Abstract
Obesity is a common disease worldwide that often results in serious conditions including hypertension, diabetes, and hyperlipidemia. Many herbal medicines have been examined with regard to ameliorating obesity. We investigated the anti-obesity effects of 50% EtOH extract of Triticum aestivum sprout (TAEE) in high-fat-diet (HFD)-induced obese mice. TAEE administration (10, 50, or 200 mg/kg) for 6 weeks significantly decreased the body weights, serum total cholesterol (TC), and low-density lipoprotein cholesterol levels in HFD-fed mice. TAEE treatment reduced lipid accumulation in epididymal white adipose tissue (EWAT) and liver. Moreover, TC and lipid levels were decreased by TAEE treatment in liver. Serum leptin and adiponectin concentrations were reduced by TAEE treatment. TAEE-treated mice showed decreases in peroxisome proliferator-activated receptor γ (PPARγ) and fatty acid synthase expression in EWAT. Furthermore, TAEE administration elevated levels of PPARα protein in the liver of HFD-induced obese mice. These results suggest that TAEE supplementation might be beneficial for the treatment and prevention of obesity and related diseases.
Graphical abstract
Obesity induces serious health problem in many developed countries, and the prevalence of obesity has been increasing dramatically for several decades.Citation1) The disease is characterized by increased numbers and sizes of adipocytes, followed by rising adipose tissue mass and may lead to various diseases, such as cardiovascular disease, non-alcoholic fatty liver disease, and type II diabetes.Citation2–5) A positive energy balance from life style, environmental, and genetic issues is key factors raising the risk of obesity.Citation6)
For controlling severe obesity, several drugs, including orlistat and sibutramine, have been developed and prescribed.Citation7) However, these drugs have undesirable side effects such as dry mouth, anorexia, constipation, insomnia, dizziness, and nausea.Citation8) Thus, there has been increasing interest in potent and safe anti-obesity materials. Recent studies have found that (−)-epigallocatechin-3-gallate, quercetin, resveratrol, and curcumin, natural products have shown anti-obesity effects in experimental mouse models.Citation9–12)
Triticum aestivum (TA) is one of the most important crops in the world. The seeds have been consumed for many dietary purposes and manufacturing products. The grass of TA is known to have several physiological activities, including anti-cancer, anti-colitis, anti-inflammatory, and anti-oxidant activities.Citation13–16)
A recent study shows that water and 100% EtOH extracts of TA sprout reduce blood glucose and body weight in vivo.Citation17) Furthermore, 50% EtOH extract of TA has anti-adipogenic effects in vitro.Citation18) However, the anti-obesity effects of TA sprout extract have not been examined in the high-fat-diet (HFD)-induced mice model. In this study, we investigated the anti-obesity effects of 50% EtOH extract of TA sprout in HFD-induced obese mice.
Materials and methods
Materials
All reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless noted otherwise. Triglyceride (TG), total cholesterol (TC), and high-density lipoprotein cholesterol (HDL-C) commercial kits were purchased from Asan Pharma (Seoul, Korea). Specific-pathogen-free male C57BL/6 mice (6 weeks old) were purchased from Samtako Bio Korea Co., Ltd. (Gyeonggi-do, Korea) and acclimated for 1 week before use. The mice were given free access to food and water. The animal room was maintained at a temperature of 23 ± 2 °C with a humidity of 60 ± 5%, keeping a 12/12-h light/dark cycle.
Preparation of Triticum aestivum (TA) sprout extract
TA sprouts were obtained from the National Institute of Crop Science (Gyeonggi-do, Korea). The seeds were germinated on organic sterile peat moss and cultivated. After 2 weeks, TA sprouts were harvested, freeze-dried, and milled in a laboratory-scale mill. The powder of TA sprout (30 g) was ultrasonically extracted with 50% EtOH for 1 h and filtered. After evaporation on a rotary vacuum evaporator (N-000, EYELA, Tokyo, Japan), 10 g of T. aestivum sprout 50% EtOH extract (TAEE) was obtained. TAEE was stored at 4 °C before use and dissolved in purified water for experiments.
Animal treatment
Mice were divided randomly into six dietary groups of 10 mice each. Mice of the control group were fed with a normal diet (ND, SAM #31, Samtako, Inc.). HFD mice (D12451, Research Diets, Inc.) were orally administered orlistat (ORT, 20 mg/kg/day) or TAEE (10, 50, or 200 mg/kg/day) for 6 weeks in the other groups. The protocol for this experiment was approved by the Institutional Animal Care and Use Committee at the Korea Research Institute of Bioscience and Biotechnology (WKU13-11).
Measurement of body weight and food intake
Body weight and dietary intake were recorded once per week and twice per week, respectively, using an electric balance (Precisa, Swiss). Unlimited amounts of water and food were given to animals. Food efficiency was calculated by division of the whole weight gain by the amount of food. All food was stored at 4 °C before use.
Collection of serum and tissue samples
At the end of experiment, blood was collected after 12 h fasting from the heart and held on ice for 1 h. Serum was separated from the blood by centrifugation (15,000 rpm, 20 min, 4 °C) and kept at −70 °C until subjected to biochemical analyses and an enzyme-linked immunosorbent assay (ELISA). Liver and epididymal white adipose tissue (EWAT) were removed from animals and weighed quickly and then stored at −70 °C before use.
Biochemical analysis of serum and blood glucose
The levels of TG, TC, HDL-C, low-density lipoprotein cholesterol (LDL-C) in serum were measured using commercial kits (Asan Pharma. Co., Ltd., Korea). Leptin and adiponectin levels in serum were assayed using a mouse leptin EIA kit (ENZO Life Sciences, Inc., Japan) and a mouse adiponectin Quantikine Immunoassay kit (R & D Systems, USA), respectively. All processes were performed according to the manufacturers’ instructions. Blood glucose level was determined using blood samples from the tail vein with a glucometer (ACCU-CHEK active, Roche).
Hepatic lipid extraction and measurement
Hepatic lipids were extracted as described by Freedman et al.Citation19). Liver tissue (0.5 g) was homogenized in 0.5 mL of 1 M NaCl. The homogenate was extracted with 3 mL of chloroform/methanol (2:1) plus 0.5 mL of 1 M NaCl. The organic phase was collected, dried, and suspended in 0.5 mL of Triton X-100/methanol (2:1). The TC and TG levels in liver were determined using the enzymatic method using commercial kits (Asan Pharma. Co., Korea).
Histopathology
Liver and EWAT were fixed in 10% buffered formaldehyde solution for 24 h at room temperature. The fixed tissues were subsequently embedded in paraffin wax and sectioned at 4 μm. For the detection of lipid deposition, the sectioned liver and EWAT were stained with hematoxylin and eosin (H & E) staining and examined by optical microscopy (Leica, Japan).
Western blot analysis
Liver tissue and EWAT were homogenized in lysis buffer (iNtRON Biotech, Korea) on ice. The homogenates were centrifuged (13,000 rpm, 20 min, 4 °C). The supernatants were transferred to new tubes and stored at −70 °C until analysis. Protein concentrations were determined using the Bio-Rad protein assay reagent (Bio-Rad, Hercules, CA, USA). Aliquots of 60 μg protein from tissue homogenates were separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto PVDF membranes (Hybond-PVDF, Amersham Pharmacia Biotech, Piscataway, NJ, USA) for 2 h. The membrane was incubated in blocking buffer with 5% skim milk in Tris-buffered saline and Tween 20 (TBS-T) for 2 h, washed in TBS-T for 10 min, four times, and then incubated with primary antibodies (1:2000) for overnight at 4 °C. After washing with TBS-T, the membranes were incubated with anti-rabbit IgG or anti-mouse IgG horseradish peroxidase-linked secondary antibodies at 1:5000 dilution in 5% skim milk in TBS-T for 1 h at room temperature. Reactive bands were visualized using enhanced chemiluminescence reagents (Amersham, Milan). Protein expression levels were determined by analyzing the signals captured on the PVDF membranes using a FluorChem E image analyzer (Cell Biosciences, CA, USA).
Ultra pressure liquid chromatography (UPLC) analysis
Ultra pressure liquid chromatography (UPLC) was performed on a Waters Acquity system (Waters, Milford, MA, USA). Analysis of TAEE was performed under the following conditions: column, Waters Acquity UPLC BEH C18 (Waters, Milford, MA, USA, 2.1 mm × 50 mm, 1.7 μm), mobile phase, 0.1% H3PO4 (pH 2.87, solvent system A) and CH3CN (solvent system B) in a gradient mode (B 3% from 0 to 0.5 min, B from 3 to 25% from 0.5 to 8 min, B 25% from 8 min to 10 min, B from 25 to 70% from 10 to 15 min, B from 70% from 15 to 17 min, B from 70 to 100% from 17 to 22 min); flow rate, 0.6 mL/min detection, UV at 206 nm; temperature, 30 °C.
Statistical analysis
Values are expressed as means ± SD. Statistical significance was determined using Student’s t-test. Values of p < 0.05 were considered to indicate statistical significance.
Results
Effects of TAEE on body weight, food intake, and food efficiency in obese mice
C57BL/6 mice were fed ND or HFD with vehicle or TAEE (10, 50, or 200 mg/kg) by oral administration for 6 weeks. As shown in Table , the body weight gain of HFD mice was significantly increased compared with that of ND mice. However, TAEE-treated mice showed a reduction in body weight gain, 39.8, 68.3, and 97.4% at 10, 50, and 200 mg/kg, respectively. Administration of TAEE increased food intake and markedly decreased food efficiency, compared with HFD-treated mice.
Table 1. Effects of TAEE on body weight gain, food intake, and food efficiency in HFD-induced obese mice.
Ameliorating effect of TAEE administration on serum lipid profiles and glucose level in obese mice
To investigate whether TAEE could control serum lipid profiles or glucose levels, serum TG, TC, HDL-C, LDL-C, and blood glucose levels were measured (Table ). In the obese condition, lipid profiles and glucose levels were increased. However, TAEE administration markedly lowered serum TG, TC, LDL-C, and blood glucose levels. In particular, serum TC and blood glucose levels were reduced in a dose-dependent manner.
Table 2. Effects of TAEE on serum lipid profiles in HFD-induced obese mice.
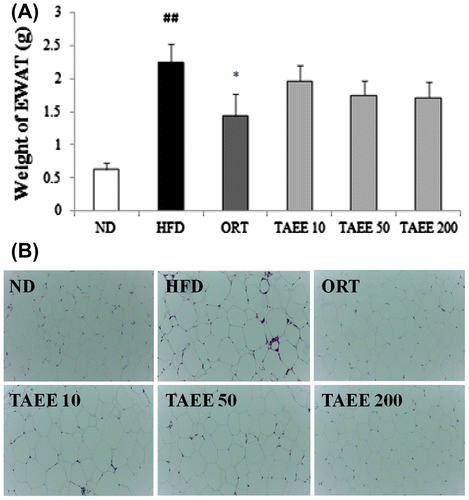
Effect of TAEE on a weight and a size of EWAT in HFD-induced obese mice
Fig. (A) shows the weight of EWAT. In HFD-induced obese mice, the weight of EWAT was increased more than three times to ND-fed mice. The weight of EWAT in TAEE-treated mice showed a tendency of decline, but the difference was not significant. As shown in the histological features, the size of EWAT in HFD-fed mice clearly increased vs. the ND-fed mice. However, TAEE administration reduced the size of EWAT (Fig. (B)).
Effects of TAEE on hepatic weight and lipid profiles in obese mice
The livers of HFD-fed mice were enlarged slightly and had a pale yellow appearance, compared with those of ND-fed mice (data not shown). Fig. (A) shows that liver weight was increased significantly in HFD-fed mice and was inhibited by TAEE treatment, dose-dependently. Moreover, TAEE treatment dramatically decreased lipid accumulation in the liver (Fig. (B)). The livers of HFD-fed mice were filled with lipid droplets and induced by steatosis, and numbers of lipid droplets were reduced by TAEE treatment. Table also shows that hepatic total lipid and TG concentration of TAEE-treated mice were decreased significantly when compared with HFD-fed mice.
Fig. 2. Effect of TAEE on a weight and histological changes of liver in HFD-induced obese mice.
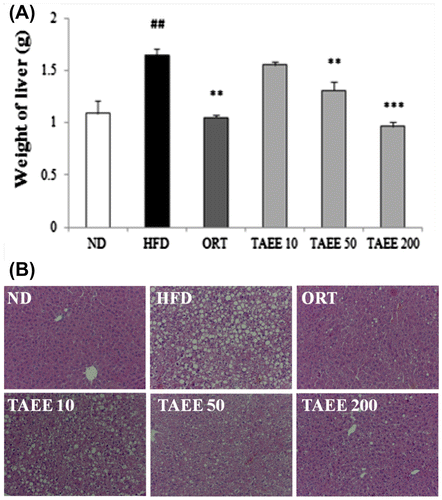
Table 3. Effects of TAEE on hepatic lipid contents in HFD-induced obese mice.
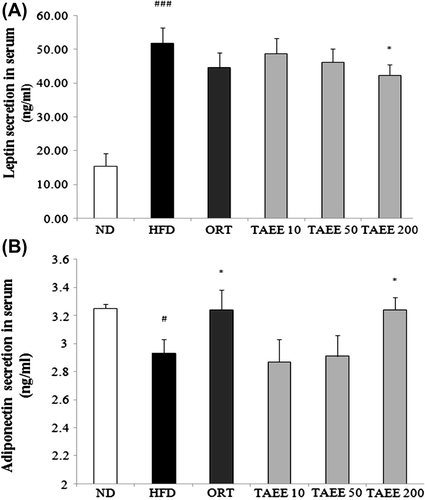
Effect of TAEE on serum leptin and adiponectin
Obesity is characterized by an increase in leptin and a decrease in adiponectin.Citation19) Thus, we examined concentrations of leptin and adiponectin in serum. Fig. shows the changes in adipokines, including leptin and adiponectin. The levels of serum leptin were increased markedly by 3.4-fold in HFD-fed mice than in ND-fed mice. Moreover, mice treated with 10, 50, and 200 mg/kg of TAEE resulted in 6.3, 12, and 22.5% decreases in serum leptin levels, respectively (Fig. (A)). Moreover, the serum adiponectin level was reduced significantly in HFD-fed mice (Fig. (B)). However, 200 mg/kg of TAEE treatment recovered adiponectin secretion up to the level of the ND-fed mice.
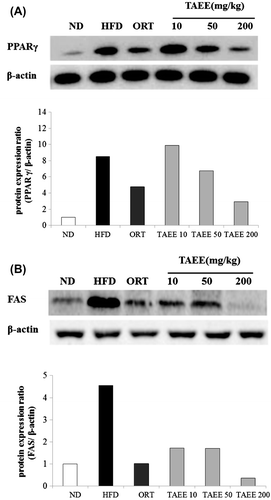
Effects of TAEE on lipogenic protein expression in HFD-fed mice
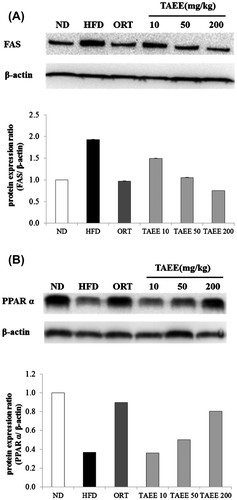
To test whether the increase in fat and liver weight in obese mice was accompanied by changes in the expression of proteins involved in lipogenesis, Western blot analysis was performed. The protein levels of peroxisome proliferator-activated receptor γ (PPARγ) and fatty acid synthase (FAS) were increased markedly in obese mice, but PPARγ expression showed a tendency to decline with TAEE treatment at 200 mg/kg and FAS expression was decreased by TAEE treatment in EWAT (Fig. ). In the liver, there was no difference in PPARγ protein expression (data not shown). The level of FAS expression was enhanced, and PPARα expression was decreased in HFD-fed mice. However, TAEE administration reduced FAS protein expression significantly. However, the expression of PPARα protein increased in a dose-dependent manner with TAEE treatment (Fig. ).
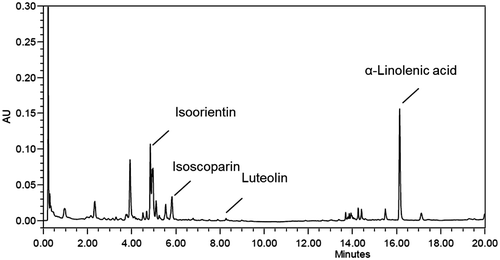
Chemical components of TAEE by UPLC analysis
When TAEE was analyzed with UPLC for the determination of chemical components, isoorientin, isoscoparin, luteorin, and alpha-linoleic acid were detected (Fig. ). The retention times were 4.8, 5.8, 8.2, and 16.2 min, respectively. Alpha-linoleic acid showed a higher peak and flavonoids were observed.
Discussion
Obesity is a serious health problem, leading to type 2 diabetes, coronary heart disease, hypertension, and metabolic syndrome.Citation2–5) To date, many herbal plants have been used to treat and ameliorate obesity. As one promising candidate, in this study, we investigated anti-obesity effects of TAEE in HFD-induced obese mice.
Obesity is characterized by body weight gain due to an increase in numbers and sizes of adipocytes. Thus, it is important to reduce body weight and body fat to prevent obesity, and the increase in body fat mass is induced by fibroblastic pre-adipocytes in adipose tissue.Citation20–22) In our study, TAEE reduced body weight gain and EWAT significantly compared with HFD-induced obese mice. A histological section of EWAT showed that TAEE markedly suppressed expansion of adipocyte tissue size. These results indicate that TAEE effectively inhibited body weight gain and body fat mass.
Hepatic lipid accumulation generally occurs with an increase in free fatty acids in the liver and the synthesis of endogenous hepatic fatty acids.Citation23) It has been reported that HFD-induced accumulation of abundant fatty droplets occurs, indicating fatty liver. The development of fatty liver is highly associated with obesity.Citation22) In our study, administration of TAEE with HFD decreased the size and weight of the liver. A histological section showed that TAEE treatment markedly reduced hepatic lipid droplets. Furthermore, serum TC, TG, and LDL-C levels were significantly decreased in TAEE-treated mice. These results suggest that TAEE improves lipid accumulation in the liver and suppresses hyperlipidemia in HFD-induced obese mice.
Two major adipokines, leptin and adiponectin, are secreted by white adipose tissue and are essential for the regulation of metabolism.Citation24–26) Leptin plays a key role in controlling the size of adipose tissue and regulates food intake and energy expenditure in the hypothalamus. Thus, the level of plasma leptin is directly associated with adiposity and body weight changes in humans and rodents.Citation27–30) Adiponectin, one of the adipokines, is known to contribute to insulin sensitivity and fatty acid oxidation. Circulating concentrations of adiponectin are inversely correlated with body mass.Citation31,32) In our study, the level of serum leptin increased significantly in HFD-induced obese mice, but decreased in TAEE-administered mice. Serum adiponectin concentrations were raised by TAEE treatment. These results indicated that TAEE effectively regulated metabolic homeostasis in HFD-induced obese mice.
Adipogenesis requires a network of transcription factors and signaling pathways, which contribute to the expression of genes for the development of the adipocyte phenotype.Citation33) C/EBPα and PPARγ are found primarily in adipose tissue and act as key transcription factors during adipogenesis and lipogenesis.Citation34–36) These transcription factors are key regulators of adipose tissue lipogenesis, which controls the expression of fatty acid biosynthetic genes such as FAS and acetyl CoA carboxylase.Citation33) In the present study, TAEE administration significantly suppressed the expression of PPARγ in EWAT. Moreover, FAS was upregulated in HFD-fed mice, but its expression was clearly inhibited by TAEE treatment.
In hepatocytes, PPARγ and PPARα are transcription factors for lipid metabolism, lipid synthesis, and fatty acid oxidation.Citation37) One of the main pathways involving PPARα regulation in mice and humans includes fatty acid metabolism. In mice, PPARα activation is important for fatty acid metabolism through the induction of genes encoding the fatty acid transporter CD36 and fatty acid binding protein 1 that brings the fatty acids from the plasma membrane to the nucleus.Citation38,39) Abnormal modulation of PPARα, associated with lipid catabolism at an epigenetic level, may contribute to hepatic lipid accumulation and eventually the development of non-alcoholic fatty liver disease (NAFLD). Indeed, in a human study, the mRNA expression level of PPARα was suppressed in the livers of obese and non-obese patients with NAFLD vs. normal controls.Citation40) Hepatic PPARγ expression also increased in obese mice. Increased PPARγ expression in liver induces fatty acid synthesis and consequently produces fatty liver.Citation41) Lipid accumulation in the liver can result from a growth in fatty acid influx, an increase in FAS expression, or a reduction of fatty acid oxidation.Citation42) In this study, PPARγ expression in liver did not show significant differences between treatment groups. However, the expression level of hepatic FAS protein was suppressed and that of PPARα was upregulated by TAEE treatment. These results explain that TAEE inhibited lipid accumulation by suppressing lipid synthesis and increasing β-oxidation.
Chemical analysis by UPLC showed that several flavonoids and α-linoleic acid were detected in TAEE. Especially, α-linolenic acid has been identified as a PPARγ and a PPARα agonist.Citation43,44) And, fermented rooibos containing several flavonoids such as isoorientin and orientin showed an inhibition of adipogenesis.Citation45) Thus, α-linolenic acid and some flavonoids may be one of the compounds contributing to the anti-obesity effect of TAEE.
Conclusion
TAEE markedly reduced body weight gain, EWAT, and hepatic lipid accumulation. TAEE effectively ameliorated hyperlipidemia by lowering serum TC, serum LDL-C, blood glucose, and plasma leptin level. TAEE not only increased PPARα expression, but also decreased FAS expression, resulting in lipogenesis inhibition. Consistent with the results described here, TAEE may be beneficial for the prevention and treatment of obesity.
Acknowledgements
This study was supported by grants from Wonkwang University in 2015.
References
- Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643.
- Couillard C, Mauriège P, Imbeault P, Prud’homme D, Nadeau A, Tremblay A, Bouchard C, Després JP. Hyperleptinemia is more closely associated with adipose cell hypertrophy than with adipose tissue hyperplasia. Int. J. Obes. Relat. Metab. Disord. 2000;24:782–788.10.1038/sj.ijo.0801227
- Glass CK, Witztum JL. Atherosclerosis. Cell. 2001;104:503–516.10.1016/S0092-8674(01)00238-0
- Orgaard A, Jensen L. The effects of Soy Isoflavones on obesity. Exp. Biol. Med. (Maywood). 2008;233:1066–1080.10.3181/0712-MR-347
- Lois K, Kumar S. Obesity and diabetes. Endocrinol. Nutr. 2009;56:38–42.10.1016/S1575-0922(09)73516-8
- Achike FI, To NH, Wang H, Kwan CY. Obesity, metabolic syndrome, adipocytes and vascular function: a holistic viewpoint. Clin. Exp. Pharmacol. Physiol. 2011;38:1–10.10.1111/cep.2010.38.issue-1
- Padwal RS, Majumdar SR. Drug treatments for obesity: orlistat, sibutramine, and rimonabant. Lancet. 2007;369:71–77.10.1016/S0140-6736(07)60033-6
- Bray GA. Drug treatment of obesity. Rev. Endocr. Metab. Disord. 2001;2:403–418.10.1023/A:1011808701117
- Klaus S, Pültz S, Thöne-Reineke C, Wolfram S. Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. Int. J. Obes. 2005;29:615–623.10.1038/sj.ijo.0802926
- Fang XK, Gao J, Zhu DN. Kaempferol and quercetin isolated from Euonymus alatus improve glucose uptake of 3T3-L1 cells without adipogenesis activity. Life Sci. 2008;82:615–622.10.1016/j.lfs.2007.12.021
- Baur JA, Pearson kJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu Vinayakumar V, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342.10.1038/nature05354
- Ejaz A, Wu D, Kwan P, Meydani M. Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. J. Nutr. 2009;139:919–925.10.3945/jn.108.100966
- Lai CN. Chlorophyll. The active factor in wheat sprout extract inhibiting the metabolic activation of carcinogens in vitro. Nutr. Cancer. 1979;1:19–21.
- Ben-Arye E, Goldin E, Wengrower D, Stamper A, Kohn R, Berry E, Scand J. Wheat grass juice in the treatment of active distal ulcerative colitis: a randomized double-blind placebocontrolled trial. Gastroenterol. 2002;37:444–449.
- Watzl B. Anti-inflammatory effects of plant-based foods and of their constituents. Int. J. Vitam. Nutr. Res. 2008;78:293–298.10.1024/0300-9831.78.6.293
- Kulkarni SD, Tilak JC, Acharya R, Rajurkar NS, Devasagayam TP, Reddy AV. Evaluation of the antioxidant activity of wheatgrass (Triticum aestivum L.) as a function of growth under different conditions. Phytother. Res. 2006;20:218–227.10.1002/(ISSN)1099-1573
- Lee SH, Lim SW, Mihn Nguyen Van, Hur JM, Song BJ, Lee YM, Lee HS, Kim DK. Administration of Triticum aestivum sprout water extracts reduce the level of blood glucose and cholesterol in leptin deficient ob/ob mice. J. Korean Soc. Food Sci. Nutr. 2011;40:401–408.10.3746/jkfn.2011.40.3.401
- Lee SH, Xin MJ, Luyen BTT, Cha JY, Im JY, Kwon SU, Lim SW, Suh JW, Kim YH, Kim DK, Lee YM. Inhibitory effect of Triticum aestivum ethanol extract on lipid accumulation in 3T3-L1 preadipocytes. Yakhak Hoeji. 2011;55:478–484.
- Freedman BD, Lee EJ, Park Y, Jameson JL. A dominant negative peroxisome proliferator-activated receptor-gamma knock-in mouse exhibits features of the metabolic syndrome. J. Biol. Chem. 2005;280:17118–17125.
- Spiegelman BM, Flier JS. Adipogenesis and obesity: rounding out the big picture. Cell. 1996;87:377–389.10.1016/S0092-8674(00)81359-8
- Ahima RS. Adipose tissue as an endocrine organ. Obesity. 2006;14:242S–249S.10.1038/oby.2006.317
- James O, Day C. Non-alcoholic steatohepatitis: another disease of affluence. Lancet. 1999;353:1634–1636.10.1016/S0140-6736(99)00163-4
- Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Invest. 2004;114:147–152.10.1172/JCI200422422
- Bradley R, Cleveland K, Cheatham B. The adipocyte as a secretory organ: mechanisms of vesicle transport and secretory pathways. Recent Prog. Horm. Res. 2001;56:329–358.10.1210/rp.56.1.329
- Guerre-Millo M. Adipose tissue and adipokines: for better or worse. Diabetes Metab. 2004;30:13–19.10.1016/S1262-3636(07)70084-8
- Klaus S. Adipose tissue as a regulator of energy balance. Curr. Drug Targets. 2004;5:241–250.10.2174/1389450043490523
- Marti A, Berraondo B, Martinez JA. Leptin: physiological actions. J. Physiol. Biochem. 1999;55:43–49.
- Moran O, Phillip M. Leptin: obesity, diabetes and other peripheral effects – a review. Pediatr. Diabetes. 2003;4:101–109.10.1034/j.1399-5448.2003.00017.x
- Trayhurn P. Leptin-a critical body weight signal and a “master” hormone? Sci. STKE. 2003;169:1–3.
- Halleux CM, Takahashi M, Delporte ML, Detry R, Funahashi T, Matsuzawa Y, Brichard SM. Secretion of adiponectin and regulation of apM1 gene expression in human visceral adipose tissue. Biochem. Biophys. Res. Commun. 2001;288:1102–1107.10.1006/bbrc.2001.5904
- Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853.10.1038/nature05483
- Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J-I, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999;257:79–83.10.1006/bbrc.1999.0255
- Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–273.10.1016/j.cmet.2006.07.001
- Rosen ED, Spiegelman BM. PPAR: a nuclear regulator of metabolism, differentiation, and cell growth. J. Biol. Chem. 2001;276:37731–37734.10.1074/jbc.R100034200
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 2001;108:1167–1174.10.1172/JCI13505
- Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol. Rev. 1998;18:783–809.
- Pu P, Gao DM, Mohamed S, Chen J, Zhang J, Zhou XY, Zhou NJ, Xie J, Jiang H. Naringin ameliorates metabolic syndrome by activating AMP-activated protein kinase in mice fed a high-fat diet. Arch. Biopchem. Biophys. 2012;518:61–70.10.1016/j.abb.2011.11.026
- Motojima K, Passilly P, Peters JM, Gonzalez FJ, Latruffe N. Expression of putative fatty acid transporter genes are regulated by peroxisome proliferator-activated receptor alpha and gamma activators in a tissue- and inducer-specific manner. J. Biol. Chem. 1998;273:16710–16714.10.1074/jbc.273.27.16710
- Poirier H, Niot I, Monnot MC, Braissant O, Meunier-Durmort C, Costet P, Pineau T, Wahli W, Willson TM. Differential involvement of peroxisome-proliferator-activated receptors α and δ in fibrate and fatty-acid-mediated inductions of the gene encoding liver fatty-acid-binding protein in the liver and the small intestine. Biochem. J. 2001;355:481–488.10.1042/0264-6021:3550481
- Nakamuta M, Kohjima M, Higuchi N, Kato M, Kotoh K, Yoshimoto T, Yada M, Yada R, Takemoto R, Fukuizumi K, Harada N, Taketomi A, Maehara Y, Nakashima M, Enjoji M. The significance of differences in fatty acid metabolism between obese and non-obese patients with non-alcoholic fatty liver disease. Int. J. Mol. Med. 2008;22:663–667.
- Ho JN, Son ME, Lim WC, Lim ST, Cho HY. Anti-Obesity effects of germinated brown rice extract through down-regulation of lipogenic genes in high fat diet-induced obese mice. Biosci. Biotechnol. Biochem. 2012;76:1068–1074.
- Schaffer JE. Lipotoxicity: when tissues overeat. Curr. Opin. Lipidol. 2003;14:281–287.10.1097/00041433-200306000-00008
- Chou YC, Prakash E, Huang CF, Lien TW, Chen X, Su IJ, Chao YS, Hsieh HP, Hsu JT. Bioassay-guided purification and identification of PPARα/γ agonists from Chlorella sorokiniana. Phytother. Res. 2008;22:605–613.10.1002/(ISSN)1099-1573
- Christensen KB, Petersen RK, Kristiansen K, Christensen LP. Identification of bioactive compounds from flowers of black elder (Sambucus nigra L.) that activate the human peroxisome proliferator-activated receptor (PPAR) γ. Phytother. Res. 2010;S2:S129–S132.10.1002/ptr.v24.2s
- Sanderson M, Mazibuko SE, Joubert E, de Beer D, Johnson R, Pheiffer C, Louw J, Muller CJ. Effects of fermented rooibos (Aspalathus linearis) on adipocyte differentiation. Phytomedicine. 2014;21:109–117.10.1016/j.phymed.2013.08.011