Abstract
Ascorbate (AsA) is an important antioxidant and an enzyme cofactor involved in various metabolic pathways. In this study, we investigated the effects of estrogen (ES)-inducible transient expression of genes encoding enzymes involved in the d-mannose/l-galactose (d-Man/l-Gal) pathway for plant AsA biosynthesis on AsA levels under light and dark conditions. No significant difference was observed in AsA levels between Arabidopsis plants transiently expressing phosphomannose isomerase (PMI1), GDP-d-Man pyrophosphorylase (GMP/VTC1), GDP-Man-3′,5′-epimerase (GME), and l-Gal 1-phosphate phosphatase (GPP/VTC4), but AsA levels in the plants transiently expressing GDP-l-Gal phosphorylase (GGP/VTC2) were 2.5-fold higher than those in control plants 7 d after ES treatment. The increase in AsA levels under continuous light conditions and the decrease in AsA levels under dark conditions were enhanced and suppressed, respectively, in the ES-treated plants. These results suggest that GGP/VTC2 acts as a rate-limiting step regulating AsA biosynthesis in response to light and dark conditions.
Graphical Abstract
The increase in ascorbate levels under continuous light and the decrease under continuous dark were enhanced and suppressed respectively by the transient expression of GGP/VTC2.
Ascorbate (AsA) is an important antioxidant that has various metabolic functions in humans and it must be incorporated into the diet. It is known as vitamin C. In planta, AsA, the main source of vitamin C for humans, is naturally an essential compound that plays important roles in many aspects of the control of cellular redox state and anti-oxidative activity as an antioxidant, and also of cell division and cell expansion and plant development and growth as an enzyme cofactor.Citation1–9) AsA was recently recognized to have specific functions, including in the redox signaling and the response to pathogen insults, and it acts as a component in the determination of flowering time.Citation10–12) In addition, intracellular AsA levels have been found to impact the expression of various genes involved in plant growth, hormonal signaling pathways, and stress defense networks.Citation13,14)
AsA is present in plant species in concentrations that range from an estimated 300 mM in the chloroplast stroma to less than 20 mM in other organelles.Citation5) Although the concentrations of AsA in the various compartments of plant cells are high and show a wide range of values, this molecule plays its crucial physiological roles adequately. Hence, the synthesis and levels of AsA in plant cells must be tightly regulated. This is achieved at various levels, as by regulating gene expression and/or modulating the enzymatic activities involved in the biosynthesis of AsA and the regeneration of its oxidized form in response to developmental and environmental cues. In addition, the compartmentation and transport of AsA is necessary to maintain AsA concentrations in various organelles and tissues.
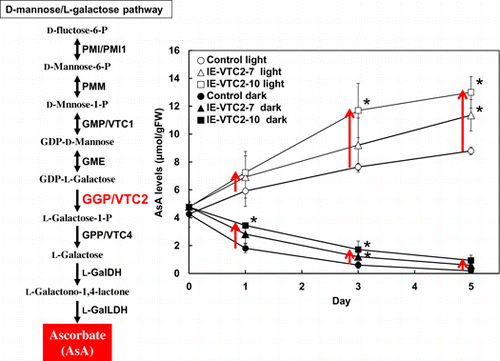
Plant cells have developed three pathways for AsA biosynthesis from d-mannose, myo-inositol, and methyl galacturonate.Citation15–18) The major AsA biosynthetic pathway in higher plants has been designated as d-mannose/l-galactose (d-Man/l-Gal) pathway. In this pathway, d-Man is synthesized from hexose phosphates, such as d-glucose-1 or 6-phosphate and d-fructose 6-phosphate, and proceeds via GDP-d-Man, l-Gal, and the final aldonolactone precursor of AsA being l-galactono-1,4-lactone (l-GalL). The d-Man/l-Gal pathway is composed of eight reaction steps, catalyzed by phosphomannose isomerase (PMI), phosphomannose mutase (PMM), GDP-d-Man pyrophosphorylase (GMP), GDP-d-Man-3′,5′-epimerase (GME), GDP-l-Gal phosphorylase/l-Gal guanylyltransferase (GGP), l-Gal-1-phosphate phosphatase (GPP), l-Gal dehydrogenase (l-GalDH), and l-GalL dehydrogenase (l-GalLDH).
Genetic and molecular biological evidence has confirmed the involvement of the d-Man/l-Gal pathway in leaf AsA levels, mainly with the Arabidopsis plant.Citation15,17,19) These analyses have confirmed that the enzymes encoded by PMI1 (At3g02570), PMM (At2g45790), GMP/VTC1 (At2g39770), GME (At5g28840), GGP/VTC2 (At4g26850), GPP/VTC4 (AT3G02870), l-GalDH (At4g33670), and l-GalLDH (At3g47930) predominantly catalyze a series of reaction steps in the d-Man/l-Gal pathway of Arabidopsis. The homologs of all the genes involved in the d-Man/l-Gal pathway are found in the other plant species, such as kiwifruit, acerola, and tomato, and in algal species such as Chlamydomonas reinhardtii.Citation20–23)
The accumulation of AsA in plant leaves is enhanced depending on light intensity and suppressed by shade.Citation24–26) There is increasing evidence confirming intricate regulation of this pathway at the transcriptional level in response to light and dark environments. We have reported that the expression levels of PMI1, GMP/VTC1, GGP/VTC2, and GPP/VTC4 in Arabidopsis changed in parallel with leaf AsA levels during dark and light periods, and that photosynthetic electron transport was closely linked to the regulation of gene expression.Citation26,27) Similar induction of VTC2/GGP expression by light was also reported by Dowdle et al.Citation25) The expression of most genes was repressed by dark and induced by light in young acerola leaves, whereas the expression of GMP/VTC1 was induced in the dark.Citation20) In addition, the activity of GME in Arabidopsis leaves was remarkably low (one or two orders of magnitude) even under strong light conditions.Citation25) These findings strongly suggest that the reactions catalyzed by PMI1, GMP/VTC1, GME, GGP/VTC2, and GPP/VTC4 in the d-Man/l-Gal pathway are rate-limiting step regulating AsA levels in response to light and dark environments.
Several studies have confirmed increases in AsA levels in plants due to constitutive overexpression of the genes involved in the d-Man/l-Gal pathway. AsA levels in transgenic tobacco plants overexpressing the acerola PMM gene were approximately 2-fold higher than those in wild-type plants.Citation28) Overexpression of the kiwifruit GGP/VTC2 gene in Arabidopsis resulted in a 4-fold increase in AsA levels.Citation21) In addition, Bulley et al.Citation29) found that overexpression of GGP/VTC2 led to an increase in AsA levels not only in the leaves of tomato and strawberry plants, but also in their fruits and in potato tubers. These findings indicate that transcriptional regulation of the genes involved in the d-Man/l-Gal pathway is important in determining intracellular AsA levels.
It is likely that the regulation of AsA biosynthesis is accomplished not only by transcriptional levels but also by other levels in cells, and that such regulatory mechanisms are influenced by each other in the natural environment. Exogenous addition of AsA resulted in a marked decrease in GMP expression in tobacco suspension cells.Citation30) Regarding the enzymatic properties of l-GalDH, GME, and PMI, which are inhibited by high concentrations of AsA, feedback inhibition of AsA biosynthesis was also found to be involved in regulation of intracellular AsA levels.Citation27,31,32) Photomorphogenic factor COP9 signalosome subunit 5B (CSN5B) was recently shown to promote ubiquitination-dependent GMP/VTC1 degradation through the 26S proteasome pathway, which suppressed the overaccumulation of AsA under both light and dark conditions,Citation33) suggesting the importance of the fate of proteins in AsA biosynthesis. These findings indicate that the increase in AsA levels due to the constitutive overexpression of genes encoding AsA biosynthetic enzymes in plant cells as described above affects gene expression and the protein degradation, and causes feedback inhibition of the other enzymes throughout long-term growth period. Thus, the AsA levels observed in such plants do not directly reflect the degrees of contribution of the respective genes to AsA biosynthesis. Therefore, the rate-limiting steps of the d-Man/l-Gal pathway for AsA biosynthesis in plants must be elucidated in more detail.
In this study, we investigated the effects of estrogen (ES)-inducible transient expression of the genes encoding PMI, GMP/VTC1, GME, GGP/VTC2, and GPP/VTC4, which catalyze possible rate-limiting steps of the d-Man/l-Gal pathway, on intracellular AsA levels in Arabidopsis leaves. To demonstrate the importance of these genes in the light/dark regulation of AsA biosynthesis, the effects of transient expression of them on AsA levels under continuous light and continuous dark conditions were also investigated.
Materials and methods
Plasmid construction and transformation of Arabidopsis
To construct the plasmid for ES-inducible transient expression of the AsA biosynthetic genes, cDNAs encoding the open reading frames of PMI1 (At3g02570), GMP/VTC1 (AT2G39770), GME (AT5G28840), GGP/VTC2 (At4g26850), and GPP/VTC4 (AT3G02870) were amplified from a cDNA pool prepared from Arabidopsis thaliana ecotype Columbia-0 (Col-0) using specific primers with attB1 and attB2 sequences for GATEWAY cloning technology, as follows: PMI1-attB1 (5′-aaaaagcaggctacATGGAGATCGCTACTGTCGT-3′), PMI1-attB2 (5′-agaaagctgggttCTATAGAGGAAACAAAAACC-3′), VTC1-attB1 (5′-aaaaagcaggctacCAGATCTCTGATCCGGTGAG-3′), VTC1-attB2 (5′-agaaagctgggttTCACATCACTATCTCTGGCTTCAAG-3′), GME-attB1 (5′-aaaaagcaggctacATGGGAACTACCAATGGAAC-3′), GME-attB2 (5′-agaaagctgggttTCACTCTTTTCCATCAGCCG-3′), VTC2-attB1 (5′-aaaaagcaggctacATGTTGAAAATCAAAAGAGTTCCGACCG-3′), VTC2-attB2 (5′-agaaagctgggttTCACTGAAGGACAAGGCACT-3′), VTC4-attB1 (5′-aaaaagcaggctacATGGCGGACAATGATTCTCT-3′), and VTC4-attB2 (5′-agaaagctgggttTCATGCCCCTGTAAGCCGCA-3′) (small and capital letters indicate attB1/B2 and gene-specific sequences, respectively). Amplified cDNAs were cloned into the donor vector, pDONR201, and then recloned into the destination vector, pMDC7, in which the cDNA was placed under the control of an ES-inducible promoter by GATEWAY technology.Citation34) PCR and in vitro BP and LR recombination reactions were carried out according to the manufacturer’s instructions (Life Technologies, Carlsbad, CA). The full-length GUS gene was used as control.Citation35)
Agrobacterium tumefaciens strain C58, which was transformed with the constructs obtained by electroporation, was used to infect Arabidopsis (Col-0) via the vacuum infiltration method.Citation36) T1 seedlings were selected on basic Murashige and Skoog (MS) medium in Petri dishes containing 3% sucrose and 50 mg L−1 hygromycin for 2 weeks, and were then transferred to soil. Among the T3 seeds obtained by self-fertilization, lines (at least two lines for each gene) that highly expressed chimeric transcription factor XVE, essential for ES-inducible expression,Citation34) were used in the experiments.
Plant materials and growth conditions
Surface-sterilized Arabidopsis wild-type (Col-0) and transgenic seeds were sown on MS medium containing 3% sucrose. The plates were stratified in the dark for 2 d at 4 °C, transferred to a growth chamber, and then the plants were grown under normal conditions (16 h of light at 100 μmol photons m−2 s−1 and 8 h of dark, 23 °C). After 2 weeks, the seedlings were treated with a 100 μM ES solution containing 0.1% (v/v) Tween 20, and then grown for 3–7 d. A solution containing 0.1% (v/v) Tween 20 without ES was sprayed as the mock treatment. Continuous light (100 μmol photons m−2 s−1, 23 °C) and dark (23 °C) conditions were started 4 h after illumination under normal conditions to abolish the effect of circadian rhythm on AsA levels.
Preparation of total RNA and cDNA synthesis
Total RNA was extracted from the leaves of Arabidopsis plants by Sepasol-RNA (Nacalai Tesque, Kyoto, Japan). First-strand cDNA was synthesized with reverse transcriptase (ReverTra Ace; Toyobo, Osaka, Japan) and an oligo(dT) primer. These were performed according to the manufacturer’s instructions.
Semi-quantitative RT-PCR analysis
Semi-quantitative RT-PCR analysis was performed following Maruta et al.Citation35) using the primer sets for plasmid construction. The primer sequences used to amplify Actin2 mRNA were as follows: actin2-F (5′-GGGATGAACCAGAAGGATGC-3′) and actin2-R (5′-ATGCTGCTTGGTGCAAGTGC-3′). PCR amplification was done through 23–28 cycles of 95 °C for 60 s, 55 °C for 60 s, and 72 °C for 60 s, followed by 72 °C for 10 min. Aliquots of the PCR products were analyzed on 1% agarose gels. All experiments were repeated at least three times with cDNA prepared from three batches of plant leaves.
Quantitative real-time PCR experiments
Quantitative RT-PCR experiments were run following Maruta et al.Citation35) Primer sequences were as follows: GGP/VTC2-F (5′-TGCTTCAGAGGATAACGCGTG-3′), GGP/VTC2-R (5′-AAAGGCGAGAGCAGTAACCTCC-3′), Actin2-F (5′-GGCAAGTCATCACGATTGG-3′), and Actin2-R (5′-CAGCTTCCATTCCCACAAAC-3′). Quantitative RT-PCR was performed with an Applied Biosystems 7300 Real-Time PCR System (Life Technologies) with SYBR Premix ExTaq (Takara, Kyoto, Japan). Actin2 mRNA was used as internal standard in all experiments, and relative expression levels normalized to Actin2 mRNA are shown. All experiments were repeated at least three times with cDNA prepared from three batches of plant leaves.
Measurement of AsA and dehydroascorbate (DHA) levels
The levels of AsA and the oxidized form of it, DHA, were measured as described by Maruta et al.Citation27) Leaves from Arabidopsis plants (0.2 g fresh weight) frozen in liquid N2 were ground using a mortar and pestle with 5 mL of 6% (v/v) HClO4, and this was centrifuged at 10,000 × g for 10 min at 4 °C. A 100-μL aliquot of the leaf extract obtained was added directly to 900 μL of 200 mM succinate buffer (pH 12.7, adjusted with NaOH) in the spectrophotometer. The final pH was approximately 6.0. A265 was immediately recorded, and again 5 min after the addition of 5 units of AsA oxidase from Cucurbita sp. (Wako, Osaka, Japan). To determine total AsA, the leaf extract was adjusted to pH 6.0 with 1.25 M K2CO3 and this was centrifuged at 10,000 × g for 5 min. The supernatant was incubated with 10 mM dithiothreitol (DTT) dissolved in 50 mM HEPES-KOH buffer with pH 7.5 for 30 min at 25 °C. A 100-μL aliquot of the solution was added directly to 900 μL of 200 mM succinate buffer with pH adjusted to 6.0 in the spectrophotometer. The resulting solution was assayed as described above. The difference between total AsA and the AsA levels was taken as the DHA level.
Enzymatic assay
Leaf extracts were prepared as described by Dowdle et al.Citation25) for the assay of GGP activity. Leaves from Arabidopsis plants (0.2 g fresh weight) frozen in liquid N2 were ground using a mortar and pestle with 1 mL of 50 mM Tris–HCl (pH 7.5) containing 10% glycerol, 2 mM DTT, 1 mM aminocaproic acid, 1 mM benzamidine, 1 mM phenylmethanesulfonylfluoride, and 1% insoluble polyvinylpolypyrrolidone (PVP). After centrifugation at 12,000 × g for 5 min at 4 °C, the supernatant was used in the assay to measure the GGP activity.
The GGP enzyme can phosphorylate not only GDP-l-Gal but also GDP-d-glucose, with almost equal efficiency.Citation37) Hence, GGP activity was assayed by the measurement of reduction in GDP-d-glucose as substrate and the formation of GDP as product by the method described by Linster et al.Citation37) with modifications. A 50-μL aliquot of the reaction mixture containing 50 mM Tris–HCl (pH 7.5), 5 mM sodium phosphate, 2 mM MgCl2, 10 mM NaCl, 1 mM dithiothreitol, and 20 μL of the leaf extract was incubated at 26 °C for 5–60 min. The reaction was terminated by boiling for 3 min, and the aliquot was centrifuged at 12,000 x g for 3 min. The aliquot was analyzed by HPLC using a C18 column (4.6 × 250 mm, Phenomenex Luna 5u C18(2), Shimadzu, Tokyo, Japan) at a flow rate of 1.0 mL min−1 for the mobile phase buffer, which contained 50 mM NaH2PO4 (pH 6.7), 5 mM tetrabutylammonium dihydrogen phosphate, 5 mg mL−1 EDTA, and 4% methanol. GDP-d-Glucose and GDP were detected according to their UV absorbance, 254 nm. The GDP and GDP-d-glucose concentrations were calculated by comparing the integrated peak areas with those of standard GDP or GDP-d-glucose solutions.
Results and discussion
Effects of ES-inducible transient expression of PMI1, GMP/VTC1, GME, GGP/VTC2, and GPP/VTC4 on intracellular AsA levels
The cDNAs encoding PMI1, GMP/VTC1, GME, GGP/VTC2, and GPP/VTC4 were placed under the control of an ES-inducible promoter in binary vector pMDC7 by GATEWAY technology.Citation34) The full-length GUS gene was used as control.Citation35) The plasmids obtained were introduced into Arabidopsis plants (Col-0), and more than 20 independent lines for each gene (Inducible expression of (IE)-PMI1, VTC1, GME, VTC2, and VTC4) were then selected for hygromycin resistance. Among the transgenic lines (the T3 generation), we screened lines that highly expressed chimeric transcription factor XVE essential for ES-inducible expressionCitation34) (data not shown), and then isolated at least two lines for each gene.
Two-week-old IE-PMI1, VTC1, GME, VTC2, and VTC4 plants grown under normal conditions were sprayed with 100 μM ES containing 0.1% (v/v) Tween 20 (ES treatment), and then grown for 3 d. A solution containing 0.1% (v/v) Tween 20 without ES was sprayed as a mock treatment. Semi-quantitative RT-PCR analysis indicated that the ES treatment for 3 d led to higher mRNA levels of PMI1, GMP/VTC1, GME, and GPP/VTC4 than the mock treatment in the leaves of respective transgenic plants, but not of the control plants (Supplemental Fig. 1; see doi:10.1080/09168451.2014.877831), but no significant difference was observed in AsA levels as between the mock- and ES-treated leaves of the IE-PMI1, VTC1, GME, and VTC4 plants (Supplemental Fig. 2). These results suggest that PMI1, GMP/VTC1, GME, and GPP/VTC4 are not rate-limiting enzymes for the d-Man/l-Gal pathway in Arabidopsis.
GGP/VTC2 mRNA levels increased in the leaves of the IE-VTC2 plants 3 d after ES treatment (Fig. ). Induction of GGP/VTC2 expression in the IE-VTC2-10 plants was higher than in the IE-VTC2-7 plants. Leaf AsA levels in the IE-VTC2-7 and -10 plants were markedly higher 3 d after ES treatment than after mock treatment (Fig. ). A similar increase in AsA levels was observed in the remaining five lines of the IE-VTC2 plants (data not shown). These results suggest that the reaction catalyzed by GGP/VTC2 is a rate-limiting step in the d-Man/l-Gal pathway in Arabidopsis leaves and that transcriptional regulation of it directly impacts intracellular AsA levels.
Fig. 1. Changes in the expression levels of GGP/VTC2 in the IE-VTC2 plants after treatment with ES.
Note: Two-week-old control and IE-VTC2 plants grown on MS medium under normal conditions were sprayed with 100 μM ES containing 0.1% (v/v) Tween 20 (ES treatment) and then grown for up to 7 d. A solution containing 0.1% (v/v) Tween 20 without ES was sprayed as mock treatment. Total RNAs were prepared from the mock- and the ES-treated plants at the indicated times and subjected to quantitative RT-PCR analysis with specific primers for the GGP/VTC2 and Actin2 genes. The details of the procedures are described in “Materials and methods.” Relative expression levels were normalized to Actin2 mRNA. Data are means ± SD for independent experiments (n = 3). An asterisk indicates that the mean value was significantly different from that of the respective pre-treated plants as analyzed by Student’s t test (p < 0.05).
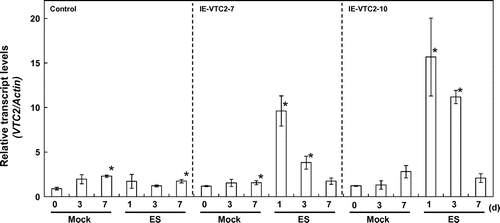
Fig. 2. Time-course effects of transient expression of GGP/VTC2 on intracellular AsA levels.
Note: The experimental conditions were as described in Fig. . The levels of AsA and DHA in the extracts prepared from the mock- and ES-treated plants at the indicated times were analyzed as described in “Materials and methods.” Data are means ± SD for independent experiments (n = 3–10). An asterisk indicates that the mean value was significantly different from that of the respective pre-treated plants as analyzed by Student’s t test (*p < 0.05; **p < 0.01).
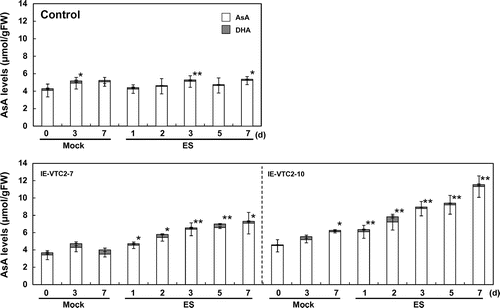
To determine whether the increase in AsA levels due to transient induction of GGP/VTC2 expression was suppressed by other regulatory systems maintaining AsA homeostasis, we analyzed the time-course effect of ES-inducible transient expression of GGP/VTC2 on intracellular AsA levels. The progression of the growth period was accompanied by slight increases in GGP/VTC2 mRNA levels, even in the control plants, after both mock and ES treatments (Fig. ), but GGP/VTC2 mRNA levels markedly increased in the leaves of the IE-VTC2 plants 1 d after ES treatment. The GGP/VTC2 mRNA levels in IE-VTC2-7 plants were approximately 8.4- and 3.3-fold higher 1 and 3 d, respectively, after ES treatment, and returned to steady-state levels at 7 d. Similarly, the GGP/VTC2 mRNA levels in the IE-VTC2-10 plants were approximately 12.9- and 9.2-fold higher 1 and 3 d, respectively, after ES treatment. Slight but significant increases in AsA levels were observed in the control plants 3–7 d after both mock and ES treatments (Fig. ). This may have been associated with plant growth. On the other hand, the AsA levels in the IE-VTC2-7 plants increased linearly under ES treatment, and were approximately 2.0-fold higher 7 d after treatment than before (Fig. ). The AsA levels in the IE-VTC2-10 plants also increased linearly but more markedly, and were approximately 2.5-fold higher 7 d after treatment. These results suggest that the expression level of GGP/VTC2 is a determinant of AsA pool size in Arabidopsis leaves.
Effects of ES-inducible transient expression of GGP/VTC2 on total GGP activity
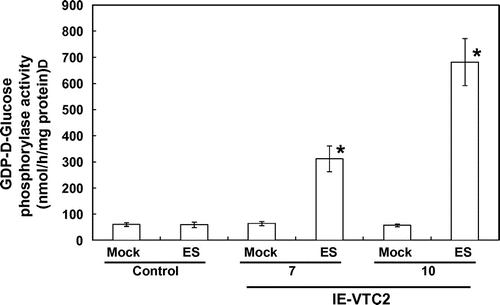
We examined changes in GGP activity in the IE-VTC2 plants. GGP catalyzes the conversion of GDP-l-Gal to l-Gal 1-phosphate in a reaction that consumes an inorganic phosphate and produces GDP.Citation25) Unfortunately, GDP-l-Gal as substrate was not available from any commercial source, but Linster et al.Citation37) have found that the GGP enzyme phosphorylates GDP-d-glucose with almost the same efficiency as GDP-l-Gal. Hence, we examined the total phosphorylase activity toward GDP-d-glucose in extracts prepared from IE-VTC2 plants (Fig. ). No significant difference was observed in total phosphorylase activity as between the mock and ES treatments in the control plants. On the other hand, consistently with the induction levels of GGP/VTC2 expression, this activity 3 d after ES treatment was higher in the IE-VTC2-10 plants (by 12.0-fold) than in the IE-VTC2-7 plants (4.9-fold). These results indicate that GGP/VTC2 activity is enhanced in the IE-VTC2 plants under ES treatment. The activities of GDP-d-glucose phosphorylase in the control and the IE-VTC2 plants 3 d after mock and ES treatments (Fig. ) correlated well (correlation coefficient, 0.9787) with the AsA levels for the respective plants (Fig. ).
Fig. 3. Effects of ES-inducible transient expression of GGP/VTC2 on GGP activity.
Note: Experimental conditions were as in Fig. . Extracts prepared from leaves of the control and IE-VTC2 plants 3 d after mock and ES treatments were used for an assay to measure GDP-d-glucose phosphorylase activity as described in “Materials and methods.” Activity was assayed by phosphate-dependent reduction of GDP-d-glucose and the production of GDP by the HPLC system. Data are means value ± SD for independent experiments (n = 3–4). An asterisk indicates that the mean value was significantly different from that of mock-treated plants as analyzed by Student’s t test (p < 0.01).
Effects of ES-inducible transient expression of GGP/VTC2 on AsA levels under continuous light and dark conditions
Although the accumulation of AsA in the plant leaf was found to be enhanced by light and suppressed by dark,Citation24,25) the importance of the d-Man/l-Gal pathway in regulating AsA levels in response to light and dark conditions has not been fully clarified. PMI1, GMP/VTC1, GGP/VTC2, and GPP/VTC4 expression levels have found to be altered in parallel with leaf AsA levels during dark and light periods,Citation25–27) which suggests the importance of these genes in the light/dark response. Hence, we examined the effects of ES-inducible transient expression of GGP/VTC2 on AsA levels under continuous light and dark conditions.
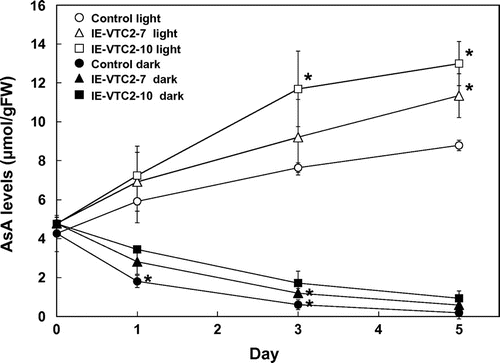
Two-week-old IE-VTC2 plants grown under normal conditions were treated with ES and then grown for 5 d under continuous light or dark conditions. The control plants showed a gradual increase in AsA levels until 5 d under continuous light (Fig. ). On the other hand, AsA levels rapidly decreased for 3 d under continuous dark, and reached nearly undetectable levels after 5 d. The increase in AsA levels under continuous light was enhanced in the IE-VTC2 plants treated with ES. The degree of this enhancement in the IE-VTC2-7 and -10 plants was consistent with the induction levels of GGP/VTC2 expression. Dowdle et al.Citation25) have reported that the activity of GGP but not those of the other enzymes of the d-Man/l-Gal pathway in Arabidopsis leaves is highly responsive to light irradiation. This suggests that the reaction catalyzed by GGP/VTC2 as a rate-limiting step in the d-Man/l-Gal pathway plays a pivotal role in enhancing the accumulation of AsA in response to light. On the other hand, the decrease in AsA levels under continuous dark was partly suppressed in the IE-VTC2 plants, suggesting that GGP/VTC2 contributes to the suppression of AsA accumulation under dark conditions. The expression not only of GGP/VTC2, but also of other genes involved in the d-Man/l-Gal pathway, such as PMI1, GMP/VTC1, GME, GPP/VTC4, and l-GalLDH, was suppressed under dark conditions.Citation26,27) In addition, Wang et al.Citation33) found that ubiquitination-dependent degradation of VTC1 is involved in the suppression of AsA levels under dark conditions.
Fig. 4. Effects of transient expression of GGP/VTC2 on AsA levels under continuous light and dark conditions.
Note: Two-week-old control and IE-VTC2 plants grown on MS medium under normal conditions were sprayed with 100 μM ES containing 0.1% (v/v) Tween 20 and then grown for 5 d under continuous light or dark conditions. The levels of AsA and DHA in extracts prepared from the control and IE-VTC2 plants at the indicated times were analyzed as described in “Materials and methods.” Data are means ± SD for independent experiments (n = 3). An asterisk indicates that the mean value was significantly different from that of the control plants under the same conditions as analyzed by Student’s t test (*p < 0.05).
Conclusion
ES-Inducible transient expression analysis revealed that GGP/VTC2 is one of the rate-limiting factors in the d-Man/l-Gal pathway in Arabidopsis leaves. Similar results were obtained by transient expression in tobacco leaves (Nicotiana benthamiana) of the GGP/VTC2 gene from kiwifruit by Agrobacterium-infiltration assay.Citation21,38) Transient expression of the Arabidopsis GGP/VTC2 gene resulted in a marked increase (50-fold) in total GGP activity in tobacco leaves, whereas the AsA levels in the leaves were only 3-fold higher than those in the control plants.Citation38) These findings raise two possibilities: (i) the actual AsA pool size in plant leaves is maximum at several-fold and (ii) the degree of enhancement in AsA levels regulated by GGP/VTC2 as a rate-limiting step differs depending on the plant species. Transient coexpression of both the GGP/VTC2 and GME genes in tobacco leaves was found to result in an 8.6-fold increase in AsA levels, while the GGP/VTC2 gene resulted only in a 4.2-fold increase, suggesting that by GME catalyze the next rate-limiting step in the pathway.Citation21) These reports and our findings indicate that control of GGP/VTC2 expression is crucial in determining the AsA pool size in plant leaves, especially under light conditions, and carries the potential to increase AsA levels several-fold.
Supplemental material
The supplemental material for this paper is available at http://dx.doi.org/10.1080/09168451.2014.877831.
Supplemental Figures 1 and 2.
Download PDF (277.4 KB)Acknowledgment
We are grateful to Dr. Nam-Hai Chua (Rockefeller University) for donating the pMDC7 vector. We thank Ms Mami Kondo, Mr Tsuyoshi Hirata, Ms Kanako Misaki, Mr Shigeyoshi Ishimoto, and Mr Ren Goto for technical assistance.
Funding
This work was supported by JSPS KAKENHI [grant number 24770046] (to K.Y.); Grant-in-Aid for Young Scientists (B), and by MEXT KAKENHI [grant number 22248042] (to S.S.); Grant-in-Aid for Scientific Research (A).
Notes
Abbreviations: DHA, dehydroascorbate; d-Man, d-mannose; GME, GDP-Man-3′,5′-epimerase; GMP, GDP-Man pyrophosphorylase; GGP, GDP-l-Gal phosphorylase/l-Gal guanylyltransferase; GPP, l-Gal-1-phosphate phosphatase; l-Gal, l-galactose; l-GalDH, l-Gal dehydrogenase; l-GalL, l-galactono-1,4-lactone; l-GalLDH, l-GalL dehydrogenase; PMI, phosphomannose isomerase; PMM, phosphomannose mutase.
References
- Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998;49:249–279.
- Loewus FA. Biosynthesis and metabolism of ascorbic acid in plants and of analogs of ascorbic acid in fungi. Phytochemistry. 1999;52:193–210.
- Arrigoni O, De Tullio MC. The role of ascorbic acid in cell metabolism: between gene-directed functions and unpredictable chemical reactions. J. Plant Physiol. 2000;157:481–488.
- Davey MW, Van Montagu M, Inze D, Sanmartin M, Kanellis A, Smirnoff N, Benzie IJJ, Strain JJ, Favell D, Fletcher J. Plant l-ascorbic acid: chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 2000;80:825–860.
- Smirnoff N. Ascorbic acid: metabolism and functions of a multi-facetted molecule. Curr. Opin. Plant Biol. 2000;3:229–235.
- Smirnoff N, Wheeler GL. Ascorbic acid in plants: biosynthesis and function. Crit. Rev. Biochem. Mol. Biol. 2000;35:291–314.
- Conklin PL. Recent advances in the role and biosynthesis of ascorbic acid in plants. Plant Cell Environ. 2001;24:383–394.
- Smirnoff N, Conklin PL, Loewus FA. Biosynthesis of ascorbic acid in plants: a renaissance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001;52:437–467.
- Smirnoff N, Gatzek S. Ascorbate biosynthesis: a diversity of pathways. In: Asard H, May JM, Smirnoff N, editors. Vitamin C: its functions and biochemistry in animals and plants. Oxford: Bios Scientific Publishers; 2004. p. 7–29.
- Conklin PL, Barth C. Ascorbic acid, a familiar small molecule intertwined in the response of plants to ozone, pathogens, and the onset of senescence. Plant Cell Environ. 2004;27:959–970.
- Barth C, De Tullio M, Conklin PL. The role of ascorbic acid in the control of flowering time and the onset of senescence. J. Exp. Bot. 2006;57:1657–1665.
- Noctor G. Metabolic signalling in defence and stress: the central roles of soluble redox couples. Plant Cell Environ. 2006;29:409–425.
- Pastori GM, Kiddle G, Antoniw J, Bernard S, Veljovic-Jovanovic S, Verrier PJ, Noctor G, Foyer CH. Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. Plant Cell. 2003;15:939–951.
- Gao Y, Nishikawa H, Badejo AA, Shibata H, Sawa Y, Nakagawa T, Maruta T, Shigeoka S, Smirnoff N, Ishikawa T. Expression of aspartyl protease and C3HC4-type RING zinc finger genes are responsive to ascorbic acid in Arabidopsis thaliana. J. Exp. Bot. 2011;62:3647–3657.
- Valpuesta V, Botella MA. Biosynthesis of l-ascorbic acid in plants: new pathways for an old antioxidant. Trends Plant Sci. 2004;9:573–577.
- Hancock RD, Viola R. Improving the nutritional value of crops through enhancement of lascorbic acid (vitamin C) content: rationale and biotechnological opportunities. J. Agric. Food Chem. 2005;53:5248–5257.
- Ishikawa T, Dowdle J, Smirnoff N. Progress in manipulating ascorbic acid biosynthesis and accumulation in plants. Physiol. Plant. 2006;126:343–355.
- Ishikawa T, Shigeoka S. Recent advances in ascorbate biosynthesis and the physiological significance of ascorbate peroxidase in photosynthesizing organisms. Biosci. Biotechnol. Biochem. 2008;72:1143–1154.
- Debolt S, Melino V, Ford CM. Ascorbate as a biosynthetic precursor in plants. Ann. Bot. 2007;99:3–8.
- Badejo AA, Fujikawa Y, Esaka M. Gene expression of ascorbic acid biosynthesis related enzymes of the Smirnoff-Wheeler pathway in acerola (Malpighia glabra). J. Plant Physiol. 2009;166:652–660.
- Bulley SM, Rassam M, Hoser D, Otto W, Schünemann N, Wright M, MacRae E, Gleave A, Laing W. Gene expression studies in kiwifruit and gene over-expression in Arabidopsis indicates that GDP-lgalactose guanyltransferase is a major control point of vitamin C biosynthesis. J. Exp. Bot. 2009;60:765–778.
- Ioannidi E, Kalamaki MS, Engineer C, Pateraki I, Alexandrou D, Mellidou I, Giovannonni J, Kanellis AK. Expression profiling of ascorbic acid-related genes during tomato fruit development and ripening and in response to stress conditions. J. Exp. Bot. 2009;60:663–678.
- Urzica EI, Adler LN, Page MD, Linster CL, Arbing MA, Casero D, Pellegrini M, Merchant SS, Clarke SG. Impact of oxidative stress on ascorbate biosynthesis in Chlamydomonas via regulation of the VTC2 gene encoding a GDP-l-galactose phosphorylase. J. Biol. Chem. 2012;287:14234–14245.
- Bartoli CG, Yu JP, Gomez F, Fernandez L, McIntosh L, Foyer CH. Inter-relationships between light and respiration in the control of ascorbic acid synthesis and accumulation in Arabidopsis thaliana leaves. J. Exp. Bot. 2006;57:1621–1631.
- Dowdle J, Ishikawa T, Gatzek S, Rolinski S, Smirnoff N. Two genes in Arabidopsis thaliana encoding GDP-lgalactose phosphorylase are required for ascorbate biosynthesis and seedling viability. Plant J. 2007;52:673–689.
- Yabuta Y, Mieda T, Rapolu M, Nakamura A, Motoki T, Maruta T, Yoshimura K, Ishikawa T, Shigeoka S. Light regulation of ascorbate biosynthesis is dependent on the photosynthetic electron transport chain but independent of sugars in Arabidopsis. J. Exp. Bot. 2007;58:2661–2671.
- Maruta T, Yonemitsu M, Yabuta Y, Tamoi M, Ishikawa T, Shigeoka S. Arabidopsis phosphomannose isomerase 1, but not phosphomannose isomerase 2, is essential for ascorbic acid biosynthesis. J. Biol. Chem. 2008;283:28842–28851.
- Badejo AA, Eltelib HA, Fukunaga K, Fujikawa Y, Esaka M. Increase in ascorbate content of transgenic tobacco plants overexpressing the acerola (Malpighia glabra) phosphomannomutase gene. Plant Cell Physiol. 2009;50:423–428.
- Bulley S, Wright M, Rommens C, Yan H, Rassam M, Lin-Wang K, Andre C, Brewster D, Karunairetnam S, Allan AC, Laing WA. Enhancing ascorbate in fruits and tubers through over-expression of the lgalactose pathway gene GDP-lgalactose phosphorylase. Plant Biotechnol. J. 2012;10:390–397.
- Tabata K, Takaoka T, Esaka M. Gene expression of ascorbic acid-related enzymes in tobacco. Phytochemistry. 2002;61:631–635.
- Wolucka BA, Van Montagu M. GDP-mannose 3',5'-epimerase forms GDP-lgulose, a putative intermediate for the de novo biosynthesis of vitamin C in plants. J. Biol. Chem. 2003;278:47483–47490.
- Mieda T, Yabuta Y, Rapolu M, Motoki T, Takeda T, Yoshimura K, Ishikawa T, Shigeoka S. Feedback inhibition of spinach lgalactose dehydrogenase by lascorbate. Plant Cell Physiol. 2004;45:1271–1279.
- Wang J, Yu Y, Zhang Z, Quan R, Zhang H, Ma L, Deng XW, Huang R. Arabidopsis CSN5B interacts with VTC1 and modulates ascorbic acid synthesis. Plant Cell. 2013;25:625–636.
- Zuo J, Niu QW, Chua NH. Technical advance. An estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J. 2000;24:265–273.
- Maruta T, Noshi M, Tanouchi A, Tamoi M, Yabuta Y, Yoshimura K, Ishikawa T, Shigeoka S. H2O2-triggered retrograde signaling from chloroplasts to nucleus plays specific role in response to stress. J. Biol. Chem. 2012;287:11717–11729.
- Bechtold N, Pelletier G. In planta Agrobacterium mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Methods Mol. Biol. 1998;82:259–266.
- Linster CL, Gomez TA, Christensen KC, Adler LN, Young BD, Brenner C, Clarke SG. Arabidopsis VTC2 encodes a GDP-lgalactose phosphorylase, the last unknown enzyme in the Smirnoff-Wheeler pathway to ascorbic acid in plants. J. Biol. Chem. 2007;282:18879–18885.
- Laing WA, Wright MA, Cooney J, Bulley SM. The missing step of the lgalactose pathway of ascorbate biosynthesis in plants, an lgalactose guanyltransferase, increases leaf ascorbate content. Proc. Nat. Acad. Sci. 2007;104:9534–9539.

