Abstract
Purpose: Facilitatory and inhibitory responses of spinal motor neurons are influenced by somatosensory input from the skin. The purpose of this study, employing electromyography, was to examine the neuromuscular changes that occur with menthol applied to the skin over the quadriceps muscle.
Methods: Forty-two healthy volunteers performed isometric knee extensions at 35% maximum voluntary contraction (MVC) in three groups (Adult Placebo, Adult Menthol, Older Adult Menthol). Stimulation used was application of 5% menthol gel to the skin. Surface electromyography (sEMG) from the vastus lateralis (VL), vastus medialis (VM), and rectus femoris (RF) was recorded using miniature pair electrodes.
Results: Root mean square electromyography (rmsEMG) in VL and VM significantly increased with menthol stimulation both in Adult and Older Adult, but no significant difference was observed between Adult Menthol and Older Adult Menthol. There was a significant decrease in mean power frequency (MPF) in VM with menthol stimulation in Older Adult, but no significant changes were observed in Adult Menthol.
Conclusion: Neuromuscular modulation was observed with the application of menthol gel at low loads in the present study. These findings could lead to a new method of muscular training that targets the recruitment of fast type muscle safe for older adults.
Introduction
Neural changes associated with strength training, skill training, and locomotor training have recently been shown to occur with cutaneous afferent input, inducing adaptive plasticity in muscle (Zehr Citation2006), further supporting the possibility of using cutaneous afferent input as a training strategy in neurorehabilitation.
It is well known that facilitatory and inhibitory responses of spinal motor neurons (MNs) occur with electrical stimulation of cutaneous nerves (Burke et al. Citation1989; Marchand-Pauvert et al. Citation2002), skin brushing (Mason Citation1985; Wood et al. Citation1998; Sugawara et al. Citation2013), skin anesthesia (Sabbahi and De Luca Citation1981; Delwaide and Crenna Citation1984; Arsenault et al. Citation1993), and skin cooling (Yona Citation1997; Sugawara et al. Citation2012; Shimose et al. Citation2014). Modulation of the MN pool differs depending on the type of stimulation and Hagbarth (Citation1960) suggests that the motor response to noxious stimulation of the skin is either facilitative or inhibitory due to interaction of the skin with homogenous muscle.
Shimose et al. (Citation2014) have shown that skin cooling improves the rate of force development at the onset of contraction (0–100 ms) by about 17% and that this may be due to selective excitation of MNs, via interneurons, from various forms of somatosensory input. Yona (Citation1997) showed that the recruitment threshold of high threshold motor units (HT-MUs) decreases with skin cooling over the working muscle. Modulation of the threshold of HT-MUs via cutaneous nerve input has also been observed in numerous other studies using electrical stimulation (Grimby and Hannerz Citation1976; Stephens et al. Citation1978; Garnett and Stephens Citation1981).
Recent studies have shown that cutaneous thermoreceptors (Transient receptor potential cation channel, subfamily M. member 8 (TRPM8) protein channels) are not only affected by cold but also chemical stimuli such as menthol (McKemy et al. Citation2002; Peier et al. Citation2002). However, the effect of menthol on neuromuscular activation has yet to be investigated (Huffman et al. Citation2010). We hypothesize that application of menthol gel on the skin over the working muscle will increase neuromuscular activation.
Therefore, the purpose of this study was to examine the neuromuscular changes that occur when a specially prepared menthol gel is applied to the skin, during 35% maximum voluntary contraction (MVC) of the quadriceps muscle.
Methods
Subjects
Forty-two healthy adults (20–65 years old) were recruited for this study. Subjects were divided into three groups: Adult Placebo, Adult Menthol, and Older Adult Menthol. The Older Adult Menthol group was determined by age and the remaining subjects were assigned to Adult Placebo and Adult Menthol groups randomly. All subjects were informed of the purpose and risks associated with the present study and gave their written informed consent before taking part.
Body composition of the subjects was measured using electronic scales (InBody720; Biospace Corporation, Cerritos, CA, USA) and these measurements are outlined in . Subjects were instructed to refrain from muscular training and maintain their regular activities of daily living (ADL) for 2–3 days before performing the experimental protocol in a room at which the temperature was set to about 25 °C. This study was approved by the Toho University Ethics Committee (No. 24058).
Table 1. Characteristics of subjects.
Procedures
Data collection
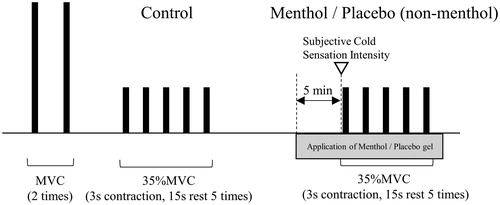
Subjects were acclimatized to the experimental room conditions for at least 30 min before carrying out the protocol outlined in . After acclimatization, MVC of the quadriceps muscle (isometric knee extension), maintained for 2–3 s, was performed twice with a rest period of 90–120 s between each contraction. The larger of these two MVCs was determined as the MVC condition in this experiment.
After a sufficient rest period, five 3-s isometric knee extension contractions (control condition: without application of gel) of the quadriceps muscle were performed at 35% MVC, with a 15-s rest period between each contraction. While surface electromyography (sEMG) was being recorded during 35% MVC contractions, subjects were provided with visual feedback via an oscilloscope (DCS-7020; Kenwood, Kanagawa, Japan). Before performing the 35% MVC contractions, subjects were given ample opportunity to practice matching their force output with the level of force indicated on the monitor.
After performing the contractions under the control conditions, subjects were given a sufficient rest period before application of 3 g of a specially prepared 5% menthol gel to the skin over the quadriceps muscle in Adult Menthol and Older Adult Menthol groups. This gel contained l-menthol (Menthol JP; Takasago International Corporation, Tokyo, Japan) dissolved in oil (Exceparl IPM; Kao Corporation, Tokyo, Japan) and homogenized in water using a surface-active polymer (Pemlen TR-1, TR-2; Lubrizol Advanced Materials, Wickliffe, OH, USA). The only ingredient in this preparation with the ability to induce a sensory response was l-menthol. Before application, the gel was maintained at 32 °C by placing the gel container in a water bath in which the temperature was controlled electronically. Five minutes after the application of this 5% menthol gel, subjects were asked to rate the level of cold sensitivity that they could feel in the area where the gel had been applied using a scale with six categories (Subjective Cold Sensation Intensity). The categories being: 0, not cold; 1, a little cold; 2, somewhat cold; 3, cold; 4, very cold; and 5, too cold and painful.
In the Adult Placebo group a non-menthol gel (the same preparation that was used in Adult Menthol and Older Adult Menthol groups but with the menthol replaced with water) was applied following the same method as menthol gel. The same Subjective Cold Sensation Intensity scale was also used for the Adult Placebo group.
Force measurement
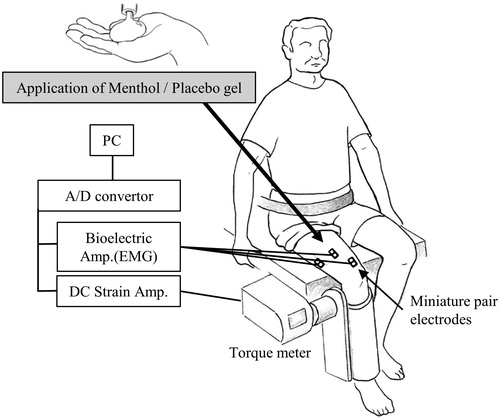
The MVC and 35% MVC isometric knee extension force were measured using a torque meter (type 9E05-B1–50; NEC Corporation, Tokyo, Japan), via a plate attached above the ankle and distal to the tibia, while the subject sat in an adjustable chair-like device with the hip at 90° of flexion and the knee at 70° of flexion (). The force output was digitized by an electronic converter (Power Lab; ADInstruments, Bella Vista, NSW, Australia) and was filtered at 100 Hz and stored at a sampling frequency of 1 kHz using computer software (LabChart7; ADInstruments).
EMG measurement
Surface EMG from the vastus lateralis (VL), vastus medialis (VM), and rectus femoris (RF) was recorded using silver/silver miniature pair electrodes 5 mm in diameter (TF207–029; Unique Medical Corporation, Tokyo, Japan) placed 10 mm apart on the belly of each muscle following Zipp’s recommendations (Zipp Citation1982). Skin impedance was maintained at a level below 5 kΩ by shaving and lightly abrading the skin with fine sandpaper prior to placement of the electrodes, which were held in place with surgical tape.
The amplifier (RMP-6004; Nippon Koden Corporation, Tokyo, Japan) was set at a high cut frequency of 1 kHz, with a time constant of 0.03 s, and the signal was digitized at a sampling rate of 1 kHz using an A-D converter (Power Lab; ADInstruments). Data analysis was then performed using computer software (LabChart7; ADInstruments). Torque output was measured simultaneously with sEMG.
Data acquisition and analysis
Root mean square EMG (rmsEMG) and mean power frequency (MPF) were analyzed for each muscle. The MVC rmsEMG analysis was performed on a 1-s data sample (from 0.5 s before to 0.5 s after peak MVC). One-way analysis of variance (ANOVA) was performed on MVC force and rmsEMG in the three groups (Adult Placebo, Adult Menthol, and Older Adult Menthol).
Chi-square tests were performed to analyze the association between Subjective Cold Sensation Intensity (six categories) and groups (Adult Placebo, Adult Menthol, and Older Adult Menthol).
A stable 1-s period of data was selected for analysis from the five 35% MVC contractions performed in both the control and placebo and menthol conditions and then the average of each of these five contractions was determined in all groups. Mean power frequency was determined using fast Fourier transform (1024 point, Hanning window).
A two-way ANOVA was also used to compare groups and test conditions (Control and Menthol/Placebo) in 35% MVC contractions. A significant main effect was analyzed utilizing a post hoc analysis using the Bonferroni correction.
The effect of rmsEMG and MPF during 35% MVC isometric knee extension in placebo and menthol conditions in relation to the control condition was converted into relative data (placebo or menthol condition/control condition ×100). One-way ANOVA was performed on the relative data of the three groups.
Level of significance was set at 5%. Statistical analysis of the data was performed with SPSS for Windows 20.0 (IBM, New York, USA).
Results
MVC and EMG activity
The MVC and rmsEMG for each group are presented in . One-way ANOVA analysis of MVC force revealed no significant difference among the three groups (F2, 39 = 2.05, p = .14). In RF one-way ANOVA analysis of MVC rmsEMG also revealed a significant difference among the three groups (F2, 39 = 4.59, p < .05) and the Bonferroni correction showed that there was a significant difference between Adult Menthol and Older Adult Menthol (p < .05).
Table 2. MVC force and rmsEMG.
Subjective Cold Sensation Intensity
Subjective Cold Sensation Intensity for each group is presented in . All subjects perceived a cold sensation after application of the placebo or menthol gel. Chi-square tests revealed a significant difference between Subjective Cold Sensation Intensity and groups (p < .01).
Table 3. Subjective cold sensation intensity.
EMG activity of 35% MVC with menthol
The EMG activity of 35% MVC with menthol for each group is presented in . A two-way ANOVA of rmsEMG in VL and VM showed a significant interaction between the groups and test conditions (F2, 39 = 7.07, p < .01 and F2, 39 = 11.4, p < .01, respectively) and a main effect was observed in the test conditions in all muscles (VL: F2, 39 = 42.23, p < .01, RF: F2, 39 = 5.53, p < .05, and VM: F2, 39 = 71.38, p < .01, respectively). Post hoc analysis of rmsEMG showed a significant increase with menthol (Adult and Older) when compared with the control condition in VL and VM (Adult Menthol: p < .01 and p < .01, and Older Adult Menthol: p < .01 and p < .01), but no significant changes were observed in Adult Placebo. A main effect was observed among the groups in VL and VM (F2, 39 = 15.79, p < .01 and F2, 39 = 12.21, p < .01, respectively).
Table 4. Changes in rmsEMG and MPF with menthol during 35% MVC.
A two-way ANOVA of MPF in VL showed a significant interaction between groups and the test conditions (F2, 39 = 3.37, p < .05) and a main effect was observed in the test conditions in RF and VM (F2, 39 = 7.89, p < .01, F2, 39 = 9.27, p < .01, respectively). Post hoc analysis of MPF showed a significant decrease in Older Adult Menthol when compared with the control condition in VM (p < .01), and a trend toward a decrease in VL and RF (p = .075 and .059, respectively). No significant changes were observed in Adult Menthol. A main effect was observed among the groups in VL (F2, 39 = 10.04, p < .01).
Relative EMG activity of 35% MVC with menthol for each group is presented in . One-way ANOVA analysis of relative rmsEMG (% of control) in VL and VM revealed a significant difference among the three groups (F2, 39 = 5.63, p < .01, F2, 39 = 8.12, p < .01, respectively) but no significant changes were observed in RF. Post hoc analysis of relative rmsEMG showed a significant increase with Adult Menthol and Older Adult Menthol when compared with Adult Placebo in VL (p < .05, p < .05, respectively) and VM (p < .01, p < .05, respectively). Relative rmsEMG showed no significant difference between Adult Menthol and Older Adult Menthol.
Figure 3. Changes in rmsEMG (A) and MPF (B) with menthol stimulation. VL: vastus lateralis, RF: rectus femoris, VM: vastus medialis. Data are mean ± SE. *,**Significant difference between groups (p < .05, p < .01, respectively) using Bonferroni post hoc test.
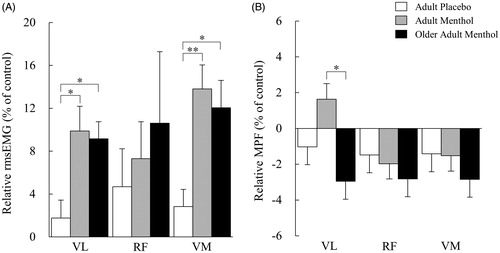
One-way ANOVA analysis of relative MPF (% of control) in VL revealed a significant difference among the three groups (F2, 39 = 3.71, p < .05) but no significant changes were observed in RF and VM. Post hoc analysis of relative MPF showed a significant difference between Adult Menthol and Older Adult Menthol in VL (p < .05).
Discussion
The purpose of this highly unique study, the first of its kind, was to investigate the effect of menthol gel, applied to the skin over the quadriceps muscle, on muscle activation using sEMG. The results showed an increase in rmsEMG and a tendency for MPF to decrease in the menthol condition and these changes were observed irrelevant of age.
In the present study, cutaneous stimulation was applied during a low load steady contraction and neuromuscular activation was measured using sEMG. Changes in neuromuscular activation that are observed in such a contraction must be due to modulation of neural control, in this study due to the application of menthol gel, as was shown by Sugawara et al. (Citation2013) in their investigation of the modulation of neuromuscular activation with skin friction during low load steady contraction (30% MVC).
It has been established that increases in rmsEMG and decreases in MPF are due to the onset of fatigue and synchronization of the firing of motor units (MUs) (Krogh-Lund and Jørgensen Citation1991, Citation1993). In the present study, due to the nature of the protocol, which was a low load contraction (35% MVC) for a short period of time (5 s) with a rest interval long enough to allow for sufficient recovery (15 s), muscle fatigue can be ruled out as having an effect on the results. Therefore, any changes observed must be due to some other form of physiological phenomenon. We believe that the increase in rmsEMG and decrease in MPF observed in this study are due to a change in MU activation from deeper regions to superficial regions of the muscle, along with early recruitment and synchronization of firing of HT-MUs. The shift in activation of MUs from deeper regions to superficial regions of the working muscle can be explained by rmsEMG increases and MPF decreases, which are observed due to a decrease in the distance between the active fibers and the electrodes (Lawrence and De Luca Citation1983). It is known that superficial regions of the quadriceps muscle are composed of mainly fast type muscle fibers whereas the deeper regions are composed of mainly slow type fibers (Johnson et al. Citation1973). Therefore, an increase in the activation of the superficial regions of the quadriceps muscle would suggest recruitment of fast type muscle fibers. Previous studies have shown that low threshold cutaneous stimulation facilitates recruitment of fast muscle fiber and inhibits recruitment of slow muscle fiber (Stephens et al. Citation1978; Garnett and Stephens Citation1981). This is very similar to the results observed in another study which suggests that there is preferential recruitment and synchronization of HT-MUs during increasing ramp contraction with skin cooling of 25 °C (Yona Citation1997). Rate of force development has also been shown to increase with the increase in rmsEMG that is induced by skin cooling of 25 °C (Shimose et al. Citation2014).
Although some studies have investigated the effects of the application of menthol, there is only one study that has investigated the effect of the application of menthol (15% menthol) on the H-reflex. In that study, a decrease in the H/M ratio was observed with the application of menthol but there was also a decrease in the H/M ratio of the control (Huffman et al. Citation2010). However, one must first consider that the H-reflex represents recruitment of MUs of a small diameter which are mainly present in slow type muscle fiber. As mentioned above, superficial regions of the quadriceps muscle are composed of mainly fast type muscle fibers whereas the deeper regions are composed of mainly slow type fibers and the H/M ratio in the H-reflex study above observed the excitation of MUs in the deeper regions of the muscle. This is in contrast to the present study which observed changes in the activation of MUs in the superficial regions of the quadriceps muscle using miniature pair electrodes, which are known to only detect the sEMG signal from a very limited area close to the surface of the muscle. A change in the activation of the small diameter MUs in the deeper regions of the muscle was not observed with the application of menthol gel. However, the results suggest facilitation of the recruitment of fast twitch muscle fiber in the superficial regions of the muscle. Therefore, it can be deducted that the effects of the application of menthol cannot be revealed by observing changes in the H/M ratio in H-reflex studies and the effect of the application of menthol on the H-reflex remains unclear.
In the present study, although there was a difference in the level, all subjects perceived a cold sensation after the application of menthol gel. Therefore, it is possible that modulation of muscle activation with menthol occurred. However, it was not possible to determine whether the level of cold had an effect on the level of neuromuscular modulation that was observed which means further quantitative analysis of afferent neuron and muscle activation will be required in future studies.
It has been shown that fast type muscle fiber significantly atrophies in older adults, therefore, the need for training that targets fast type muscle is widely recognized (Larsson Citation1982; Kirkendall and Garrett Citation1998). However, recommended training for hypertrophy of fast type muscle requires training loads at 60–80% MVC which is unsuitable for older adults (Fiatarone et al. Citation1990; Frontera et al. Citation1991; Verfaillie et al. Citation1997; Häkkinen et al. Citation1998). Repetitive training at high loads can lead to rupture of muscle fibers, which is especially to be avoided in older adults due to the possibility of inducing muscle damage and dysfunction, made worse by the reduction in their regenerative capability (Phillips et al. Citation1997). Moreover, training at high intensity can also lead to an increase in blood pressure and increase the load placed on the heart (Roth et al. Citation1999).
Previous studies have shown age-related increases in the threshold to mechanical and thermal stimuli. However, all regions of the body remain more sensitive to cold stimuli with age (Kenshalo Citation1986; Stevens and Choo Citation1998; Guergova and Dufour Citation2011). Therefore, making use of changes in sensory input from skin cooling may be a useful technique that can be used in training methods for older adults.
Limitations
Assessing the modulation of motor unit recruitment behavior with sEMG can only provide an indication of the activity from groups of MUs in a specific region of the muscle therefore explanations regarding MU activity can only be of a speculative nature. However, although we cannot come to definitive conclusions about MU activity in the present study, we can be sure that we were observing the activity of MUs in the regions of the muscle close to the surface because we were using miniature electrodes to detect the sEMG signal.
Disclosure statement
The authors have the following interests: Tadayuki Tokunaga is employed by Personal Healthcare Research Laboratories, Kao Corporation, a chemical, cosmetic, and food company headquartered in Tokyo, Japan. The remaining authors had no personal or financial conflicts of interest. Menthol and Placebo (non-menthol) gel used in this study were provided by Kao Corporation. However, the funders had no role in the study design, data collection and analysis, preparation of the manuscript, or decision to publish. The authors received no other specific funding for the present work.
Additional information
Funding
References
- Arsenault AB, Bélanger AY, Durand MJ, De Serres SJ, Fortin L, Kemp F. 1993. Effects of TENS and topical skin anesthesia on soleus H-reflex and the concomitant influence of skin/muscle temperature. Arch Phys Med Rehabil 74:48–53.
- Burke JR, Kamen G, Koceja DM. 1989. Long-latency enhancement of quadriceps excitability from stimulation of skin afferents in young and old adults. J Gerontol 44:M158–M163.
- Delwaide PJ, Crenna P. 1984. Cutaneous nerve stimulation and motoneuronal excitability. II: evidence for non-segmental influences. J Neurol Neurosurg Psychiatry 47:190–196.
- Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. 1990. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA 263:3029–3034.
- Frontera WR, Hughes VA, Lutz KJ, Evans WJ. 1991. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. J Appl Physiol 71:644–650.
- Garnett R, Stephens JA. 1981. Changes in the recruitment threshold of motor units produced by cutaneous stimulation in man. J Physiol. (Lond) 311:463–473.
- Grimby L, Hannerz J. 1976. Disturbances in voluntary recruitment order of low and high frequency motor units on blockades of proprioceptive afferent activity. Acta Physiol Scand 96:207–216.
- Guergova S, Dufour A. 2011. Thermal sensitivity in the elderly: a review. Ageing Res Rev 10:80–92.
- Hagbarth KE. 1960. Spinal withdrawal reflexes in the human lower limbs. J Neurol Neurosurg Psychiatry 23:222–227.
- Häkkinen K, Kallinen M, Izquierdo M, Jokelainen K, Lassila H, Mälkiä E, Kraemer WJ, Newton RU, Alen M. 1998. Changes in agonist-antagonist EMG, muscle CSA, and force during strength training in middle-aged and older people. J Appl Physiol 84:1341–1349.
- Huffman DH, Pietrosimone BG, Grindstaff TL, Hart JM, Saliba SA, Ingersoll CD. 2010. Effects of menthol-based counterirritant on quadriceps motoneuron-pool excitability. J Sport Rehabil 19:30–40.
- Johnson MA, Polgar J, Weightman D, Appleton D. 1973. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci 18:111–129.
- Kenshalo DR Sr. 1986. Somesthetic sensitivity in young and elderly humans. J Gerontol 41:732–742.
- Kirkendall DT, Garrett WE, Jr. 1998. The effects of aging and training on skeletal muscle. Am J Sports Med 26:598–602.
- Krogh-Lund C, Jørgensen K. 1991. Changes in conduction velocity, median frequency, and root mean square-amplitude of the electromyogram during 25% maximal voluntary contraction of the triceps brachii muscle, to limit of endurance. Eur J Appl Physiol Occup Physiol 63:60–69.
- Krogh-Lund C, Jørgensen K. 1993. Myo-electric fatigue manifestations revisited: power spectrum, conduction velocity, and amplitude of human elbow flexor muscles during isolated and repetitive endurance contractions at 30% maximal voluntary contraction. Eur J Appl Physiol Occup Physiol 66:161–173.
- Larsson L. 1982. Physical training effects on muscle morphology in sedentary males at different ages. Med Sci Sports Exerc 14:203–206.
- Lawrence JH, De Luca CJ. 1983. Myoelectric signal versus force relationship in different human muscles. J Appl Physiol Respir Environ Exerc Physiol 54:1653–1659.
- Marchand-Pauvert V, Nicolas G, Burke D, Pierrot-Deseilligny E. 2002. Suppression of the H reflex in humans by disynaptic autogenetic inhibitory pathways activated by the test volley. J Physiol 542:963–976.
- Mason CR. 1985. One method for assessing the effectiveness of fast brushing. Phys Ther 65:1197–1202.
- McKemy DD, Neuhausser WM, Julius D. 2002. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416:52–58.
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. 2002. A TRP channel that senses cold stimuli and menthol. Cell 108:705–715.
- Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. 1997. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol 273:E99–107.
- Roth SM, Martel GF, Ivey FM, Lemmer JT, Tracy BL, Hurlbut DE, Metter EJ, Hurley BF, Rogers MA. 1999. Ultrastructural muscle damage in young vs. older men after high-volume, heavy-resistance strength training. J Appl Physiol 86:1833–1840.
- Sabbahi M, De Luca CJ. 1981. Topical anesthesia: H-reflex recovery changes by desensitization of the skin. Electroencephalogr Clin Neurophysiol 52:328–335.
- Shimose R, Ushigome N, Tadano C, Sugawara H, Yona M, Matsunaga A, Muro M. 2014. Increase in rate of force development with skin cooling during isometric knee extension. J Electromyogr Kinesiol 24:895–901.
- Stephens JA, Garnett R, Buller NP. 1978. Reversal of recruitment order of signal motor units produced by cutaneous stimulation during voluntary muscle contraction in man. Nature 272:363–364.
- Stevens JC, Choo KK. 1998. Temperature sensitivity of the body surface over the life span. Somatosens Mot Res 15:13–28.
- Sugawara H, Shimose R, Tadano C, Muro M. 2012. Skin cold stimulation of the dermatome modulates activation of the quadriceps. J Phys Ther Sci 24:169–174.
- Sugawara H, Shimose R, Tadano C, Ushigome N, Muro M. 2013. Change in EMG with skin friction at different frequencies during elbow flexion. Somatosens Mot Res 30:72–80.
- Verfaillie DF, Nichols JF, Turkel E, Hovell MF. 1997. Effects of resistance, balance, and gait training on reduction of risk factors leading to falls in elders. J Aging Phys Act 5:213–228.
- Wood L, Nicol DJ, Thulin CE. 1998. The effects of skin brushing on H reflex amplitude in normal human subjects. Exp Physiol 83:175–183.
- Yona M. 1997. Effects of cold stimulation of human skin on motor unit activity. Jpn J Physiol 47:341–348.
- Zehr EP. 2006. Training-induced adaptive plasticity in human somatosensory reflex pathways. J Appl Physiol 101:1783–1794.
- Zipp P. 1982. Recommendations for the standardization of lead positions in surface electromyography. Eur J Appl Physiol 50:41–54.