Abstract
Objective
Solid composite propellants combustion, in aerospace and defense fields, can lead to complex aerosols emission containing high concentrations of alumina nanoparticles (Al2O3 NPs) and hydrogen chloride gas (HClg). Exposure to these mixtures by inhalation is thus possible but literature data toward their pulmonary toxicity are missing. To specify hazards resulting from these combustion aerosols, a pilot study was implemented.
Materials and methods
Male Wistar rats were nose-only exposed to Al2O3 NPs (primary size 13 nm, 10 g/L suspension leading to 20.0–22.1 mg/m3 aerosol) and/or to HClg aerosols (5 ppm target concentration) following two exposure scenarios (single exposures (SE) or repeated exposures (RE)). Bronchoalveolar lavage fluids (BALF) content and lungs histopathology were analyzed 24 h after exposures.
Results
Repeated co-exposures increased total proteins and LDH concentrations in BALF indicating alveolar–capillary barrier permeabilization and cytolysis. Early pulmonary inflammation was induced after RE to Al2O3 NPs ± HClg resulting in PMN, TNF-α, IL-1β, and GRO/KC increases in BALF. Both exposure scenarios resulted in pulmonary histopathological lesions (vascular congestions, bronchial pre-exfoliations, vascular and interalveolar septum edemas). Lung oxidative damages were observed in situ following SE.
Conclusion
Observed biological effects are dependent on both aerosol content and exposure scenario. Results showed an important pro-inflammatory effect of Al2O3 NPs/HClg mixtures on the lungs of rat 24 h after exposure. This pilot study raises concerns toward potential long-term pulmonary toxicity of combustion aerosols and highlights the importance for further studies to be led in order to define dose limitations and exposure thresholds for risk management at the work place.
Introduction
Combustion reactions from different sources can produce high volumes of complex aerosols containing various components as gases and particles in the environment (Sgro et al. Citation2012; Chivas-Joly et al. Citation2016). Solid composite propellants use in aerospace and defense fields lead to emission of complex combustion aerosols containing notably alumina (Al2O3) particles and hydrogen chloride gas (HClg) (Pellett et al. Citation1983; Meda et al. Citation2005). Indeed, high concentrations of Al2O3 particles and HClg were measured close to these propulsion systems under normal use conditions (classified data). Thermochemical simulations based on highly aluminized solid composite propellant (68% ammonium perchlorate and 20% aluminum) also confirm production of high quantities of Al2O3 particles and HClg during the combustion process (data not shown). Moreover, these pollutants can also be emitted, respectively, by industrial activities such as aluminum manufacturing (Pichard et al. Citation2005) and wastes incineration (Wang et al. Citation1999) increasing the risk of exposure to the workers.
Alumina (Al2O3) is the oxidized form of aluminum and exists under several crystalline phases (γ, δ, θ, and α) (Piriyawong et al. Citation2012). Among them, a majority of γ then δ polymorphs were found following solid composite propellants use (Galfetti et al. Citation2004; Meda et al. Citation2005). Alumina is mainly used for aluminum production and enters in the composition of many everyday products (paper, plastics, ceramics, flame retardants, and coatings) due to its hardness. Otherwise, alumina can also be found in foods and cosmetics (Krewski et al. Citation2007). To determine health hazard related to alumina particles exposure, only few studies were conducted in vivo by inhalation. Twenty-eight days nose-only inhalation of Al2O3 NPs (11.94 nm, 0.2–5 mg/m3, 5 days a week) showed pulmonary pro-inflammatory properties of Al2O3 NPs on rats. This was characterized by total cells, polymorphonuclear neutrophils (PMN), lymphocytes, lactate dehydrogenase (LDH), TNF-α, and IL-6 increases in bronchoalveolar lavage fluids (BALF). Alveolar macrophages accumulation was also observed in lungs after 28 days exposure (Kim et al. Citation2018). In mice, pulmonary pro-inflammatory effects were also recorded after 7 days exposure to 40 nm Al2O3 NPs (0.4 mg/m3) by inhalation. Increased concentrations of IL-6 and IL-33 were measured in BALF associated with emphysema and airway remodeling appearance. However, in this study, BALF PMN concentration was not increased following Al2O3 NPs inhalation (Li et al. Citation2017). Al2O3 NPs intratracheal (IT) instillation on rats (6.3 nm, 0.5–300 cm2/mL) also induced acute pulmonary inflammation resulting in PMN concentration increases in BALF (Cho et al. Citation2012). Moreover, mice IT instillation exposures to Al2O3 particles (4.37 μm, 40 mg) increased macrophages, PMN, and fibronectin concentrations in BALF until 12 months after exposure suggesting pulmonary inflammation persistence over time (Tornling et al. Citation1993). Another study, carried out by nasal instillation on rats, highlighted dose-dependent inflammation and alveolar–capillary barrier permeabilization after Al2O3 particles (size unknown, 1–40 mg/kg) exposure (Kwon et al. Citation2013). Besides pro-inflammatory properties, several in vitro Al2O3 particles toxicity studies’ results suggest oxidative stress induction and genotoxic potential (DNA single- and double-strand breaks induction) of these particles (Sliwinska et al. Citation2015; Zhang et al. Citation2017). These effects were also found in vivo after oral, intravenous, or peritoneal injection exposures to Al2O3 particles (Chen et al. Citation2008; Balasubramanyam et al. Citation2009; Prabhakar et al. Citation2012; Zhang et al. Citation2017). Furthermore, aluminum is a known respiratory sensitizer which can induce immune response resulting in allergies and occupational asthma (Cohen Citation2004; Zhu et al. Citation2013; Kongerud and Soyseth Citation2014).
Hydrogen chloride gas is a known irritant and caustic agent able to cause severe ocular and cutaneous lesions (Dyer and Esch Citation1976). In water, HClg dissolves to form hydrochloric acid solution (HCl). HClg and HCl exposure risk is non-negligible as these compounds are widely used in industries and laboratories and as they can be found in some household products (Dawe et al. Citation2019). After HClg inhalation by rats and mice, eyes, mucous membranes, and skin irritations were observed which is associated with respiratory system lesions (emphysema, atelectasis, and edema) (Darmer et al. Citation1974). Respiratory system necrosis as well as PMN increases were also observed on rats after 30 min inhalation (1284 and 1293 ppm of HClg) (Stavert et al. Citation1991). Nevertheless, HClg long-term inhalation (10 ppm; 6 h a day 5 days a week) performed during rats entire life did not induce neoplastic or preneoplastic lesions (Sellakumar et al. Citation1985). Similar respiratory symptoms were found in Guinea pigs and rabbits associated with severe and persistent inflammatory reactions (Machle et al. Citation1942). Mitogenic effect of HCl (40 mM) was also shown on rabbit esophageal mucosa after 1 h incubation (Carpizo et al. Citation1998). Moreover, respiratory rate increase and oxygen partial pressure in blood decrease were observed after HClg head-only inhalation in baboons (500, 5000, and 10,000 ppm; 15 min). However, no alteration of pulmonary function was reported 3 months post-exposure (Kaplan et al. Citation1988). Pro-inflammatory effect of HCl was also demonstrated in vitro on esophageal mucosa and mice macrophages (cell line RAW 264.7), resulting in an increase in the substance P, IL-8, IL-6, IL-1β, platelet-activating factor (PAF), and nitric acid levels (Kellum et al. Citation2004; Cheng et al. Citation2006; Cheng et al. Citation2006; Ma et al. Citation2010).
Taken together, these toxicity data regarding Al2O3 particles and HClg raise questions toward their potential combined toxicity due to possible exposure to the workers. The study of mixtures effects is an actual major concern in toxicology. Only few scientific data are available in literature, especially for mixtures of compounds with different physical forms (solid and gas for example). However, population is daily exposed to multiple pollutants simultaneously and a recent study highlighted mixtures effects (synergy, addition, antagonism, or inhibition) unpredictability (Hayes et al. Citation2019). Therefore, it is currently necessary to study mixtures effects in a case-by-case basis. Biological effects and resulting hazard of complex combustion aerosols exposure are poorly described in literature. To date, only one study was published concerning the combined effect of Al2O3 particles and HClg. This study was carried out in vivo on rats and mice by whole body exposure (Wohlslagel et al. Citation1976). However, during exposures, HClg was always associated with hydrogen fluoride gas (HFg) and the only studied endpoint was mortality. Conclusions did not highlight any additive or synergistic effect of Al2O3 particles addition to the mixture of gases on animals’ mortality.
Due to the lack of scientific data in literature concerning biological effects of mixtures containing NPs and other environmental pollutants, performing in vitro and in vivo studies is essential to improve knowledge on toxicological impact on human health. Following solid composite propellant combustion, high concentrations of hydrogen chloride gas (HClg) and alumina nanoparticles (Al2O3 NPs) are produced. Therefore, our pilot study focused on exposure to mixture of aerosols containing HClg and Al2O3 NPs. Inhalation is the main route of exposure to these combustion aerosols, and little is known about combined effects of HClg and Al2O3 NPs on the respiratory tract. Consequently, our objective was to assess the early pulmonary effects of model aerosols mimicking an inhalation exposure to combustion aerosols from solid composite propellants on rats. To investigate toxicological effects, animals were nose-only exposed 4 h (single exposures; SE) or 4 h a day for 4 days (repeated exposures; RE) to aerosols. To assess pro-inflammatory response and oxidative stress induction, BALF content, and lungs histopathology were analyzed 24 h after exposures end.
Material and methods
Test materials
Alumina nanoparticles (Al2O3 NPs) were provided by Sigma-Aldrich (St. Louis, MO, USA) (718475) with the following physico-chemical specifications: primary size 13 nm, crystalline phase gamma/delta (γ/δ), specific surface (85–115 m2/g) experimentally confirmed by the Brunauer, Emmet, and Teller method (BET) (97 m2/g), pH (4.5–5.5), and purity 99.8%. HClg was calibrated, certified, and provided by Air Liquide (0023159805-000010; “Saphir” quality). Initial HClg concentration is 500 ppm in the gas cylinder.
Animals
All in vivo experiments were approved by the “Comité Régional d’Ethique en Matière d’Expérimentation Animale de Picardie” (CREMEAP, CNREEA registration code: C2EA-96). Healthy 7-week-old male rats WISTAR RjHan:WI, Janvier Labs; 250 g) were housed in polycarbonate cages, at a temperature of 20–24 °C, and a relative humidity of 40–70% controlled room, and had free access to food and water ad libitum. A 12 h light–12 h dark cycle was respected. Animals (2–3 rats/cage) were acclimatized for at least 5 days before experiments. Wistar rats were selected for this project as they are often used in inhalation studies (Parasuraman Citation2011).
Animal exposure
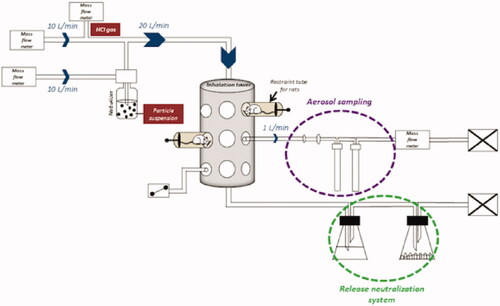
Two exposure scenarios were tested in this study. Single exposures (SE) of 4 h and repeated exposures (RE) of 4 h a day for 4 days were performed. These scenarios were adapted from OECD guidelines 403 “Acute Inhalation Toxicity” and 436 “Acute Inhalation Toxicity – Acute Toxic Class Method” (OECD/OCDE403 Citation2009a; OECD/OCDE436 Citation2009c) recommendations. The 96 animals were distributed in 8 groups of 12 rats (6 rats for biochemistry analysis, 3 rats for histopathology analysis, and 3 rats for aluminum quantification in lungs among each group), respectively, exposed to air (control group, inhaling air mixed with nebulized ultrapure water), HClg, Al2O3 NPs or Al2O3 NPs/HClg for each exposure scenario. They were exposed in a nose-only inhalation tower (randomized placement on the inhalation tower) to nebulized Al2O3 NPs suspensions in ultrapure water (10 g/L, a high dose to simulate a worst case scenario) using a PALAS AGK 2000E (Palas) and/or to HClg (target concentration 5 ppm, French Occupational Exposure Limit (OEL)). For Al2O3 NPs, the choice was based on a preliminary study results in which intratracheal instillations of 10 µg of Al2O3 NPs did not induce biological effect while instillations of 500 µg of Al2O3 NPs led to pro-inflammatory response (data not shown). HClg French OEL was selected to conduct this pilot study in the absence of field data related to HClg precise concentrations generated by solid composite propellants use. Exposure flow was controlled using regularly checked mass flow meters (TYLAN). Both compounds were mixed (Al2O3 NPs/HClg exposures) prior to entering into the inhalation tower at a 20 L/min flow rate. Samples were taken on bubblers (two bubblers in series) or cellulose filters using mass flow meters to allow aerosols characterization. A neutralization system (bubblers with caustic soda and desiccant) was placed at the exposure device exit to eliminate generated aerosols (). Rats were euthanized by isoflurane inhalation 24 h after the last exposure end and biological samples were collected for further analysis according to the OECD guidelines 412 “28-day (subacute) inhalation toxicity study” (OECD/OCDE412 Citation2009b).
Figure 1. Nose-only inhalation exposure system. Al2O3 NPs aggregates suspension in water and HClg bottle were connected to the system on two parallel paths that joined to create the mixture. Mass flow meters allowed to generate and calibrate the air flow in order to avoid re-inhalation phenomena of the air exhaled by the animals (dynamic air circulation, 10 L/min). During exposures, rats were placed in individual containment tubes positioned on the oro-nasal inhalation tower. Al2O3 NPs and HClg mixture reached the inhalation chamber at a flow rate of 20 L/min. Bubblers and samplers connected to the inhalation tower allowed the collection of samples during the exposures in order to characterize the generated aerosol. A release neutralization system (bubbler with soda and desiccant) was set up at the inhalation tower exit.
Monitoring and characterization of aerosols
To precisely characterize generated aerosols and ensure their stability over time, samples were collected directly from the inhalation tower (empty animal exposure tube location) regularly during exposures (30 min, 2 h and 3h30 after exposure beginning). Aerosols characterization was only performed during rats single 4-h exposures assuming that repeated exposures would result in similar exposures. Al2O3 NPs aggregates size, form, and chemical composition were studied. Transmission electron microscopy (TEM) imaging on Al2O3 NPs bulk powder was performed using electron microscope JEOL JEM 1400 operated at 120 kV equipped with an CCD camera (Gatan Orius 1000, Gatan Inc., Warrendale, PA, USA). Aerosol samples were collected on copper grids and TEM observations were performed using a CM12 80 kV microscope (Philips, Tokyo, Japan) equipped with a Megaview 3 camera (EMSIS) and an EDX probe. Al2O3 NPs crystal structure and size of crystallites were determined by X-ray powder diffraction (XRD) using a D4 ENDEAVOR X-ray diffractometer (Bruker, Karlsruhe, Germany). XRD patterns were acquired with the use of Cu-Kα radiation (λ = 1.5406 Å) from powders directly hand-pressed on sample holders. Sioutas (SKC Inc., Seoul, South Korea) cascade impactor was used to precise the NPs sizes distribution and concentrations in the aerosols. Sampling was performed during aerosol generation (Al2O3 NPs alone or with HClg) without animals as it requires a 9 Lpm (liter per minute) flow rate during 15 min. The mean aerodynamic diameter was calculated from Sioutas data using the following formula: aerodynamic diameter (nm) = ∑ (mass % x stage size (µm)) × 1000. The stability of Al2O3 NPs concentrations during exposures was assessed using inductively coupled plasma optical emission spectrometry (ICP-OES) to quantify aluminum on cellulose filters after samples collection (1 Lpm, 15 min), as recommended by AFNOR standard NF-EN-ISO-11885. Analyses were performed on ICP-OES 5110 (Agilent, San Deigo, CA, USA) with ICP Expert II 7.3 software (Agilent).
HClg content was assessed indirectly by chloride ions (Cl−) dosing on bubbler samples (free fraction, two bubblers placed in series) and on cellulose filters (particulate fraction) using ion exchange chromatography according to AFNOR standard NF-EN-1911. The sampling was performed over 15 min at a flow rate of 1 Lpm.
Aluminum quantification in lungs
Rats (n = 3) were euthanized 24 h after the last exposure end and perfused with phosphate-buffered saline (PBS) to remove blood from the lungs. Aluminum burden was then quantified using ICP-OES. Briefly, tissue samples were digested in a mixture of concentrated nitric acid (HNO3; Analpure® Analytika 69% for SE and Normatom VWR 67–69% for RE) and hydrofluoric acid (HF; Analpure® Analytika 48% for SE and Normatom VWR 47–51% for RE) using a microwave oven (Mars Xpress CEM for SE and Ethos One Milestone for RE). After cooling at room temperature, boric acid solution was added to neutralize digests (H3BO4; Ultra-pure Chem Lab 99.9% for SE and Suprapur® Merck 99.9999% for RE) and samples were diluted in ultrapure water (at a final volume of 50 mL). Aluminum quantification was then performed using ICP-OES 5110 (Agilent, for SE, LLOQFootnote1 = 0.25 µg/g of lung) or ICP-OES Optima 8300 (PerkinElmer, Waltham, MA, USA, for RE, LLOQ = 1.25 µg/g of lung). Software used to realize spectra analysis were ICP Expert II 7.3 (Agilent, for SE) or WinLab32 ICP v5.5 (PerkinElmer, for RE). The equipment and reagents change should not have influenced experimental results as LLOQ were in each case was lower than measured values and as the reagents used had the same quality and concentration.
Clinical symptoms and body weight
Daily monitoring of clinical symptoms was performed throughout the experiment. Respiratory, behavioral, and dermal visible changes were watched. Type, date of occurrence, and symptoms severity were individually recorded. Rats were weighted at the arrival, before, and after each 4 h exposure and before euthanasia.
BALF analysis
Rats (n = 6) were euthanized 24 h after the last exposure end and bronchoalveolar lavages were performed with PBS. A first bronchoalveolar lavage was performed using 5 mL of PBS. Two other lavages were then performed with 10 mL of PBS to collect more cells. Collected BALF were centrifuged at 350 g (4 °C) for 10 min.
Cell pellets of the three lavages were suspended in Hank's Balanced Salt Solution (HBSS) and immune cells were counted with Cellometer auto2000 (Ozyme, Saint-Cyr-l'École, France) using propidium iodide and orange acridine. BALF cytospins were prepared by methanol fixation and May-Grünwald Giemsa staining (MGG) to allow immune cells differential count (macrophages, lymphocytes, polymorphonuclear neutrophils (PMN), polymorphonuclear eosinophils (PME), and polymorphonuclear basophils (PMB)).
Supernatants recovered from the first lavage were aliquoted and stored at −80 °C until biochemical analysis. BALF lactate dehydrogenases (LDH) and total proteins contents were assessed using commercial kits (respectively, 74048 and 74230, Siemens Healthineers, Erlangen, Germany). Absorbances were measured at, respectively, 340/410 nm for LDH and 596/694 nm for total proteins on ADVIA 1800 clinical chemistry automaton (Siemens Healthineers). BALF pro-inflammatory cytokines were quantified by ELISA assay. IL-1β, TNF-α, and IL-6 concentrations (respective LLOQ= 6 pg/mL, 16 pg/mL, and 13 pg/mL) were measured using Bio-Plex ProTM Rat kit (64178837, Bio Rad, Hercules, CA, USA). GRO/KC, INF-γ, and MIP-2 concentrations (respective LLOQ = 19.7 pg/mL, 6.2 pg/mL, and 11.3 pg/mL) were measured using Milliplex MAP Rat kit (RECYTMAG-65K, Merck, Kenilworth, NJ, USA). Measurements were performed on Bio-Plex® MAGPIXTM System (Bio Rad). 8-Isoprostane (8-iso PGF2α) levels in BALF were quantified using ELISA assay (ADI-900-010, Enzo, LLOQ= 6.1 pg/mL). Measurements were performed on Multiskan Go plate reader (Thermo Scientific, Waltham, MA, USA) at 405 nm.
Lungs histopathology
After euthanasia, rats (n = 3) were perfused with PBS then with 4% formalin. Injections of 4% formalin were also performed directly inside the lungs to inflate them. After 48 h incubation in 4% formalin, lungs were transferred to PBS and included in paraffin. Five micro-meter slices were cut from paraffin blocks with Autocut 2045 (Leica, Norcross, GA, USA) and stained with hematoxylin, phloxine, and saffron (HPS staining). Lungs histopathology study was carried out on cranial lobes of right lungs to insure reproducibility. Samples were observed under DMI6000B light microscope (Leica) with a DFC 450 C color camera (Leica).
After staining, microscopy slides were sent to Novotec R&D (Bron, France) who performed anatomo-histopathological reading and lesions scoring. Localization (alveolar, vascular or bronchial) and severity of pulmonary lesions resulting from exposures were assessed () and lesion scores were calculated individually. Lesion scores means for each experimental group are presented in arbitrary units (A.U.).
Table 1. Novotec scoring and corresponding pulmonary lesions.
8-OHDG immunofluorescence labeling in situ on rat lung
To perform lungs immunofluorescence labeling, 5 μm lungs slices were cut from paraffin blocks with Autocut 2045 (Leica). After toluene dewaxing, citrate buffer (pH 6; 10 mM; C9999, Sigma-Aldrich, St. Louis, MO, USA) incubation enable antigen unmasking. To reduce lung tissue autofluorescence (Davis et al. Citation2014), samples were stained with Sudan black (0.3%; 30 min; 199664, Sigma-Aldrich). Cells were then permeabilized with Triton X100 (0.025%; 10 min; X100, Sigma-Aldrich) and aspecific sites saturation was performed using bovine serum albumin (BSA; 6%; 1 h; A2153, Sigma-Aldrich). Lung slices were then incubated with primary antibody overnight at 4 °C (anti-8-OHdG antibody, Abcam ab48508; dilution 1/400), before shorter incubation (2 h) with secondary antibody (goat anti-mouse IgG secondary antibody, Life Technologies A11029; dilution 1/500). Finally, slides were mounted using Vectashield© Hardset containing Di Aminido Phenyl lndol (DAPI; H-1500,Vector). Lung sections without primary antibody incubation were used as negative controls to ensure specificity of observed labeling.
Statistical analysis
Results are presented as means ± standard deviations (SD) for each experimental group. Statistical analysis was performed using GraphPad Prism 7 software (GraphPad Software, La Jolla, CA). Within the same exposure scenario, comparison with the control group (air) or other experimental groups means was performed using one-way ANOVA variance tests and Tukey’s post-tests. To compare exposure scenarios for the same aerosol two-way ANOVA variance tests and Sidak’s post-tests were implemented. Two-way ANOVA variance tests and Dunnett’s post-tests were used to compare weights losses of rats to the control ones. Statistical analysis was performed using an α-risk of 0.05 and the p-value was used to assess differences significance. p-values were represented on figures using stars or circles (* or ¤ p-value < 0.05, **or ¤¤ p-value < 0.01, ***or ¤¤¤ p-value < 0.001, ****or ¤¤¤¤ p-value < 0.0001).
Results
Aerosols physico-chemical characterization
Transmission electron microscopy (TEM) observations showed micronic spherical agglomerates of Al2O3NPs from bulk powder (). To characterize alumina crystalline structure, XRD experiments were performed on bulk powder. Results confirmed majority of γ and δ polymorphs as indicated by the supplier ().
Figure 2. Bulk powder and aerosols alumina nanoparticles physico-chemical characterization. Transmission electron microscopy (TEM) images of Al2O3 NPs in bulk powder (A) and generated aerosol (C) (respective scale bars = 200 nm and 500 nm). Crystalline phases (γ and δ) identification using X-ray diffraction (XRD) on bulk Al2O3 NPs (B). NPs elementary chemical composition (in %) using energy-dispersive X-ray (EDX) spectroscopy on Al2O3 NPs aggregates retrieved in the generated aerosol (D). Table E summarizes Al2O3 NPs representative particles size distribution in the generated aerosols measuring using Sioutas cascade impactor (mg/m3 for each size class in μm).
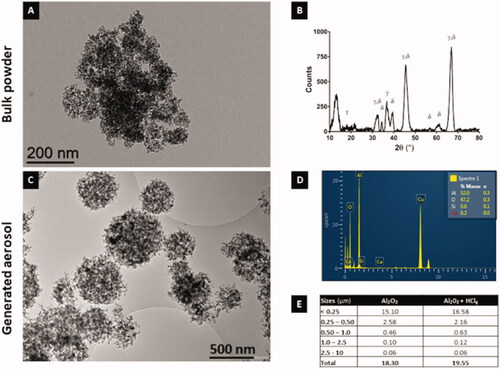
Once nebulized, Al2O3 NPs formed spherical agglomerates (). The presence of HClg did not modify the shape of agglomerates in aerosols (data not shown). Energy dispersive X-ray (EDX) analysis confirmed aluminum and oxygen atoms presence without chemical impurities. The copper spike on EDX spectra corresponds to TEM grids used for aerosol sampling (). Results obtained with Sioutas (SKC Inc., Seoul, South Korea) cascade impactor revealed that a majority of particles in the agglomerates in generated aerosols had a size below 250 nm (). Mean aerodynamic diameters of 383.07 nm and 343.59 nm were, respectively, calculated for Al2O3 NPs aerosol and for the mixture aerosol. Based on these data, the presence of HClg did not seem to modify particles’ concentrations and size distribution over 250 nm ().
To deepen and complete these analytical data, the content of aerosol particles was assessed using Al quantification (ICP-OES) after filter samplings. Mean Al2O3 concentrations of 22.1 ± 4.5 mg/m3 and 20.0 ± 2.9 mg/m3 were, respectively, measured for aerosols containing Al2O3 NPs and Al2O3 NPs associated with HClg (). Therefore, we confirmed that the addition of HClg to aerosol did not significantly modify NPs concentrations. HClg concentrations in generated aerosols were estimated through the measurements of chlorine (Cl−) levels carried out on filters (particulate fraction) and bubbler (free fraction) samples. Results showed 10 times smaller Cl− levels than target concentrations (5 ppm = 7.4 mg/m3). The addition of Al2O3 NPs to the HClg aerosol did not seemed to modify significantly Cl− levels but this statement should be taken cautiously due to the variability in the chloride measurements. However, it has to be noted that in the presence of Al2O3 NPs, Cl− in the aerosols was exclusively found in particulate fraction whereas it was exclusively in free fraction when alone (). Moreover, variations in Cl− concentrations were noted when HClg was alone, with concentrations ranging from 0 to 2145 µg/m3 (). These variations were not observed in presence of Al2O3 NPs.
Table 2. Generated aerosols alumina and HClg contents.
Lungs aluminum content increasing after repeated exposures
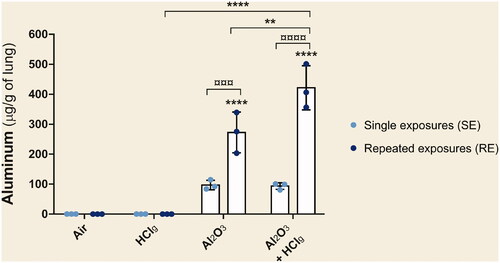
Pulmonary aluminum load was measured by ICP-OES after aerosols inhalation. Following single exposures (SE) to Al2O3 NPs alone or associated with HClg, concentrations around 100 μg of aluminum per g of lung were measured (). However, these mean quantities were not significantly different from air exposed animals. Repeated exposures (RE) to Al2O3 NPs alone or in association with HClg-induced significant aluminum quantities increases in lungs compared to air exposed rats or to those exposed to the same conditions in SE (). RE to Al2O3 NPs with HClg significantly increased pulmonary aluminum load compared to repeated exposures to each component alone ().
Figure 3. Pulmonary aluminum deposition after nose-only exposures. Rats were exposed to air, HClg, Al2O3 or to the mixture Al2O3 + HClg. Clear blue points symbolize single exposures (SE) and dark blue points symbolize repeated exposures (RE) to each aerosol. Mean aluminum quantities (μg/g of lung) ± SD were measured in lungs 24 h after the last exposure using inductively coupled plasma optical emission spectrometry (ICP-OES). One-way ANOVA and Tukey’s post-test (n = 3, ** p-value < 0.01, **** p-value < 0.0001, α risk = 0.05) were performed to compare each experimental condition to the control within the same exposure scenario and Two-way ANOVA and Sidak’s post-test (n = 3, ¤¤¤p-value < 0.001, ¤¤¤¤ p-value < 0.0001, α risk = 0.05) were performed to compare the two exposure scenarios for the same aerosol.
Clinical symptoms and body weight
Animal clinical follow-up did not highlight any treatment-related clinical symptom following exposures. Moreover, even if exposures (including air) induced significant animals weight losses, no biologically relevant change in body weight were observed after SE () and RE (). Indeed, these weight decreases were always less than 10% of rats’ initial weight (data not shown) and no particular trend was noted for the different experimental groups ().
Table 3. Rats body weight changes during the experimental period.
Alveolar–capillary barrier and pulmonary inflammation
No change in total proteins or lactate dehydrogenases (LDH) concentrations was observed following SE compared to air exposed rats (). Only RE to Al2O3 NPs alone or associated to HClg induced significant concentrations increases of total proteins and LDH in rats BALF compared to air exposed control animals (). Total protein concentrations of 0.18 ± 0.03 g/L and 0.22 ± 0.04 g/L and LDH concentrations of 99 ± 43 U/L and 112 ± 42 U/L were measured in rats BALF following, respectively, RE to Al2O3 NPs and toAl2O3 NPs + HClg.
Figure 4. Total proteins, LDH and immune cells populations in bronchoalveolar lavage fluids (BALF). Rats were exposed to air, HClg, Al2O3 or to the mixture Al2O3 + HClg. Clear blue points symbolize single exposures (SE) and dark blue points symbolize repeated exposures (RE) to each aerosol. Total proteins (g/L) (A) and lactate dehydrogenases (LDH, U/L) (B) concentrations were measured in rats BALF 24 h after the last exposure. Total cells, polymorphonuclear neutrophils (PMN), macrophages and lymphocytes (cells × 106) were counted on BALF cytospins (respectively C, D, E, and F). Results are expressed as mean values ± SD. One-way ANOVA and Tukey’s post-test (n = 6, * p-value < 0.05, ** p-value < 0.01, *** p-value < 0.001, **** p-value < 0.0001, α risk = 0.05) were performed to compare each experimental condition to the control within the same exposure scenario and two-way ANOVA and Sidak’s post-test (n = 6, ¤ p-value < 0.05, ¤¤ p-value < 0.01, ¤¤¤ p-value < 0.001, ¤¤¤¤ p-value < 0.0001, α risk = 0.05) were performed to compare the two exposure scenarios for the same aerosol.
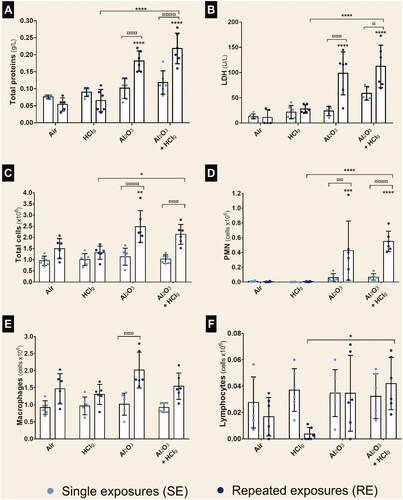
The number of total cells was increased in rats BALF after RE to Al2O3 NPs. Moreover, significantly higher total cell numbers were counted following RE than SE to Al2O3 NPs and to Al2O3 NPs + HClg aerosols (). RE to the same aerosols also induced significant polymorphonuclear neutrophils (PMN) number increases in BALF compared to air exposed control animals (). In contrast, no significant change in macrophages and lymphocytes numbers was highlighted by the carried out counts ().
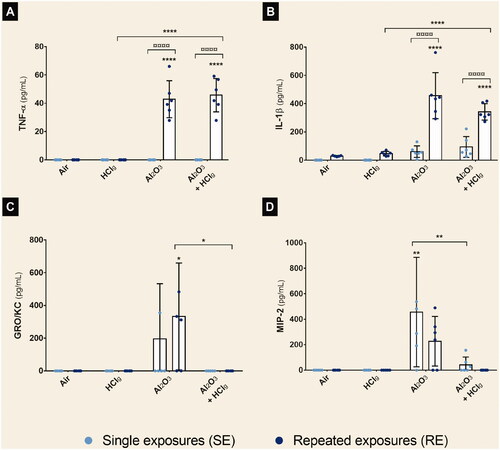
RE to Al2O3 NPs or Al2O3 NPs + HClg aerosols led to significant TNF-α and IL-1β concentration increases in rats BALF (). TNF-α concentrations of 42.86 ± 13.07 pg/mL and 45.73 ± 11.77 pg/mL, and IL-1β concentrations of 456.2 ± 163.2 pg/mL and 341.6 ± 58.11 pg/mL were, respectively, measured. Significant increased concentrations of GRO/KC and MIP-2 were also measured in rats BALF after, respectively, RE to Al2O3 NPs () and SE to Al2O3 NPs () compared to air exposed control rats.
Figure 5. Aerosols pro-inflammatory potential on rat lungs after nose-only inhalations. Rats were exposed to air, HClg, Al2O3 or to the mixture Al2O3 + HClg. Clear blue points symbolize single exposures (SE) and dark blue points symbolize repeated exposures (RE) to each aerosol. Pro-inflammatory cytokines concentrations (pg/mL) of TNF-α (A), IL-1β (B), GRO/KC (C) and MIP-2 (D) were measured in rats BALF 24 h after the last exposure by ELISA multiplex. Results are expressed as mean values ± SD. One-way ANOVA and Tukey’s post-test (n = 6, * p-value < 0.05, ** p-value < 0.01, **** p-value < 0.0001, α risk = 0.05) were performed to compare each experimental condition to the control within the same exposure scenario and two-way ANOVA and Sidak’s post-test (n = 6, ¤¤¤¤ p-value < 0.0001, α risk = 0.05) were performed to compare the two exposure scenarios for the same aerosol.
Histopathology observations
Lung parenchyma observations revealed the presence of pathological lesions following experimental aerosols inhalation (). However, no peribronchial or perivascular fibrosis, resorption hyperplasia (phagocytosis phenomena), or pleura inflammation was reported in this study. Lesions scoring (Novotec R&D, Cincinnati, OH, USA) was performed in order to compare the different experimental aerosols and exposure scenarios’ effects (). No statistical analysis was performed on these data as groups were composed of only three animals and scores are not a measured biological data (values estimated from observations and without unit). A table with scoring details including lesions localizations is provided as Supplementary data.
Figure 6. Pulmonary histopathological lesions observations following inhalation exposures. Rats lung parenchyma stained in HPS were observed in optical microscopy for histopathological analysis. Images show, respectively, lung parenchyma 24 h after single exposure (SE) to air (A), HClg (C), Al2O3 (E) and Al2O3 + HClg (G) and 24 h after repeated exposures (RE) to air (B), HClg (D), Al2O3 (F) and Al2O3 + HClg (H). Scale bar = 200 μm. Pictures legends: a = alveoli; b = bronchioles or bronchi; v = blood vessel; c = vascular congestion; blue arrows = vascular edema; red arrows = bronchial (pre)-exfoliation; i = inflammatory area; black squares = velamentous epithelium and black circles = interalveolar septum edema.
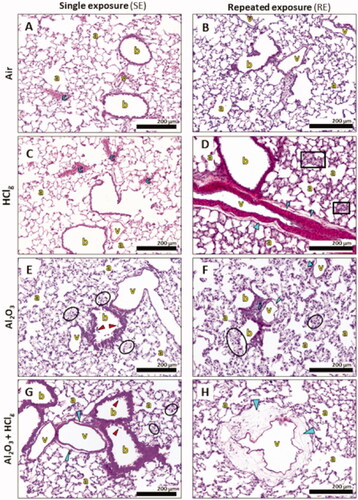
Figure 7. Pulmonary histopathological lesions scoring. Rats were exposed to air, HClg, Al2O3 or to the mixture Al2O3 + HClg. Scoring was performed on HPS stained lungs slices of three rats of each group. Lesion scores (mean ± SD, n = 3) were estimated for each experimental aerosol and exposure scenario based on the presence and the gravity of vascular, alveolar and bronchial lesions observed in rats lungs 24 h after the last exposure. Empty and striped bars represent, respectively, single exposures (SE) and repeated exposures (RE) results.
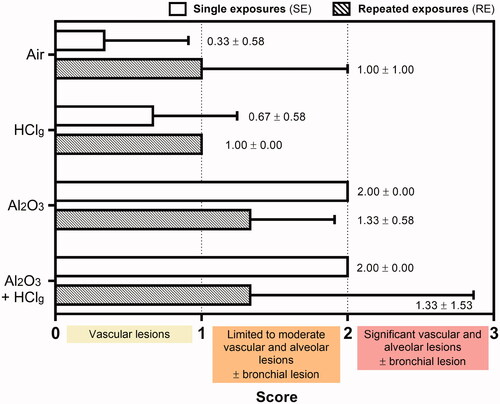
In the lungs of control rats, vascular congestions were occasionally found and considered as physiological lesions (histological alteration which would have no functional consequence and which can be observed in normal tissue, ). HClg exposures mainly induced vascular lesions. SE increased vascular congestions (), while RE induced vascular edema and, in some cases, velamentous epithelium, pneumocytes edema, and moderate walls thickening were observed in the alveolar regions (). Al2O3NPs inhalation mainly induced alveolar interstitial and inflammatory lesions (). Indeed, marked interalveolar septum edemas were found within rats pulmonary epithelium after SE and RE. Slight bronchial pre-exfoliation phenomena () and vascular edemas () were also observed. In some cases, localized inflammatory areas, composed of polymorphonuclear cells and lymphocytes, were also found in rats’ lungs (). Co-exposures to Al2O3 NPs and HClg induced vascular and bronchial interstitial lesions at a time (). Marked bronchial pre-exfoliation phenomena (), vascular, and interalveolar septum edemas () were found in rats’ lungs after SE. RE to the same aerosol mainly induce vascular edemas and interalveolar septum edemas were observed in some animals but no bronchial lesions was reported (). Moreover, in one animal, repeatedly exposed to mixture pre-pulmonary edema stage was observed (significant vascular edema, dilated capillaries, and incipient alveolar septa rupture). Therefore, higher mean lesion scores were calculated in animals exposed to aerosols containing Al2O3 NPs alone or associated with HClg ().
In conclusion, results did not suggest major influence of exposure scenario toward histopathological lesions induction as close scores were calculated for SE and RE to the same aerosol. The presence of Al2O3 NPs in the aerosol induced lung irritation increasing lesion score, but adding HClg to Al2O3 NPs aerosol did not seem to worsen lesions comparing to those resulting from Al2O3 NPs inhalation alone. An exception was noted for SE as the addition of HClg to Al2O3 NPs aerosol lead to bronchial lesion worsening.
Oxidative stress analysis
To assess oxidative stress induction following experimental aerosols exposures, 8-OHdG (8-hydroxy-2′-deoxyguanosine, oxidative nucleic acids’ damages marker) was labeled in situ in the lung tissue of the rats using immunofluorescence. Microscopic observations revealed 8-OHdG fluorescent signal after SE (), whereas no specific signal was observed after RE to the different aerosols. After SE to the experimental aerosols, cytoplasmic and nuclear labeling were found both in alveolar () and bronchial epithelia (). Aerosols containing Al2O3 NPs ± HClg seemed to induce more labeling on bronchial regions than on alveolar regions whereas HClg alone lead to both bronchial and alveolar labeling. However, no quantitative analysis was performed to compare labeling in the two regions. These labeling localizations referred to RNA (cytoplasmic) and DNA (nuclear) oxidations.
Figure 8. Oxidative stress induction by experimental aerosols inhalation exposures. Immunofluorescence was performed to detect 8-OHdG (8-hydroxy-2'-deoxyguanosine, green fluorescence) on rat lungs slices 24 h after the last exposure. DNA was stained by DAPI, which emits in blue fluorescence. Pictures represent obtained results following single exposures (SE) to air (A), HClg (B), Al2O3 (C) and Al2O3 + HClg (D). Scale bars = 50 μm. Pictures legends: a = alveoli; b = bronchioles or bronchi. Red blood cells could also emit in green fluorescence, but red arrows and red circles point out specific 8-OHdG staining. 8-Isoprostane concentrations (pg/mL) were measured in rats BALF 24 h after the last exposure (E). Clear blue points symbolize single exposures (SE) and dark blue points symbolize repeated exposures (RE) to each aerosol. Results are expressed as mean values ± SD. One-way ANOVA and Tukey’s post-test (n = 3, * p-value < 0.05, ** p-value < 0.01, **** p-value < 0.0001, α risk = 0.05) were performed to compare each experimental condition to the control within the same exposure scenario and two-way ANOVA and Sidak’s post-test (n = 3, α risk = 0.05) were performed to compare the two exposure scenarios for the same aerosol.
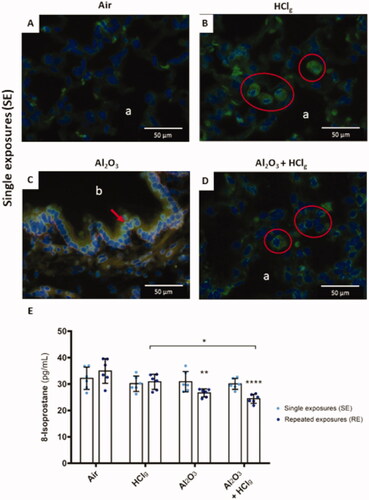
To complete our investigations on oxidative stress induced by inhalations, 8-isoprostane (lipid peroxidation marker) concentrations were measured in rats BALF 24 h after the last exposure end. Results did not reveal any significant increase in 8-isoprostane concentrations following inhalations (). Significant 8-isoprostane concentration decreases were measured after RE to Al2O3 NPs and to Al2O3 NPs + HClg compared to control, with respective mean values of 26.6 ± 1.5 pg/mL and 24.4 ± 1.7 pg/mL versus 34.9 ± 4.7 pg/mL for the control group ().
Discussion
A combined study of gas and particles toxicity is an innovative concept. In this pilot study, nose-only inhalation system was selected to ensure better control of the inhaled dose. Only one dose of HClg and Al2O3 NPs could be assessed. In the case of Al2O3 NPs, a high dose was used (10 g/L suspension leading to 20.0–22.1 mg/m3 aerosol) to ensure biological response following inhalation in order to identify toxic effects.
However, this choice could have masked subtle HClg effects on Al2O3 NPs when mixed together. Indeed, observed effect in this study could mainly be due to the delivered Al2O3 NPs load to the lungs, especially since HClg measured concentrations in the experimental aerosols were lower than expected (617–791 µg/m3 versus 5 ppm = 7.4 mg/m3). This difference and the experimental variability in chlorides dosing repetitions could be explained by both HClg high reactivity with water, leading to HClg dissolution in water drops on the exposure system walls, and bubblers saturation by other compounds, sometimes causing a non-optimal chlorides trapping. Moreover, in our study, Al2O3 NPs were nebulized from a NPs suspension in water. This method is different from combustion process in a solid composite propellant and could promote aggregates formation. We can, therefore, assume that animals were exposed to particles aggregates with different size distributions than those which could be found on the field.
Specific physico-chemical characterization is a mandatory step to better understand the biological effects of mixture aerosols. Their characterization revealed the presence of spherical Al2O3 NPs agglomerates with respective mean aerodynamic diameters of 383 nm for Al2O3 NPs alone and of 344 nm for mixtures. Al2O3 NPs concentrations in aerosols were high and comprised between 20.0 mg/m3 and 22.1 mg/m3. Indeed, Paur and colleagues reported in their literature review that 5 mg/m3 could be considered as an upper limit for workplace NPs mass concentration, according to Occupational Safety and Health Administration (OSHA) standard for respirable nuisance dust (Paur et al. Citation2011). Based on this value, they estimated a mass of NPs per surface area deposition in human lungs of 5 × 10−3 μg/cm2/h, which would correspond in our exposure scenarios to theoretical particles deposition in human lungs of 0.02 μg/cm2 (5 × 10−3 × 4) after SE and of 0.08 μg/cm2 (5 × 10−3 × 16) after RE. Considering alveolar surface of 4000 cm2 in rats, exposures to NPs aerosols led to higher measured pulmonary depositions. Indeed, for Al2O3 NPs exposures, values of 0.04 μg/cm2 and 0.13 μg/cm2 were, respectively, obtained for SE and RE, while concentrations for co-exposures with HClg were, respectively, 0.03 μg/cm2 and 0.18 μg/cm2. Therefore, these experimental values are higher than theoretical values but consistent with aerosols high NPs concentrations. Even if we observed high NPs pulmonary depositions, these scenarios are relevant in a context of accidental exposures where specific workers (e.g. military or aerospace industry personnels) could be exposed to combustion aerosols.
To quantify Al2O3 NPs biodistribution in lungs after exposures, aluminum concentrations were measured using ICP-OES. Obtained values were approximately four times higher in lungs after RE compared to SE. These results are consistent with exposure scenarios as RE correspond to four repetitions of SE. NPs accumulated in lungs after exposures and were still retained by lungs after 100 h in the case of RE, suggesting absence or slow clearance mechanisms early after Al2O3 NPs aerosols inhalation. However, in this study, rats were euthanized 24 h after the last exposure bringing no information about long-term Al2O3 NPs clearance. This result seems consistent with a study carried out on Sprague–Dawley rats by intratracheal instillations, which concluded to a very slow clearance, gradual accumulation, and pulmonary retention of alumina particles 19 weeks after repeated exposures (Schlesinger et al. Citation2000). Moreover, aluminum concentration was significantly increased in the case of co-exposure to Al2O3 NPs with HClg compared to Al2O3 NPs aerosols, while NPs concentration and size distribution were not modified. Therefore, we hypothesized that acidification of mixture aerosols may promote Al2O3 NPs dissolution (Franke et al. Citation1987), increasing aluminum pulmonary bioavailability. Indeed, in aqueous environment, Al2O3 NPs could hydrated and react with hydrogen ions to form water and aluminum ions as follow: Al2O3 + 6 H + → 2 Al 3+ + 3 H2O (Diggle et al. Citation1970; Franke et al. Citation1987). Particles overload, especially after RE, and HClg local effect in lungs could also have both contribute to hinder particles lungs clearance as previously shown in different studies (Lippmann et al. Citation1980; Pritchard Citation1989).
Pro-inflammatory properties of Al2O3 particles have already been reported in literature following inhalation (Sikkeland et al. Citation2016; Li et al. Citation2017; Kim et al. Citation2018). To date and to our knowledge, no literature data were found concerning the potential inflammatory response induced by HClg alone or Al2O3 NPs/HClg inhalation exposures. Therefore, we studied pro-inflammatory biomarkers following Al2O3 NPs, HClg or Al2O3 NPs/HClg mixtures nose-only exposures. Different secretion profiles were observed for each cytokine, depending on aerosol composition and exposure scenario. No increase of immune cells or cytokines concentrations was measured in animals BALF exposed to air or HClg. Our results showed early inflammation triggered by RE to aerosols containing Al2O3 NPs with or without HClg. Indeed, significant amount of alveolar macrophages and PMN were measured in BALF with increased secretion of IL-1β, TNF-α, and GRO/KC (except for Al2O3 NPs and HClg mixture). These results corroborate with histopathological observations with mainly interstitial and inflammatory lesions (localized areas composed of PMN and lymphocytes) in the case of Al2O3 NPs inhalation. Therefore, RE induced more potent pro-inflammatory response compared to SE. Increased cytokine concentrations observed in BALF also corroborate with PMN influx as they have chemoattractant properties (Huang et al. Citation1992; Toews Citation2001; Kim et al. Citation2018). IL-1β and TNF-α seem to orchestrate the pulmonary pro-inflammatory response. On the one hand, it has been described that Al2O3 NPs could stimulate the nuclear factor NFκB pathway (Simon-Vazquez et al. Citation2016). IL-1β concentration increases could thus be associated with this hypothesis, suggesting the inflammasome pathway involvement in the pulmonary pro-inflammatory response initiated by Al2O3 NPs. Indeed, the nuclear factor NFκB can contribute to the inflammasome regulation (Liu et al. Citation2017) which is involved in IL-1β synthesis (Lamkanfi and Dixit Citation2014). This conjecture is reinforced by the fact that this pathway is also known to be activated by pulmonary inflammation in patients with chronic obstructive pulmonary disease (Barnes Citation2016). On the other hand, Al2O3 NPs can also induce TNF-α release in BALF. This result corroborate with the study of Kim et al. which showed neutrophils, LDH, TNF-α, and IL-6 increased concentrations in BALF after Al2O3 NPs nose-only exposure of rats for 28 days (5 days/week) (Kim et al. Citation2018). TNF-α secretion after exposure to Al2O3 NPs may be linked to IL-1β release. Indeed, in a context of acute pulmonary inflammation, Saperstein and colleagues demonstrated that IL-1β contributes, in part, to TNF-α-mediated chemokine release, and neutrophil recruitment to the lung (Saperstein et al. Citation2009). Besides, in our study, GRO/KC and MIP-2 secretion in BALF were only measured after Al2O3 NPs exposure. These two cytokines possess neutrophils chemoattractant properties (Tanino et al. Citation2010). We hypothesized that co-exposure to Al2O3 NPs/HClg mixtures could modify their kinetic of secretions in BALF. A significant increase of MIP-2 concentrations was found in BALF only after SE to Al2O3 NPs. As a consequence, MIP-2 may act as an early step contributing to PMN recruitment. Furthermore, Pirela and colleagues also demonstrated down-regulation of GRO/KC and MIP-2 in the nasal lavage fluid after repeated whole-body inhalation exposure to printer-emitted engineered NPs in rats (containing aluminum oxide) (Pirela et al. Citation2019). Otherwise, IL-6 and INF-γ were not detected in BALF after SE and RE to the different aerosols of the study (respective detection limits: 13 pg/mL and 6.2 pg/mL). INF-γ promotes pulmonary inflammation through oxidative stress induction (Yin et al. Citation1999) and could have been secreted earlier than 24 h post-exposure to the aerosols, inducing nucleic acids oxidative damages (8-OHdG), which were still detected at that time. Therefore, we hypothesized that aluminum oxide-based nano-objects are notably able to induce secretion of different cytokine secretion profiles in BALF depending on their shape, time of exposure, and used inhalation system. Given the sensitizing properties of aluminum and HClg (Darmer et al. Citation1974; Cohen Citation2004; Zhu et al. Citation2013; Kongerud and Soyseth Citation2014), studies on immune effects could also be considered later (allergic response and other immune-related cytokines).
As oxidative stress is a physiological mechanism closely linked to pulmonary inflammation induced by NPs exposure, and indirectly to the increase of PMN number potentially releasing free radicals in the alveoli, this endpoint was investigated (Lugrin et al. Citation2014; Salzano et al. Citation2014; Khanna et al. Citation2015; Biswas Citation2016). Nucleic acids oxidation (8-OHdG) were only detected in situ following SE to HClg, Al2O3 NPs or mixture exposures while no increase of lipid peroxidation was detected in BALF (8-isoprostane). The absence of 8-OHdG following RE could be explained by lesions repairing systems induction after 24 h which would limit their later appearance and persistence over time. The main mechanism implied in these oxidative damages repair is base excision repair (Whitaker et al. Citation2017), but other intracellular actors such as p53 protein (Silva et al. Citation2014) or PARP-1 enzyme (Qin et al. Citation2008) may also contribute to damages repair. As previously shown, NPs inhalation could also initiate increased production of enzymes such as endonuclease III homolog 1 (NTH1) or apurinic/apyrimidinic endonuclease 1 (APE1) (Ma et al. Citation2013), p53 (Silva et al. Citation2014), or poly [ADP-ribose] polymerase 1 (PARP-1) (Qin et al. Citation2008), involved in various repair mechanisms and consequently limit side effects on nucleic acids. Lack of lipid peroxidation, assessed by 8-isoprostane dosing, in BALF might be due to the activation of the antioxidant response following exposures. Significant decreases of 8-isoprostane after RE to Al2O3 NPs or mixtures may also be due to changes of antioxidant balance. It would be interesting to perform glutathione peroxidase dosing in BALF in further studies to assess these antioxidant mechanisms (Romero et al. Citation1998). Moreover, reverse transcriptase polymerase chain reaction (RT-PCR) study of several antioxidant enzyme genes, such as superoxide dismutase 1 (SOD1), heme oxygenase-1 (Ho-1), or glutathione peroxidase1 (Gpx1), could also be performed to confirm this hypothesis (Constantino et al. Citation2014; Liu et al. Citation2017). In complement, western Blot analysis and immunohistochemistry could be performed to assess other repairing mechanisms such as PARP-1, p53, APE1, and NTH1 (Qin et al. Citation2008; Ma et al. Citation2013; Silva et al. Citation2014).
As obtained results highlighted Al2O3 NPs lung retention 24 h after aerosols exposures, pro-inflammatory, and oxidative stress induction, the aerosols effect on alveolar–capillary barrier permeability was also studied. Significant elevations of LDH and total protein concentrations were measured in BALF only after RE to Al2O3 NPs and mixture aerosols containing HClg, indicating impairment of alveolar–capillary barrier permeability and cell cytolysis. These biological effects could be linked to pro-inflammatory mechanisms and PMN influx observed in lungs following RE to Al2O3 NPs alone or combined with HClg. Indeed, as previously demonstrated, PMN recruitment and pulmonary inflammation trigger alveolar–capillary barrier permeabilization (Williams and Chambers Citation2014; Wittekindt Citation2017). Alveolar–capillary barrier permeabilization could promote translocation of inhaled particles into bloodstream leading to potential systemic effects or local toxicity on other organs such as the brain, the liver, the spleen, or kidneys. A study is currently performed on these organs to assess local histopathological effects induced by aerosols inhalation.
Conclusion
In conclusion, inhalation exposure to Al2O3 NPs with or without HClg aerosols induce early pulmonary effects (24 h post-exposure) resulting in inflammation, oxidative stress, and alveolar–capillary barrier permeabilization. No synergistic or additive effect between Al2O3 NPs and HClg was observed on studied endpoints except for lung histopathology lesions nature (mainly vascular lesions observed with HClg, interstitial lesions observed with Al2O3 NPs and both observed after co-exposure). Obtained results underline that the exposure scenario mainly influences toxicological effects of mixtures. Al2O3 NPs presence in the aerosols also seems to play an essential role in driving toxicity (pro-inflammatory response, PMN, LDH and total protein increases, histopathological lesions and oxidative stress induction) compared to HClg (slight histopathological lesions and oxidative stress). Despite an increased pulmonary aluminum biodistribution in the case of RE to mixtures compared to Al2O3 NPs, similar pro-inflammatory responses were observed in the two conditions. SE induce oxidative stress resulting in nucleic oxidative damages, while RE essentially promote pulmonary inflammation. Special attention should be paid to pulmonary pro-inflammatory and oxidative stress induction as these phenomena are known to be closely linked to long-term pathologies such as pulmonary fibrosis or cancers (Reuter et al. Citation2010; Sesti et al. Citation2012; Khanna et al. Citation2014). Therefore, this pilot study raises concerns toward potential long-term pulmonary toxicity of combustion mixture aerosols. Further studies will have to be led to determine the potential toxicity of the mixture at longer times and lower doses. The latter information would then help to define dose limitations and exposure thresholds for workplace risk management. At the same time, better characterization of personnel exposure is still necessary, especially for military personnel who can be exposed due to operational constraints.
Ethics approval and consent to participate
In vivo experiments were approved by the “Comité Régional d’Ethique en Matière d’Expérimentation Animale” (CREMEAP) (C2EA-96).
Acknowledgments
The authors would like to thank the LASEM and Jean-Ulrich Mullot (LASEM, Toulon, FR) for aluminum ICP-OES dosing in lungs. They are indebted to Armel Descamps (UMS Castaing, Toulouse, FR) for TEM analyses and to Benjamin Duployer (CIRIMAT, Université de Toulouse, CNRS, Toulouse, FR) for Al2O3 NPs crystal structure determination. They acknowledge David Crouzier (IRBA, Brétigny-sur-Orge, FR) for his involvement in this project initiation. The authors also gratefully thank Marco Valente (IRBA, Brétigny-sur-Orge, FR), Claire Le Galliot (DGA, Paris, FR) and Noël Perrier (DGA, Paris, FR) for their reviewing and constructive feedback which helped to improve the manuscript.
Disclosure statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Funding
Notes
1 LLOQ: lower limit of quantification.
References
- Balasubramanyam A, Sailaja N, Mahboob M, Rahman MF, Hussain SM, Grover P. 2009. In vivo genotoxicity assessment of aluminium oxide nanomaterials in rat peripheral blood cells using the comet assay and micronucleus test. Mutagenesis. 24:245–251.
- Barnes PJ. 2016. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 138(1):16–27.
- Biswas SK. 2016. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid Med Cell Longev. 2016:5698931.
- Carpizo DR, Reaka AJ, Glaws WR, Pooley N, Schmidt L, Halline AG, Goldstein JL, Layden TJ. 1998. Acute acid exposure increases rabbit esophageal cell proliferation. J Lab Clin Med. 131(2):157–162.
- Chen L, Yokel RA, Hennig B, Toborek M. 2008. Manufactured aluminum oxide nanoparticles decrease expression of tight junction proteins in brain vasculature. J Neuroimmune Pharmacol. 3(4):286–295.
- Cheng L, Cao W, Behar J, Fiocchi C, Biancani P, Harnett KM. 2006. Acid-induced release of platelet-activating factor by human esophageal mucosa induces inflammatory mediators in circular smooth muscle. J Pharmacol Exp Ther. 319(1):117–126.
- Cheng L, Cao W, Fiocchi C, Behar J, Biancani P, Harnett KM. 2006. HCl-induced inflammatory mediators in cat esophageal mucosa and inflammatory mediators in esophageal circular muscle in an in vitro model of esophagitis. Am J Physiol Gastrointest Liver Physiol. 290(6):G1307–G1317.
- Chivas-Joly C, Gaie-Levrel F, Motzkus C, Ducourtieux S, Delvallée A, De Lagos F, Nevé SL, Gutierrez J, Lopez-Cuesta J-M. 2016. Characterization of aerosols and fibers emitted from composite materials combustion. J Hazard Mater. 301:153–162.
- Cho W-S, Duffin R, Thielbeer F, Bradley M, Megson IL, MacNee W, Poland CA, Tran CL, Donaldson K. 2012. Zeta potential and solubility to toxic ions as mechanisms of lung inflammation caused by metal/metal oxide nanoparticles. Toxicol Sci. 126(2):469–477.
- Cohen MD. 2004. Pulmonary immunotoxicology of select metals: aluminum, arsenic, cadmium, chromium, copper, manganese, nickel, vanadium, and zinc. J Immunotoxicol. 1(1):39–69.
- Constantino L, Goncalves RC, Giombelli VR, Tomasi CD, Vuolo F, Kist LW, de Oliveira GM, Pasquali MA, Bogo MR, Mauad T, et al. 2014. Regulation of lung oxidative damage by endogenous superoxide dismutase in sepsis. Intensive Care Med Exp. 2(1):17.
- Darmer KI Jr, Kinkead ER, DiPasquale LC. 1974. Acute toxicity in rats and mice exposed to hydrogen chloride gas and aerosols. Am Ind Hyg Assoc J. 35(10):623–631.
- Davis AS, Richter A, Becker S, Moyer JE, Sandouk A, Skinner J, Taubenberger JK. 2014. Characterizing and diminishing autofluorescence in formalin-fixed paraffin-embedded human respiratory tissue. J Histochem Cytochem. 62(6):405–423.
- Dawe KER, Furlani TC, Kowal SF, Kahan TF, VandenBoer TC, Young CJ. 2019. Formation and emission of hydrogen chloride in indoor air. Indoor Air. 29(1):70–78.
- Diggle JW, Downie TC, Goulding CW. 1970. The dissolution of porous oxide films on aluminium. Electrochim Acta. 15:1079–1093.
- Dyer RF, Esch VH. 1976. Polyvinyl chloride toxicity in fires. Hydrogen chloride toxicity in fire fighters. JAMA. 235(4):393–397.
- Franke MD, Ernst WR, Myerson AS. 1987. Kinetics of dissolution of alumina in acidic solution. Alche J. 33(2):267–273.
- Galfetti L, Severini F, DeLuca L, Marra G, Meda L, Braglia R. 2004. Ballistics and condensed residues of aluminized solid rocket propellants. 9-IWCP "Novel Energetic Materials and Applications". Lerici, SP, Italy.
- Hayes AW, Li R, Hoeng J, Iskandar A, Peistch MC, Dourson ML. 2019. New approaches to risk assessment of chemical mixtures. Toxicol Res Appl. 3(6):239784731882076
- Huang S, Paulauskis JD, Godleski JJ, Kobzik L. 1992. Expression of macrophage inflammatory protein-2 and KC mRNA in pulmonary inflammation. Am J Pathol. 141(4):981–988.
- Kaplan HL, Anzueto A, Switzer WG, Hinderer RK. 1988. Effects of hydrogen chloride on respiratory response and pulmonary function of the baboon. J Toxicol Environ Health. 23(4):473–493.
- Kellum JA, Song M, Li J. 2004. Lactic and hydrochloric acids induce different patterns of inflammatory response in LPS-stimulated RAW 264.7 cells. Am J Physiol Regul Integr Comp Physiol. 286(4):R686–R692.
- Khanna H, Karki K, Pande D, Negi R, Khanna R. 2014. Inflammation, free radical damage, oxidative stress and cancer. Interdiscip J Microinflammation. 1(1):109.
- Khanna P, Ong C, Bay BH, Baeg GH. 2015. Nanotoxicity: an interplay of oxidative stress, inflammation and cell death. Nanomaterials (Basel). 5(3):1163–1180.
- Kim YS, Chung YH, Seo DS, Choi HS, Lim CH. 2018. Twenty-eight-day repeated inhalation toxicity study of aluminum oxide nanoparticles in male Sprague–Dawley rats. Toxicol Res. 34(4):343–354.
- Kongerud J, Soyseth V. 2014. Respiratory disorders in aluminum smelter workers. J Occup Environ Med. 56(5 Suppl):S60–S70.
- Krewski D, Yokel RA, Nieboer E, Borchelt D, Cohen J, Harry J, Kacew S, Lindsay J, Mahfouz AM, Rondeau V. 2007. Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. J Toxicol Environ Health B Crit Rev. 10(Suppl 1):1–269.
- Kwon JT, Seo GB, Lee MJ, Kim HM, Shim I, Jo E, Kim P, Choi K. 2013. Pulmonary toxicity assessment of aluminum oxide nanoparticles via nasal instillation exposure. J Environ Health Sci. 39:48–55.
- Lamkanfi M, Dixit VM. 2014. Mechanisms and functions of inflammasomes. Cell. 157(5):1013–1022.
- Li X, Yang H, Wu S, Meng Q, Sun H, Lu R, Cui J, Zheng Y, Chen W, Zhang R, et al. 2017. Suppression of PTPN6 exacerbates aluminum oxide nanoparticle-induced COPD-like lesions in mice through activation of STAT pathway. Part Fibre Toxicol. 14(1):53.
- Lippmann M, Yeates DB, Albert RE. 1980. Deposition, retention, and clearance of inhaled particles. Br J Ind Med. 37(4):337–362.
- Liu Y, Lu F, Kang L, Wang Z, Wang Y. 2017. Pirfenidone attenuates bleomycin-induced pulmonary fibrosis in mice by regulating Nrf2/Bach1 equilibrium. BMC Pulm Med. 17(1):63.
- Liu T, Zhang L, Joo D, Sun SC. 2017. NF-kappaB signaling in inflammation. Signal Transduct Target Ther. 2: 17023.
- Lugrin J, Rosenblatt-Velin N, Parapanov R, Liaudet L. 2014. The role of oxidative stress during inflammatory processes. Biol Chem. 395(2):203–230.
- Ma J, Altomare A, de la Monte S, Tong M, Rieder F, Fiocchi C, Behar J, Shindou H, Biancani P, Harnett KM. 2010. HCl-induced inflammatory mediators in esophageal mucosa increase migration and production of H2O2 by peripheral blood leukocytes. Am J Physiol Gastrointest Liver Physiol. 299:G791–G798.
- Machle W, Kitzmiller KV, Scott EW, Treon JF. 1942. The effect of the inhalation of hydrogen chloride. J Ind Hyg Toxicol. 24:222–225.
- Ma H, Wang J, Abdel-Rahman SZ, Boor PJ, Khan MF. 2013. Induction of base excision repair enzymes NTH1 and APE1 in rat spleen following aniline exposure. Toxicol Appl Pharmacol. 267(3):276–283.
- Meda L, Marra G, Galfetti L, Inchingalo S, Severini F, Deluca L. 2005. Nano-composites for rocket solid propellants. Compos Sci Technol. 65:769–773.
- OECD/OCDE403. 2009a. Guidelines for the Testing of Chemicals – Acute Inhalation Toxicity. 403. OECD/OCDE.
- OECD/OCDE412. 2009b. Guidelines for the Testing of Chemicals – Subacute Inhalation Toxicity: 28-Day Study. 412. OECD/OCDE.
- OECD/OCDE436. 2009c. Guidelines for the Testing of Chemicals – Acute Inhalation Toxicity – Acute Toxic Class Method. 436. OECD/OCDE.
- Parasuraman S. 2011. Toxicological screening. J Pharmacol Pharmacother. 2(2):74–79.
- Paur H-R, Cassee FR, Teeguarden JG, Fissan HJ, Diabate S, Aufderheide M, Kreyling WG, Hänninen O, Kasper G, Riediker M, et al. 2011. In-vitro cell exposure studies for the assessment of nanoparticle toxicity in the lung—A dialog between aerosol science and biology. J Aerosol Sci. 42(10):668–692.
- Pellett GL, Sebacher DI, Bendura RJ, Wornom DE. 1983. HCI in rocket exhaust clouds: atmospheric dispersion, acid aerosol characteristics, and acid rain deposition. J Air Pollut Control Assoc. 33:304–311.
- Pichard A, Bisson M, Gay G, Houeix N, Jolibois B, Lefevre JP, Magaud H, Migné V, Morin A, Tissot S. 2005. Fiche de données toxicologiques et environnementales des substances chimiques: Aluminium et Dérivés – INERIS.
- Pirela SV, Bhattacharya K, Wang Y, Zhang Y, Wanga G, Christophi CA, Godleski J, Thomas T, Qiane Y, Orandlee MS, et al. 2019. A 21-day sub-acute, whole-body inhalation exposure to printer-emitted engineered nanoparticles in rats: Exploring pulmonary and systemic effects. NanoImpact. 15:100176.
- Piriyawong V, Thongpool V, Asanithi P, Limsuwan P. 2012. Preparation and characterization of alumina nanoparticles in deionized water using laser ablation technique. J Nanomater. 2012:1–6.
- Prabhakar PV, Reddy UA, Singh SP, Balasubramanyam A, Rahman MF, Indu Kumari S, Agawane SB, Murty US, Grover P, Mahboob M. 2012. Oxidative stress induced by aluminum oxide nanomaterials after acute oral treatment in Wistar rats. J Appl Toxicol. 32(6):436–445.
- Pritchard JN. 1989. Dust overloading causes impairment of pulmonary clearance: evidence from rats and humans. Exp Pathol. 37(1–4):39–42.
- Qin XJ, Hudson LG, Liu W, Ding W, Cooper KL, Liu KJ. 2008. Dual actions involved in arsenite-induced oxidative DNA damage. Chem Res Toxicol. 21(9):1806–1813.
- Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. 2010. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 49(11):1603–1616.
- Romero FJ, Bosch-Morell F, Romero MJ, Jareno EJ, Romero B, Marin N, Roma J. 1998. Lipid peroxidation products and antioxidants in human disease. Environ Health Perspect. 106(Suppl 5):1229–1234.
- Salzano S, Checconi P, Hanschmann EM, Lillig CH, Bowler LD, Chan P, Vaudry D, Mengozzi M, Coppo L, Sacre S, et al. 2014. Linkage of inflammation and oxidative stress via release of glutathionylated peroxiredoxin-2, which acts as a danger signal. Proc Natl Acad Sci U S A. 111(33):12157–12162.
- Saperstein S, Huyck H, Kimball E, Johnston C, Finkelstein J, Pryhuber G. 2009. The effects of interleukin-1beta in tumor necrosis factor-alpha-induced acute pulmonary inflammation in mice. Mediators Inflamm. 2009:958658.
- Schlesinger RB, Snyder CA, Chen LC, Gorczynski JE, Menache M. 2000. Clearance and translocation of aluminum oxide (alumina) from the lungs. Inhal Toxicol. 12(10):927–939.
- Sellakumar AR, Snyder CA, Solomon JJ, Albert RE. 1985. Carcinogenicity of formaldehyde and hydrogen chloride in rats. Toxicol Appl Pharmacol. 81:401–406.
- Sesti F, Tsitsilonis OE, Kotsinas A, Trougakos IP. 2012. Oxidative stress-mediated biomolecular damage and inflammation in tumorigenesis. In Vivo. 26(3):395–402.
- Sgro LA, D'Anna A, Minutolo P. 2012. On the characterization of nanoparticles emitted from combustion sources related to understanding their effects on health and climate. J Hazard Mater. 211–212:420–426.
- Sikkeland L, Alexis NE, Fry RC, Martin E, Danielsen TE, Sostrand P, Kongerud J. 2016. Inflammation in induced sputum after aluminium oxide exposure: an experimental chamber study. Occup Environ Med. 73(3):199–205.
- Silva AR, Santos AC, Farfel JM, Grinberg LT, Ferretti RE, Campos AH, Cunha IW, Begnami MD, Rocha RM, Carraro DM, et al. 2014. Repair of oxidative DNA damage, cell-cycle regulation and neuronal death may influence the clinical manifestation of Alzheimer's disease. PLoS One. 9(6):e99897.
- Simon-Vazquez R, Lozano-Fernandez T, Davila-Grana A, Gonzalez-Fernandez A. 2016. Analysis of the activation routes induced by different metal oxide nanoparticles on human lung epithelial cells. Future Sci OA. 2(2)FSO:118.
- Sliwinska A, Kwiatkowski D, Czarny P, Milczarek J, Toma M, Korycinska A, Szemraj J, Sliwinski T. 2015. Genotoxicity and cytotoxicity of ZnO and Al2O3 nanoparticles. Toxicol Mech Methods. 25:176–183.
- Stavert DM, Archuleta DC, Behr MJ, Lehnert BE. 1991. Relative acute toxicities of hydrogen fluoride, hydrogen chloride, and hydrogen bromide in nose- and pseudo-mouth-breathing rats. Fundam Appl Toxicol. 16(4):636–655.
- Tanino Y, Coombe DR, Gill SE, Kett WC, Kajikawa O, Proudfoot AE, Wells TN, Parks WC, Wight TN, Martin TR, et al. 2010. Kinetics of chemokine-glycosaminoglycan interactions control neutrophil migration into the airspaces of the lungs. J Immunol. 184(5):2677–2685.
- Toews GB. 2001. Cytokines and the lung. Eur Respir J Suppl. 34:3s–17s.
- Tornling G, Blaschke E, Eklund A. 1993. Long term effects of alumina on components of bronchoalveolar lavage fluid from rats. Br J Ind Med. 50(2):172–175.
- Wang K-S, Chiang K-Y, Lin S-M, Tsai C-C, Sun C-J. 1999. Effects of chlorides on emissions of hydrogen chloride formation in waste incineration. Chemosphere. 38:1571–1582.
- Whitaker AM, Schaich MA, Smith MR, Flynn TS, Freudenthal BD. 2017. Base excision repair of oxidative DNA damage: from mechanism to disease. Front Biosci (Landmark Ed). 22:1493–1522.
- Williams AE, Chambers RC. 2014. The mercurial nature of neutrophils: still an enigma in ARDS? Am J Physiol Lung Cell Mol Physiol. 306(3):L217–L230.
- Wittekindt OH. 2017. Tight junctions in pulmonary epithelia during lung inflammation. Pflugers Arch. 469(1):135–147.
- Wohlslagel J, Dipasquale LC, Vernot EH. 1976. Toxicity of solid rocket motor exhaust: effects of HC1, HF and alumina on rodents. J Combustion Toxicol. 3:61–70.
- Yin K, Hock CE, Lai PS, Ross JT, Yue G. 1999. Role of interferon-gamma in lung inflammation following cecal ligation and puncture in rats. Shock. 12(3):215–221.
- Zhang Q, Wang H, Ge C, Duncan J, He K, Adeosun SO, Xi H, Peng H, Niu Q. 2017. Alumina at 50 and 13 nm nanoparticle sizes have potential genotoxicity. J Appl Toxicol. 37(9):1053–1064.
- Zhu YZ, Liu DW, Liu ZY, Li YF. 2013. impact of aluminum exposure on the immune system: a mini review. Environ Toxicol Pharmacol. 35(1):82–87.

