutch Expert Committee on Occupational Safety. The Hague: Health Council of the Netherlands. Publication no. 2011/29.
- De Roos AJ, Martínez-Maza O, Jerome KR, et al. (2013). Investigation of Epstein-Barr virus as a potential cause of B-cell non-Hodgkin lymphoma in a prospective cohort. Cancer Epidemiol Biomarkers Prev 22:1747–55.
- Deschler B, Lübbert M. (2006). Acute myeloid leukemia: epidemiology and etiology. Cancer 107:2099–107.
- Dotson GS. (2006). Characterization of asbestos exposure among automotive mechanics servicing and handling asbestos-containing materials. PhD Thesis, Department of Environmental and Occupational Health College of Public Health University of South Florida.
- Drummond G, Bevan R, Harrison P. (2016). A comparison of the results from intra-pleural and intra-peritoneal studies with those from inhalation and intratracheal tests for the assessment of pulmonary responses to inhalable dusts and fibres. Regul Toxicol Pharm 81:89–105.
- Egilman D, Billings MA. (2005). Abuse of epidemiology: automobile manufacturers manufacture a defense to asbestos liability. Int J Occup Environ Health 11:360–71.
- Egilman D, Longo WE. (2012). Egilman's assessment rewarding exposures of auto mechanics to amphiboles is correct. Inhal Toxicol 24:614–8.
- Environmental Resources Management (ERM). 1995. Description and characterization of the ceramic fibres industry of the European Union (Reference 3059). ERM is a trading name of ERL, Environmental Resources Limited. Brussels, Belgium: Environmental Resources Management (ERM).
- Eun HC, Lee HG, Paik NW. (1991). Patch test responses to rockwool of different diameters evaluated by cutaneous blood flow measurement. Contact Dermatitis 24:270–3.
- Everest Consulting Associates, Cranbury, NJ. (1996). Refractory ceramic fibers: a substitute study. Washington, DC: Refractory Ceramic Fibers Coalition (RCFC).
- Everest Consulting Associates, Princeton Junction, NJ. (2017). PSP 2012: a voluntary product stewardship program (PSP), 2016 Annual Report by HTIW Coalition to the Occupational Safety and Health Administration (OSHA) Complete Edition. Washington, DC: HTIW Coalition.
- Fernberg P, Odenbro Å, Bellocco R, et al. (2007). Tobacco use, body mass index, and the risk of leukemia and multiple myeloma: a nationwide cohort study in Sweden. Cancer Res 67:5983–6.
- Finkelstein MM. (2008). Asbestos fibre concentrations in the lungs of brake workers: another look. Ann Occup Hyg 52:455–61.
- Finley BL, Pierce JS, Paustenbach DJ, et al. (2012). Malignant pleural mesothelioma in US automotive mechanics: reported vs expected number of cases from 1975 to 2007. Regul Toxicol Pharmacol 64:104–16.
- Fircanis S, Merriam P, Khan N, Castillo JJ. (2014). The relation between cigarette smoking and risk of acute myeloid leukemia: an updated meta‐analysis of epidemiological studies. Am J Hematology 89:E125–32.
- Floderus B, Persson T, Stenlund C, et al. (1993). Occupational exposure to electromagnetic fields in relation to leukemia and brain tumors: a case-control study in Sweden. Cancer Causes Control 4:465–76.
- Franke K, Paustenbach D. (2011). Government and Navy knowledge regarding health hazards of asbestos: a state of the science evaluation (1900 to 1970). Inhal Toxicol 23 Suppl 3:1–20.
- Freeman MD, Kohles SS. (2012). Assessing specific causation of mesothelioma following exposure to chrysotile asbestos-containing brake dust. Int J Occup Environ Health 18:329–36.
- Freedman ND, Silverman DT, Hollenbeck AR, et al. (2011). Association between smoking and risk of bladder cancer among men and women. J Am Med Assoc 306:737–45.
- Garabrant DH, Alexander DD, Miller PE, et al. (2016). Mesothelioma among motor vehicle mechanics: an updated review and meta-analysis. Ann Occup Hyg 60: 8–26.
- Garshick E, Schenker MB, Woskie SR, Speizer FE. (1987). Past exposure to asbestos among active railroad workers. Am J Ind Med 12:399–406.
- Gasparrini A, Pizzo AM, Gorini G, et al. (2008). Prediction of mesothelioma and lung cancer in a cohort of asbestos exposed workers. Eur J Epidemiol 23:541–6.
- Gérazime A, Stücker I, Luce D. (2015). Exposition professionnelle aux fibres céramiques réfractaires et cancers respiratoires: résultats de l’étude Icare. Arch Maladies Profess L’Environ 76:399.
- Gerosa A, Ietri E, Belli S, et al. (2000). High risk of pleural mesothelioma among the state railroad carriage repair workers. Epidemiol Prev 24:117–9.
- Ghadimi T, Gheitasi B, Nili S, et al. (2015). Occupation, smoking, opium, and bladder cancer: a case-control study. South Asian J Cancer 4:111–4.
- Glass DC, Gray CN, Jolley DJ, et al. (2003). Leukemia risk associated with low-level benzene exposure. Epidemiology 14:569–77.
- Goldsmith JR, Goldsmith DF. (1993). Fiberglass or silica exposure and increased nephritis or ESRD (end-stage renal disease). Am J Ind Med 28:873–81.
- Golka K, Goebell PJ, Rettenmeier AW. (2007). Bladder cancer: etiology and prevention. Deutsches Ärzteblatt 104:719–23.
- Golka K, Wiese A, Assennato G, Bolt HM. (2004). Occupational exposure and urological cancer. World J Urol 21:382–91.
- González CA, López-Abente G, Errezola M, et al. (1989). Occupation and bladder cancer in Spain: a multi-centre case-control study. Int J Epidemiol 18:569–77.
- Goodman M, Morgan RW, Ray R, et al. (1999). Cancer in asbestos-exposed occupational cohorts: a meta-analysis. Cancer Causes Control 10:453–65.
- Goodman M, Teta MJ, Hessel PA, et al. (2004). Mesothelioma and lung cancer among motor vehicle mechanics: a meta-analysis. Ann Occup Hyg 48:309–26.
- Greaves MF. (1997). Aetiology of acute leukaemia. Lancet 349:344–9.
- Greenberg M. (1991). Cancer mortality in merchant seamen. Ann N Y Acad Sci 643:321–32.
- Greim H, Utell MJ, Maxim LD, Niebo R. (2014). Perspectives on refractory ceramic fiber (RCF) carcinogenicity: comparisons with other fibers. Inhal Toxicol 26:789–810.
- Gross P, Westrick ML, Schrenk HH, McNerney JM. (1956). The effects of synthetic ceramic fiber dust upon the lungs of rats. AMA Arch Indust Health 13:161–6.
- Gustavsson P, Plato N, Axelson O, et al. (1992). Lung cancer risk among workers exposed to man-made mineral fibers (MMMF) in the Swedish prefabricated house industry. Am J Ind Med 21:825–34.
- Hansen ES. (1989). Mortality of auto mechanics. A ten-year follow-up. Scand J Work Environ Health 15:43–6.
- Harling M, Schablon A, Schedlbauer G, et al. (2010). Bladder cancer among hairdressers: a meta-analysis. Occup Environ Med 67:351–8.
- Harries PG. (1968). Asbestos hazards in naval dockyards. Ann Occup Hyg 11:135–45.
- Harrison P, Holmes P, Bevan R, et al. (2015). Regulatory risk assessment approaches for synthetic mineral fibres. Regul Toxicol Pharm 43:425–41.,
- Health and Safety Executive (HSE), (2008). Hazards from the use of Refractory Ceramic Fibre, Report OC 267/3. Suffolk, United Kingdom: HSE, 15 p. Available from: http://www.hse.gov.uk/foi/internalops/ocs/200-299/267_3v2.htm [last accessed 19 Dec 2016].
- Heck JE, Charbotel B, Moore LE, et al. (2010). Occupation and renal cell cancer in Central and Eastern Europe. Occup. Environ Med 67:47–53.
- Heck HDA, Casanova M. (2004). The implausibility of leukemia induction by formaldehyde: a critical review of the biological evidence on distant-site toxicity. Regul Toxicol Pharm 40:92–106.
- Heisel EB, Hunt FE. (1968). Further studies in cutaneous reactions to glass fibers. Arch Environ Health 17:705–11.
- Henshaw DL, Eatough JP, Richardson RB. (1990). Radon as a causative factor in induction of myeloid leukaemia and other cancers. Lancet 335:1008–12.
- Hemelt M, Yamamoto H, Cheng KK, Zeegers MPA. (2009). The effect of smoking on the male excess of bladder cancer: a meta-analysis and geographical analyses. Int J Cancer 124:412–9.
- Hessel PA, Teta MJ, Goodman M, Lau E. (2004). Mesothelioma among brake mechanics: an expanded analysis of a case-control study. Risk Anal 24:547–52.
- Hesterberg TW, Chase G, Axten C, et al. (1998). Biopersistence of synthetic vitreous fibers and amosite asbestos in the rat lung following inhalation. Toxicol Appl Pharmacol 151:262–75.
- Hesterberg TW, Hart GA. (2001). Synthetic vitreous fibers: a review of toxicology research and its impact on hazard classification. Crit Rev Toxicol 31:1–53.
- Hill AB. (1965). The environment and disease: association or causation? Proc R Soc Med 58:295–300.
- Hjortsberg U, Orbeak P, Arborelius M, et al. (1988). Railroad workers with pleural plaques: 1. spirometric and nitrogen washout investigation on smoking and nonsmoking asbestos-exposed workers. Am J Ind Med 14:635–41.
- Hoffmann S, de Vries RB, Stephens ML, et al. (2017). A primer on systematic reviews in toxicology. Arch Toxicol 91:2551–75.
- Hosoda Y, Hiraga Y, Sasagawa S. (2008). Railways and Asbestos in Japan (1928-1987)-epidemiology of pleural plaques, malignancies and pneumoconioses-. J Occup Health 50:297–307.
- Hsieh MY, Guo YL, Shiao JS, Sheu HM. (2001). Morphology of glass fibers in electronics workers with fiberglass dermatitis–scanning electron microscopy study. Int J Dermatol 40:258–61.
- Hu J, Mao Y, White K. (2002). Renal cell carcinoma and occupational exposure to chemicals in Canada. Occup Med (Lond) 52:157–64.
- Huncharek M. (1987). Asbestos-related mesothelioma risk among railroad workers. Am Rev Respir Dis 135: 983–4.
- Huncharek M. (1990). Brake mechanics, asbestos, and disease risk. Am J Forensic Med Pathol 11:236–40.
- Huncharek M. (1992). Changing risk groups for malignant mesothelioma. Cancer 69:2704–11.
- Hunt JD, van der Hel OL, McMillan GP, et al. (2005). Renal cell carcinoma in relation to cigarette smoking: meta-analysis of 24 studies. Int J Cancer 114:101–8.
- Imbernon E, Marchand JL, Garras L, Goldberg M. (2005). Quantitative assessment of the risk of lung cancer and pleural mesothelioma among automobile mechanics. Rev D'epidemiol Sante Publique 53:491–500.
- Industrial Injuries Advisory Council [IIAC]. (2008). Position Paper 23 – Pleural Plaques. London, UK: IIAC. 60 p. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/328553/iiac-pp23.pdf [last accessed 10 Apr 2017].
- Institute National de la Santé et de la Recherche Médicale (INSERM), (1999). Chapter 5 - Effets sur la santé humaine – Dermatoses induites par les fibres artificielles. Fibres de substitution à l'amiante. Rapport. Paris: Les editions, 247–65. Available from: http://www.ipubli.inserm.fr/bitstream/handle/10608/192/?sequence = 10.
- International Agency for Research on Cancer (IARC). 2002. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume 81, Man-Made Vitreous Fibres. Lyon, France: IARC. 433 p. Available from: https://monographs.iarc.fr/ENG/Monographs/vol81/mono81.pdf [last accessed 10 Apr 2017].
- International Agency for Research on Cancer (IARC). (2012). Chemical agents and related occupations, volume 100 F, A review of human carcinogens. Lyon, France: IARC.
- International Agency for Research on Cancer (IARC). (2004). IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Tobacco smoke and involuntary smoking. IARC Working Group On The Evaluation Of Carcinogenic Risks To Humans. Lyon, France: IARC. 1476 p. Available from: https://monographs.iarc.fr/ENG/Monographs/vol83/mono83.pdf [last accessed 10 Apr 2017].
- Iwatsubo Y, Pairon JC, Boutin C, et al. (1998). Pleural mesothelioma: dose-response relation at low levels of asbestos exposure in a French population-based case-control study. Am J Epidemiol 148:133–42.
- Janout V, Janoutova G. (2004). Epidemiology and risk factors of kidney cancer. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 148:95–101.
- Jolanki R, Mäkinen I, Suuronen K, et al. (2002). Occupational irritant contact dermatitis from synthetic mineral fibres according to Finnish statistics. Contact Dermatitis 47:329–33.
- Kakooei H, Hormozy M, Marioryad H. (2011). Evaluation of asbestos exposure during brake repair and replacement. Ind Health 49:374–80.
- Kane EV, Roman E, Cartwright R, et al. (1999). Tobacco and the risk of acute leukaemia in adults. Br J Cancer 81:1228–33.
- Kanerva L, Wahlberg JE, Elsner P, Maibach HI (eds.). (2013). Handbook of occupational dermatology. Berlin, Heidelberg: Springer. 1300 p.
- Kannio A, Ridanpää M, Koskinen H, et al. (1996). A molecular and epidemiological study on bladder cancer: p53 mutations, tobacco smoking, and occupational exposure to asbestos. Cancer Epidemiol Biomarkers Prev 5:33–9.
- Karakosta M, Delicha EM, Kouraklis G, Manola KN. (2016). Association of various risk factors with chronic lymphocytic leukemia and its cytogenetic characteristics. Arch Environ Occup Health 71:317–29.
- Karami S, Boffetta P, Stewart PS, et al. (2011). Occupational exposure to dusts and risk of renal cell carcinoma. Br J Cancer 104:1797–803.
- Karami S, Colt JS, Schwartz K, et al. (2012). A case-control study of occupation/industry and renal cell carcinoma risk. BMC Cancer 12:344.
- Kerper LE, Lynch HN, Zu K, et al. (2015). Systematic review of pleural plaques and lung function. Inhal Toxicol 27:15–44.
- Khoubi J, Pourabdian S, Mohebbi I, et al. (2013). Association between the high risk occupations and bladder cancer in Iran: a case-control study. Int J Occup Med Environ Health 26:205–13.
- Kieć-Świerczyńska M, Wojtczak J. (2000). Occupational ceramic fibres dermatitis in Poland. Occup Med (Lond) 50:337–42.
- Kieć-Świerczyńska M, Szymczk W. (1995). The effect of the working environment on occupational skin disease development in workers processing rockwool. Int J Occup Med Environ Health 8:17–22.
- Kim I, Ha J, Lee J-H, et al. (2014). The relationship between the occupational exposure of trichloroethylene and kidney cancer. Ann Occup Environ Med 26:12.
- Kishimoto T, Ono T, Okada K. (1988). Acute myelocytic leukemia after exposure to asbestos. Cancer 62:787–90.
- Kishimoto T. (1992). Cancer due to asbestos exposure. Chest 101:58–63.
- Koebnick C, Michaud D, Moore SC, et al. (2008). Body mass index, physical activity, and bladder cancer in a large prospective study. Cancer Epidemiol Biomarkers Prev 17:1214–21.
- Kogevinas M, Mannetje A, Cordier S, et al. (2003). Occupation and bladder cancer among men in Western Europe. Cancer Causes Control 14:907–14.
- Koh D, Aw TC, Foulds IS. (1992). Fiberglass dermatitis from printed circuit boards. Am J Ind Med 21:193–8.
- Konzen JL. (1987). Fiberglass and the skin. In: Maibach HI (ed.) Occupational and industrial dermatology, 2nd ed. Chicago: Yearbook Medical Publishers,283–5.
- Kurumatani N, Natori Y, Mizutani R, et al. (1999). A historical cohort mortality study of workers exposed to asbestos in a refitting shipyard. Ind Health 37:9–17.
- Lachapelle JM. (2006). Chapter 8: irritant dermatitis. In: Chew A-L, HI Maibach (eds.) Airborne irritant dermatitis. Berlin: Springer, 71–9.
- Lachapelle JM. (1986). Industrial airborne irritant or allergic contact dermatitis. Contact Dermatitis 3:137–45.
- Lacourt A, Gramond C, Audignon S, et al. (2013). Pleural mesothelioma and occupational coexposure to asbestos, mineral wool, and silica. Am J Respir Crit Care Med 187:977–82.
- Lacourt A, Rinaldo M, Gramond C, et al. (2014). Co-exposure to refractory ceramic fibres and asbestos and risk of pleural mesothelioma. Eur Respir J 44:725–33.
- Laden F, Stampfer MJ, Walker AM. (2004). Lung cancer and mesothelioma among male automobile mechanics: a review. Rev Environ Health 19:39–61.
- Lauriola M, Bua L, Chiozzotto D, et al. (2012). Urinary apparatus tumours and asbestos: the Ramazzini Institute caseload. Arch Ital Urol Androl 84:189–96.
- Larsson SC, Wolk A. (2008). Overweight and obesity and incidence of leukemia: a meta-analysis of cohort studies. Int J Cancer 122:1418–21.
- Lee TV, Lam TH. (1992). Occupational fiberglass dermatitis in Hong Kong. Contact Dermatitis 27:341–3.
- LeMasters GK, Lockey JE, Levin LS, et al. (1998). An industry-wide pulmonary study of men and women manufacturing refractory ceramic fibers. Am J Epidemiol 148:910–9.
- LeMasters GK, Lockey JE, Yiin JH, et al. (2003). Mortality of workers occupationally exposed to refractory ceramic fibers. J Occup Environ Med 45:440–50.
- LeMasters GK, Lockey JE, Hilbert TJ, et al. (2017). A thirty-year mortality and respiratory morbidity study of Refractory Ceramic Fiber workers. Inhal. Toxicol 29: 462–70.
- Lemen RA. (2004). Asbestos in brakes: exposure and risk of disease. Am J Ind Med 45:229–37.
- Leuraud K, Richardson DB, Cardis E, et al. (2015). Ionising radiation and risk of death from leukaemia and lymphoma in radiation-monitored workers (INWORKS): an international cohort study. Lancet Haematol 2:e276–81.
- Lewis JD, Ferrara A, Peng T, et al. (2011). Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care 34:916–22.
- Lippmann M. (2014). Toxicological and epidemiological studies on effects of airborne fibers: coherence and public health implications. Crit Rev Toxicol 44:643–95.
- Lipworth L, Tarone RE, Lund L, McLaughlin JK. (2009). Epidemiologic characteristics and risk factors for renal cell cancer. Clin Epidemiol 1:33–43.
- Ljungberg B, Campbell SC, Cho HY, et al. (2011). The epidemiology of renal cell carcinoma. Eur Urol 60:615–21.
- Lockey J, LeMasters G, Rice C, et al. (1996). Refractory ceramic fiber exposure and pleural plaques. Am J Respir Crit Care Med 154:1405–10.
- Lockey JE, Levin LS, LeMasters GK, et al. (1998). Longitudinal estimates of pulmonary function in refractory ceramic fiber manufacturing workers. Am J Respir Crit Care Med 157:1226–33.
- Lockey JE, LeMasters GK, Levin LS, et al. (2002). A longitudinal study of chest radiographic changes of workers in the refractory ceramic fiber industry. Chest 121:2044–51.
- Lockey JE, Roggli VL, Hilbert TJ, et al. (2012). Biopersistence of refractory ceramic fiber in human lung tissue and a 20-year follow-up of radiographic pleural changes in workers. J Occup Environ Med 54:781–8.
- Lundgren L, Moberg C, Lidén C. (2014). Do insulation products of man-made vitreous fibres still cause skin discomfort? Contact Dermatitis 70:351–60.
- Lynge E, Andersen A, Rylander L, et al. (2006). Cancer in persons working in dry cleaning in the Nordic countries. Environ Health Perspect 114:213–9.
- Malker HS, McLaughlin JK, Malker BK, et al. (1985). Occupational risks for pleural mesothelioma in Sweden, 1961-79. J Natl Cancer Inst 74:61–6.
- Maltoni C, Pinto C, Carnuccio R, et al. (1995). Mesotheliomas following exposure to asbestos used in railroads: 130 Italian cases. Med Lav 86:461–77.
- Mancuso TF. (1983). Mesothelioma among machinists in railroad and other industries. Am J Ind Med 4:501–13.
- Mancuso TF. (1988). Relative risk of mesothelioma among railroad machinists exposed to chrysotile. Am J Ind Med 13: 639–57.
- Mancuso TF. (1991). Mesotheliomas among railroad workers in the United States. Ann N Y Acad Sci 643:333–46.
- Mangold CA, Beckett RR, Bessmer DJ. (1970). Asbestos exposure and control at Puget Sound Naval Shipyard. Bremerton, WA: Industrial Hygiene Division Medical Department, Puget Sound Naval Shipyard.
- Marchand JL, Luce D, Leclerc A, et al. (2000). Laryngeal and hypopharyngeal cancer and occupational exposure to asbestos and man-made vitreous fibers: results of a case-control study. Am J Ind Med 37:581–9.
- Mariusdottir E, Ingimarsson JP, Jonsson E, et al. (2016). Occupation as a risk factor for renal cell cancer: a nationwide, prospective epidemiological study. Scand J Urol 50:181–5.
- Marsh GM, Enterline PE, Stone RA, Henderson VL. (1990). Mortality among a cohort of US man-made mineral fiber workers: 1985 follow-up. J Occup Med 32:594–604.
- Marsh G, Stone R, Youk A, et al. (1996). Mortality among United States rock wool and slag wool workers: 1989 update. J Occup Health Saf Aust NZ 12:297–312.
- Marsh GM, Youk AO, Stone RA, et al. (2001a). Historical cohort study of US man-made vitreous fiber production workers: I. 1992 fiberglass cohort follow-up: initial findings. J Occup Environ Med 43:741–56.
- Marsh GM, Gula MJ, Youk AO, et al. (2001b). Historical cohort study of US man-made vitreous fiber production workers: II. Mortality from mesothelioma. J Occup Environ Med 43:757–66.
- Mast RW, McConnell EE, Anderson R, et al. (1995b). Studies on the chronic toxicity (inhalation) of four types of refractory ceramic fiber in male Fischer 344 rats. Inhal Toxicol 7:425–67.
- Mast RW, McConnell EE, Hesterberg TW, et al. (1995a). Multiple-dose chronic inhalation toxicity study of size-separated kaolin refractory ceramic fiber in male Fischer 344 rats. Inhal Toxicol 7:469–502.
- Mast RW, Maxim LD, Utell MJ, Walker AM. (2000a). Refractory ceramic fiber: toxicology, epidemiology, and risk analyses – a review. Inhal Toxicol 12:359–99.
- Mast RW, Yu CP, Oberdörster G, et al. (2000b). A retrospective review of the carcinogenicity of refractory ceramic fiber in two chronic Fischer 344 rat inhalation studies: an assessment of the MTD and implications for risk assessment. Inhal Toxicol 12:1141–72.
- Matsuoka M, Jeang KT. (2007). Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat Rev Cancer 7:270–80.
- Matsuzaki H, Lee S, Kumagai-Takei N, et al. (2015). Biological effects of refractory ceramic fiber. Kawasaki Med J 41:57–63.
- Maxim LD, Allshouse JN, Chen SH, et al. (2000). Workplace monitoring of refractory ceramic fiber in the United States. Regul Toxicol Pharm 32:293–309.,
- Maxim LD, Allshouse JN, Deadman J, et al. (1998). CARE: a European programme for monitoring and reducing refractory ceramic fibre dust at the workplace: initial results. Gefahrstoffe-Reinhaltung Der Luft 58:97–103.
- Maxim LD, Allshouse J, Fairfax RF, et al. (2008). Workplace monitoring of occupational exposure to refractory ceramic fiber – a 17-year retrospective. Inhal Toxicol 20:289–309.
- Maxim LD, Allshouse JN, Kelly WP, et al. (1997). A multiyear workplace-monitoring program for refractory ceramic fibers: findings and conclusions. Regul Toxicol Pharmacol 26:156–71.
- Maxim LD, Hadley JG, Potter RM, Niebo R. (2006). The role of fiber durability/biopersistence of silica-based synthetic vitreous fibers and their influence on toxicology. Regul Toxicol Pharm 46:42–62.
- Maxim LD, Kelly WP, Walters T, Waugh R. (1994). A multiyear workplace-monitoring program for refractory ceramic fibers. Regul Toxicol Pharmacol 20:S200–S15.
- Maxim LD, Niebo R, Utell MJ. (2015). Are pleural plaques an appropriate endpoint for risk analyses? Inhal Toxicol 27:321–34.
- Maxim LD, Yu CP, Oberdörster G, Utell MJ. (2003). Quantitative risk analyses for RCF: survey and synthesis. Regul Toxicol Pharmacol 38:400–16.
- McConnell EE, Mast RW, Hesterberg TW, et al. (1995). Chronic inhalation toxicity of a kaolin based refractory ceramic fiber (RCF) in Syrian Golden hamsters. Inhal Toxicol 7:503–32.
- McDonald C, McDonald A. (1989). Mesothelioma in railroad workers. Am J Ind Med 15:487–90.
- McDonald TA, Holland NT, Skibola C, et al. (2001). Hypothesis: phenol and hydroquinone derived mainly from diet and gastrointestinal flora activity are causal factors in leukemia. Leukemia 15:10–20.
- McKay RT, LeMasters GK, Hilbert TJ, et al. (2011). A long term study of pulmonary function among US refractory ceramic fibre workers. Occup Environ Med 68:89–95.
- McLaughlin JK, Lipworth L, Tarone RE. (2006). Epidemiologic aspects of renal cell carcinoma. Semin Oncol 33:527–33.
- Michaels D, Monforton C. (2007). How litigation shapes the scientific literature: asbestos and disease among automobile mechanics. J Law Policy 15:1137–70.
- Minamoto K, Nagano M, Inaoka T, et al. (2002). Skin problems among fiber-glass reinforced plastics factory workers in Japan. Ind Health 40:42–50.
- Morfeld P, Mundt KA, Dell LD, et al. (2016). Meta-analysis of cardiac mortality in three cohorts of carbon black production workers. Int J Environ Res Public Health 13:302–31.
- Moolgavkar SH, Anderson EL, Chang ET, et al. (2014). A review and critique of U.S. EPA's risk assessments for asbestos. Crit Rev Toxicol 44:499–522.
- Moore LE, Boffetta P, Karami S, et al. (2010). Occupational trichloroethylene exposure and renal carcinoma risk: evidence of genetic susceptibility by reductive metabolism gene variants. Cancer Res 70:6527–36.
- Morinaga K, Nakamura K, Kohyama N, Kishimoto T. (1999). A retrospective cohort study of male workers exposed to PVA fibers. Ind Health 37:18–21.
- Mostafa MH, Sheweita SA, O’Connor PJ. (1999). Relationship between schistosomiasis and bladder cancer. Clin Microbio Rev 12:97–111.
- Moyad MA. (2001). Review of potential risk factors for kidney (renal cell) cancer. Semin Urol Oncol 19:280–93.
- National Institute for Occupational Safety and Health (NIOSH). 2006. Criteria for a Recommended Standard Occupational Exposure to Refractory Ceramic Fibers. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control Prevention, National Institute for Occupational Safety and Health, 204 p.
- National Research Council (NRC). 2000. Review of the U.S. Navy’s Exposure Standard for Manufactured Vitreous Fibers, Subcommittee on Manufactured Vitreous Fibers, Committee on Toxicology, Board on Environmental Studies and Toxicology, Commission on Life Sciences National Research Council. Wsshington, DC: National Academy Press. 73 p.
- Ohlson C-G, Klaesson B, Hogstedt C. (1984). Mortality among asbestos-exposed workers in a railroad workshop. Scand J Work Environ Health 10:283–91.
- Olfert SM, Felknor SA, Delclos GL. (2006). An updated review of the literature: risk factors for bladder cancer with focus on occupational exposures. Southern Med J 99:1256–63.
- Oliver LC, Eisen EA, Greene RE, Sprince NL. (1985). Asbestos-related disease in railroad workers: a cross-sectional study. Am Rev Respir Dis 131:499–504.
- Olsen JH, Jensen OM. (1987). Occupation and risk of cancer in Denmark. An analysis of 93,810 cancer cases, 1970-1979. Scand J Work Environ Health 13(Suppl 1):1–91.
- Orth SR. (1997). The renal risks of smoking. Kidney Int 51:1669–7.
- Partanen T, Heikkilä P, Hernberg S, et al. (1991). Renal cell cancer and occupational exposure to chemical agents. Scand J Work Environ Health 17:231–9.
- Pascual D, Borque A. (2008). Epidemiology of kidney cancer. Adv Urol 5:1–7.
- Patiwael JA, Wintzen M, Rustemeyer T, Bruynzeel DP. (2005). Airborne irritant contact dermatitis due to synthetic fibres from an air-conditioning filter. Contact Dermatitis 52:126–9.
- Paustenbach DJ, White KR. (2012). Egilman's assessment regarding exposures of auto mechanics to amphiboles is not accurate. Inhal Toxicol 24:609–13.
- Pesch B, Haerting J, Ranft U, et al. (2000). Occupational risk factors for renal cell carcinoma: agent-specific results from a case-control study in Germany. Int J Epidemiol 29:1014–24.
- Petersen R, Sabroe S. (1991). Irritative symptoms and exposure to mineral wool. Am J Ind Med 20:113–22.
- Peto J, Rake C, Gilham C, Hatch J. (2009). Occupational, domestic and environmental mesothelioma risks in Britain: a case-control study. Norwich: HSE Books, 76 p.
- Plato N, Martinsen JI, Sparén P, et al. (2016). Occupation and mesothelioma in Sweden: updated incidence in men and women in the 27 years after the asbestos ban. Epidemiol Health 38: e2016039.
- Plato N, Westerholm P, Gustavsson P, et al. (1995). Cancer incidence, mortality and exposure-response among Swedish man-made vitreous fiber production workers. Scand J Work Environ Health 21:353–61.
- Polychronakis I, Dounias G, Makropoulos RE, Linos A. (2013). Work-related leukemia: a systematic review. J Occup Med Toxicol 8:14.
- Possick PA, Gellin GA, Key MM. (1970). Fibrous glass dermatitis. Am Ind Hyg Assoc J 31:12–5.
- Qayyum T, Oades G, Horgan P, et al. (2012). The epidemiology and risk factors for renal cancer. Curr Urol 6:169–74.
- Qin Q, Xu X, Wang X, Zheng X-Y. (2013). Obesity and risk of bladder cancer: a meta-analysis of cohort studies. Asian Pacific J Cancer Prev 14:3117–21.
- Rake C, Gilham C, Hatch J, et al. (2009). Occupational, domestic and environmental mesothelioma risks in the British population: a case-control study. Br J Cancer 100:1175–83.
- Rauscher GH, Sandler DP, Poole C, et al. (2002). Family history of cancer and incidence of acute leukemia in adults. Am J Epid 156:517–26.
- Reulen RC, Kellen E, Buntinx F, et al. (2008). A meta-analysis on the association between bladder cancer and occupation. Scand J Urol Nephrol 42(Suppl):64–78.
- Richardson DB, Cardis E, Daniels RD, et al. (2015). Risk of cancer from occupational exposure to ionising radiation: retrospective cohort study of workers in France, the United Kingdom, and the United States (INWORKS). BMJ 351:h5359.
- Richardson K, Band PR, Astrakianakis G, Le ND. (2007). Male bladder cancer risk and occupational exposure according to a job-exposure matrix – a case-control study in British Columbia, Canada. Scand J Work Environ Health 33:454–64.
- Richter RO, Finley BL, Paustenbach DJ, et al. (2009). An evaluation of short-term exposures of brake mechanics to asbestos during automotive and truck brake cleaning and machining activities. J Expos Sci Environ Epidemiol 19:458–74.
- Rinsky RA. (1989). Benzene and leukemia: an epidemiologic risk assessment. Environ Health Perspect 82:189–91.
- Rödelsperger K, Jöckel KH, Pohlabeln H, et al. (2001). Asbestos and man‐made vitreous fibers as risk factors for diffuse malignant mesothelioma: results from a German hospital‐based case‐control study. Am J Ind Med 39:262–75.
- Rodriguez-Abreu D, Bordoni A, Zucca E. (2007). Epidemiology of hematological malignancies. Ann Oncol 18:i3–8.
- Roelofs CR, Kernan GJ, Davis LK, et al. (2013). Mesothelioma and employment in Massachusetts: analysis of cancer registry data 1988-2003. Am J Ind Med 56:985–92.
- Rogers A, Yeung P, Berry G, et al. (1997). Exposure and respiratory health of workers in the Australian refractory ceramic fibre production industry. Ann Occup Hyg 41(Suppl 1):261–6.
- Roggli VL, Sharma A, Butnor KJ, et al. (2002). Malignant mesothelioma and occupational exposure to asbestos: a clinicopathological correlation of 1445 cases. Ultrastruct Pathol 26:55–65.
- Rolland P, Gramond C, Lacourt A, PNSM Study Group, et al. (2010). Occupations and industries in France at high risk for pleural mesothelioma: a population-based case-control study (1998-2002). Am J Ind Med 53:1207–19.
- Rom WN, Markowitz SB (eds.). (2004). Environmental and occupational medicine. 4th ed. Philadelphia: Wolters Kluwer, Lippincott Williams and Wilkins. 1884 p.
- Ross JA, Kasum CM, Davies SM, et al. (2002). Diet and risk of leukemia in the Iowa Women’s Health Study. Cancer Epidemiol Biomarkers Prev 11:777–81.
- Rubagotti A, Martorana G, Boccardo FM. (2006). Epidemiology of kidney cancer. Eur Urol Suppl 5:558–65.
- Rushworth DH. (2005). The navy and asbestos thermal insulation. Naval Eng J 17:35–48.
- Russo P. (2012). End stage and chronic kidney disease: associations with renal cancer. Front Oncol 2:1–7.
- Sali D, Boffetta P. (2000). Kidney cancer and occupational exposure to asbestos: a meta-analysis of occupational cohort studies. Cancer Causes Control 11:37–47.
- Sali D, Boffetta P, Andersen A, et al. (1999). Non-neoplastic mortality of European workers who produce man-made vitreous fibers. Occup Environ Med 56:612–7.
- Sankila R, Karjalainen S, Pukkala E, et al. (1990). Cancer risk among glass factory workers: an excess of lung cancer? Br J Ind Med 47:815–8.
- Scarselli A, Scanno P, Marinaccio A, Iavicoli S. (2011). Bladder cancer and occupational exposure: estimating the workers potentially at risk in Italy. Ann Ist Super Sanitaà 47:200–6.
- Schenker MB, Garshick E, Muñoz A, et al. (1986). A population-based case-control study of mesothelioma deaths among U.S. railroad workers. Am Rev Respir Dis 134:461–5.
- Schwilk E, Zhang L, Smith MT, et al. (2010). Formaldehyde and leukemia: an updated meta-analysis and evaluation of bias. J Occup Environ Med 52:878–86.
- Scientific Committee on Occupational Exposure Limits (SCOEL). (2011). Recommendation from the Scientific Committee on Occupational Exposure Limits for Refractory Ceramic Fibres SCOEL/SUM/165. Brussels: European Commission- Employment, Social Affairs and Inclusion, 21 p.
- Seidler A, Becker N, Nieters A, et al. (2010). Asbestos exposure and malignant lymphoma: a multicenter case–control study in Germany and Italy. Int Arch Occup Environ Health 83:563–70.
- Selikoff IJ. (1990). Historical developments and perspectives in inorganic fiber toxicity in man. Environ Health Perspect 88:269–76.
- Selikoff IJ, Hammond EC. (1978). Asbestos-associated disease in United States shipyards. CA Cancer J Clin 28:87–99.
- Selikoff IJ, Lilis R, Levin G. (1990). Asbestotic radiological abnormalities among United States merchant marine seamen. Br J Ind Med 47:292–7.
- Serra C, Kogevinas M, Silverman DT, et al. (2008). Work in the textile industry in Spain and bladder cancer. Occup Environ Med 65:552–9.
- Sertoli A, Giorgini S, Farli M. (1992). Fiberglass dermatitis. Clin Dermatol 10:167–74.
- Shannon H, Hayes M, Julian JA, Muir DCF. (1984). Mortality experience of glass fibre workers. Br J Ind Med 41:35–8.
- Shannon H, Muir A, Haines T, Verma D. (2005). Mortality and cancer incidence in Ontario glass fiber workers. Occup Med 55:528–34.
- Siemiatycki J, Richardson L, Gérin M, et al. (1986). Associations between several sites of cancer and nine organic dusts: results from a hypothesis-generating case-control study in Montreal, 1979-1983. Am J Epidemiol 123:235–49.
- Silverman D, Devesa S, Moore L, Rothman N. (2006). Bladder cancer. In: Schottenfeld D, Fraumeni JJ (eds.) Cancer epidemiology and prevention, 3rd ed.). Chapter 58. New York, NY: Oxford University Press.
- Sim MR, Echt A. (1996). An outbreak of pruritic skin lesions in a group of laboratory workers – a case report. Occup Med (Lond) 46:235–8.
- Simonato L, Fletcher AC, Cherrie J, et al. (1986). The man-made mineral fiber European historical cohort study: extension of the follow-up. Scand J Work Environ Health 12:34–47.
- Skotnicki-Grant S. (2008). Allergic Contact Dermatitis versus Irritant Contact Dermatitis. Discussion Paper prepared for Workplace Safety and Insurance Appeals Tribunal. Toronto: University of Toronto – Division of Dermatology and Occupational Health – St. Michael’s Hospital. Available from: http://www.wsiat.on.ca/english/mlo/allergic.htm [last accessed 19 Apr 2017].
- Smith DM, Ortiz LW, Archuleta RF, Johnson NF. (1987). Long term health effects in hamsters and rats exposed chronically to man-made vitreous fibers. Ann Occup Hyg 31:731–43.
- Smith MT, Zhang L. (1998). Biomarkers of leukemia risk: benzene as a model. Environ Health Perspect 106(Suppl 4): 937–43.
- Song X, Pukkala E, Dyba T, et al. (2014). Body mass index and cancer incidence: the FINRISK study. Eur J Epidemiol 29:477–87.
- Spinelli J. (1989). Mortality and cancer incidence in workers at the Alcan aluminum plant in Kitimat, B.C.: final report. British Columbia: Cancer Control Agency of British Columbia.
- Stam-Westerveld EB, Coenraads PJ, Van der Valk PGM, et al. (1994). Rubbing test responses of the skin to man-made mineral fibres of different diameters. Contact Dermatitis 31:1–4.
- Stone RA, Youk AO, Marsh GM, et al. (2004). Historical cohort study of U.S. man-made vitreous fiber production workers IX: summary of 1992 mortality follow up and analysis of respiratory system cancer among female workers. J Occup Environ Med 46:55–67.
- Strand L, Martinsen JI, Koefoed VF, et al. (2010). Asbestos-related cancers among 28,300 military servicemen in the Royal Norwegian Navy. Am J Ind Med 53:64–71.
- Strom SS, Yamamura Y, Kantarijian HM, Cortes-Franco JE. (2009). Obesity, weight gain, and risk of chronic myeloid leukemia. Cancer Epidemiol Biomarkers Prev 18:1501–6.
- Sun JW, Zhao LG, Yang Y, et al. (2015). Obesity and risk of bladder cancer: a dose-response meta-analysis of 15 cohort studies. PLoS One 10:1–11.
- Tarvainen K, Estlander T, Jolanki R, Kanerva L. (1994). Occupational dermatoses caused by man-made mineral fibers. Dermatitis 5:22–9.
- Teppo L, Kojonen E. (1986). Mortality and cancer risk among workers exposed to man-made mineral fibers in Finland. Scand J Work Environ Health 12:61–4.
- Teschke K, Morgan MS, Checkoway H, et al. (1997). Mesothelioma surveillance to locate sources of exposure to asbestos. Can J Public Health 88:163–8.
- Tessari R, Canova C, Simonato L. (2004). Epidemiological investigation on the health status of employees in two factories manufacturing and repairing railway rolling stock: a historical perspective study of mortality. Med Lav 95:381–91.
- Thermal Insulation Manufacturers Association (TIMA) Nomenclature Committee. 1993. Man-made vitreous fibers: nomenclature, chemical and physical properties. Washington, DC: Refractory Ceramic Fibers Coalition (RCFC). 72 p.
- Travis LB, Curtis RE, Glimelius B, et al. (1995). Bladder and kidney cancer following cyclophosphamide therapy for non-Hodgkin’s lymphoma. J Natl Cancer Inst 87:524–30.
- Trethowan WN, Burge PS, Rossiter CE, et al. (1995). Study of the respiratory health of employees in seven European plants that manufacture ceramic fibers. Occup Environ Med 52:97–104.
- Tsai RJ, Luckhaupt SE, Schumacher P, et al. (2014). Acute myeloid leukemia risk by industry and occupation. Leuk Lymphoma 55:2584–91.
- Twight C. (1991). From claiming credit to avoiding blame: the evolution of congressional strategy for asbestos management. J Public Policy 153:155–6.
- U.S. Environmental Protection Agency (U.S. EPA). (2009). Toxicological review of tetrachloroethylene (perchloroethylene) (CAS No. 127-18-4), in Support of Summary Information on the Integrated Risk Information System. Report EPA/635/R-08/011A. Washington, DC: U.S. EPA. 537 p.
- U.S. Environmental Protection Agency (U.S. EPA). (2012). Toxicological Review of Tetrachloroethylene (Perchloroethylene) (CAS No. 127-18-4) in Support of Summary Information on the Integrated Risk Information System (IRIS). EPA/635/R-08/011F U.S. Washington DC: EPA. 1077 p.
- Utell MJ, Maxim LD. (2010). Refractory ceramic fiber (RCF) toxicity and epidemiology: a review. Inhal Toxicol 22:500–21.
- Vucenik I, Stains JP. (2012). Obesity and cancer risk: evidence, mechanisms, and recommendations. Ann N Y Acad Sci 1271:37–43.
- Walker AM, Maxim LD, Utell M. (2012a). Are airborne refractory ceramic fibers similar to asbestos in their carcinogenicity? Inhal Toxicol 24:416–24.
- Walker AM, Maxim LD, Utell M. (2012b). Corigendum. Are airborne refractory ceramic fibers similar to asbestos in their carcinogenicity? Inhal Toxicol 24:928–9.
- Wang BJ, Lee JY, Wang RC. (1993). Fiberglass dermatitis: report of two cases. J Formos Med Assoc 92:755–8.
- Wang P, Liu H, Jiang T, Yang J. (2015). Cigarette smoking and the risk of adult myeloid disease: a meta-analysis. PLoS One 10:e0137300.
- Weikert S, Ljungberg B. (2010). Contemporary epidemiology of renal cell carcinoma: perspectives of primary prevention. World J Urol 28:247–52.
- Welch LS. (2007). Asbestos exposure causes mesothelioma, but not this asbestos exposure: an amicus brief to the Michigan Supreme Court. Int J Occup Environ Health 13:318–27.
- Wild P, Gonzalez M, Bourgkard E, et al. (2012). Occupational risk factors have to be considered in the definition of high-risk lung cancer populations. Br J Cancer 106:1346–52.
- Winer A, Holtgren WD. (1976). Asbestos – a case study of the U. S. Navy's response to upgraded safety and health requirements. Safety Division Naval Sea Systems Command. Naval Eng J 88:41–8.
- Wong O. (2001). Malignant mesothelioma and asbestos exposure among auto mechanics: appraisal of scientific evidence. Regul Toxicol Pharmacol 34:170–7.
- Wong O. (2006). The interpretation of occupational epidemiologic data in regulation and litigation: studies of auto mechanics and petroleum workers. Regul Toxicol Pharmacol 44:191–7.
- Wu X, Lin J, Grossman HB, et al. (2007). Projecting individualized probabilities of developing bladder cancer in white individuals. J Clin Oncol 25:4975–81.
- Zeeb H, Blettner M. (1998). Adult leukaemia: what is the role of currently known risk factors? Radiat Environ Biophys 36:217–28.
- Zeegers MP, Swaen GM, Kant I, et al. (2001). Occupational risk factors for male bladder cancer: results from a population based case- cohort study in the Netherlands. Occup Environ Med 58:590–6.
- Zeegers MP, Tan F, Dorant E, Van Den Brandt P. (2000). The impact of characteristics of cigarette smoking on urinary tract cancer risk: a meta-analysis of epidemiologic studies. Cancer 89:630–9.
- Zhang L, Steinmaus C, Eastmond DA, et al. (2009). Formaldehyde exposure and leukemia: a new meta-analysis and potential mechanisms. Mutat Res Rev Mutat Res 681:150–68.
- Zhu X, Li T, Wang H. (2015a). Relationship between dust mass concentration and fiber number concentration of refractory ceramic fibers. Chin J Ind Hyg Occup Dis 33:309–12.
- Zhu X, Li T, Wang H. (2015b). Effects of refractory ceramic fibers on workers’ pulmonary ventilation function. J Environ Occup Med (Chinese) 32:399–403.
- Zhu X, Li T, Wang H. (2015c). Effects of refractory ceramic fibers on workers’ skin. J Environ Occup Med (Chinese) 5:404–8.
- Zhu X, Li T, Wang H. (2015d). Analysis of occupational exposure levels to fibrous dust for refractory ceramic fiber workers. Chin J Ind Med 2:98–101.
Appendix
Table A1. Known risk factors for kidney cancer.
Table A2. Known risk factors for bladder cancer.
Table A3. Studies on irritant contact dermatitis and fiber exposure.
Table A4. Causes, contributing factors and risk factors for adult leukemia.

