Abstract
The purpose of this study was to investigate the effect of repeated harvesting on the content of caffeic acid (CA) and seven species of caffeoylquinic acids (CQAs) in sweet potato leaves using a newly developed high-performance liquid chromatography method. Six cultivars and two breeding lines were used in this study. Leaves were collected at monthly intervals from 1st harvest (May) to 4th harvest (August) in 2011 and 2012. ANOVA analysis revealed that the contents of CQAs were significantly different among all cultivars and breeding lines, but no significant differences were found for CA. No annual variation was confirmed in CA and CQAs. Repeated harvest of sweet potato leaves affected the content of only 4-CQA and 5-CQA. Post-hoc comparisons using Tukey’s method indicated that the contents of 4-CQA and 5-CQA in sweet potato leaves harvested at first time were significantly higher compared to those at the other harvest times.
Graphical Abstract
Contents of the article titled “Effect of repeated harvesting on the content of caffeic acid and seven species of caffeoylquinic acids in sweet potato leaves”
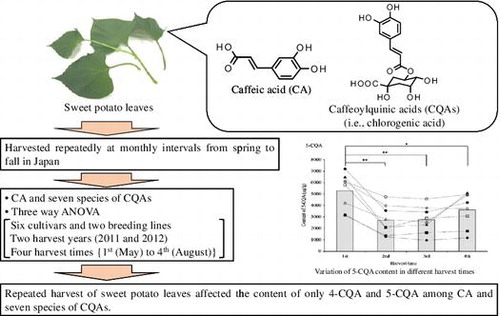
Sweet potato [Ipomoea batatas (L.) Lam.] leaves are used as edible vegetables and consumed in many parts of the world.Citation1–3) Compared to other green leafy vegetables, sweet potato leaves can be harvested at monthly intervals from spring to fall in Japan, and, furthermore, they are more tolerant to high humidity levels. The annual yield of sweet potato leaves is higher than that of other green leafy vegetables, which are harvested only in the summer months. Therefore, sweet potato leaves could replace other green leafy vegetables when the latter are off-season, and they could potentially alleviate food shortages during natural disasters, such as floods or typhoons.Citation4)
Sweet potato leaves are considered to be a good source of protein, fiber, and minerals, particularly K, P, Ca, Mg, Fe, Mn, and Cu.Citation5) Previous studies have revealed that sweet potato leaves are an excellent source of anti-oxidative compounds, such as caffeic acid (CA) and all known caffeoylquinic acids (CQA).Citation6–8) CQAs are found in plant-derived foods and beverages, and play a crucial role in human health. In recent years, the health benefits of CQAs have attracted particular interest because of their anti-oxidative, anti-inflammatory, anti-microbial, hepatoprotective, anti-cardioprotective, low-density lipoprotein oxidation activities, and neuroprotective effect.Citation9–15)
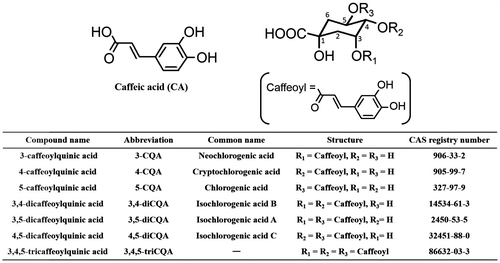
A decade ago, NARO Kyushu Okinawa Agricultural Research Center (NARO/KARC) developed a sweet potato cultivar, “Suioh”, the leaves of which are edible.Citation16) It is reported that “Suioh” leaves contain CA and the following five species of CQAs: 5-caffeoylquinic acid (5-CQA, chlorogenic acid), 3,4-dicaffeoylquinic acid (3,4-diCQA, isochlorogenic acid B), 3,5-dicaffeoylquinic acid (3,5-diCQA, isochlorogenic acid A), 4,5-dicaffeoylquinic acid (4,5-diCQA, isochlorogenic acid C), and 3,4,5-tricaffeoylquinic acid (3,4,5-triCQA).Citation6) However, we have developed a new high-performance liquid chromatography (HPLC) method that can determine the amount of CA and seven species of CQAs {3-caffeoylquinic acid (3-CQA, neochlorogenic acid), 4-caffeoylquinic acid (4-CQA, cryptochlorogenic acid), 5-CQA, 3,4-diCQA, 3,5-diCQA, 4,5-diCQA, and 3,4,5-triCQA, whose chemical structures were shown in Fig. } using CA and 5-CQA as relative response factors (RRF), which is calculated by the molar extinction coefficient and the molecular mass of CQAs standards and used to compute absolute but not relative concentration of CQAs in sweet potato leaves.Citation17) Furthermore, a single-laboratory validation study of the proposed method was successfully performed, and the high accuracy and precision of the method were confirmed.Citation17)
Fig. 1. Chemical structures of CA and CQAs in sweet potato leaves.
Note: All data for CQAs presented in this manuscript use the recommended IUPAC numbering system.Citation23)
Previous research has shown that cultivars/breeding lines and environmental conditions, such as temperature and intensity of solar radiation, affect CA and CQAs content in sweet potato leaves,Citation18) but limited information is available on their variation as a result of repeated harvesting several times in a year, which is one of the their unique properties. The aim of this work was to examine the effect of repeated harvesting on the content of CA and seven species of CQAs in sweet potato leaves using a single-laboratory validated HPLC method.
Materials and methods
Plant material
Six sweet potato cultivars, “Elegant Summer,” “Kogane Sengan,” “Kokei-14,” “Kyuikukan-1,” “Kyuikuyou-2,” and “Suioh,” along with two breeding lines, “06382-1” and “06385-21,” were grown in a greenhouse at the Miyakonojyo branch of NARO/KARC (131°1′E, 31°45′N). Sweet potato leaves were harvested four times, at monthly intervals, from May through August in 2011 and 2012. Harvested materials were washed with tap water and leaves were separated from petioles and stems. Leaf samples were lyophilized, powdered with a grinder mill (IFM-800DG, Iwatani, Japan), and stored at −20 °C until use. Leaves of “Elegant Summer,” “Kogane Sengan,” “Kokei-14,” “Kyuikuyou-2,” and “Suioh” are green in color, whereas those of “Kyuikukan-1,” “06382-1,” and “06385-21” are purple because of the presence of anthocyanin.
Reagents
CA (Funakoshi Co., Ltd, Tokyo, Japan) and 5-CQA (Sigma-Aldrich, St. Louis, MO, USA) were used as standards for CA and CQAs determination, respectively. HPLC-grade acetonitrile (AcCN) was used in the mobile phase. Deionized water that used had a resistivity of 18.2 MΩ-cm at 25 °C.
Extraction of CA and CQAs
Lyophilized sweet potato leaf powder (250 mg) was placed into a 50 mL centrifuge tube with 9 mL of 80% (v/v) aqueous ethanol solution. All tubes were vortexed, capped tightly, immersed in a 37 °C water bath, and sonicated for 5 min at an oscillating frequency of 40 kHz. The tubes were then incubated for 10 min in a 37 °C water bath and centrifuged at 1870 × g for 15 min. Each supernatant was transferred into a 25 mL volumetric flask. The extraction procedure was repeated twice, and the final volume was brought to 25 mL. The extract was filtered through a 0.45-μm polyvinylidene difluoride (PVDF) membrane, and stored at 4 °C before HPLC analysis.
Determination of CA and CQA by HPLC
As described in a previous study,Citation17) 5 μL of filtered extract was injected into an HPLC system for analysis. The HPLC system that was used in this study consisted of a model PU-2080i plus pump, a model LG-2080-04 low-pressure gradient unit, a model DG-2080-54 degasser, a model AS-2057 plus auto injector, a model CO-2060 plus column oven, and a model UV-2070 plus detector with a conventional flow cell of 17 μL intrinsic volume (JASCO, Tokyo, Japan). A Cadenza CD-C18 column (250 × 4.6 mm i.d., 3 μm; Imtakt, Kyoto, Japan) heated at 40 °C in the column oven was used. The mobile phase was composed of water containing 0.4% (v/v) formic acid (A) and 100% (v/v) AcCN (B). Elution was conducted with a linear gradient as follows: 7–40% (B) from 0 to 33 min, with a flow rate of 1 mL/min. The wavelength was set at 326 nm for monitoring CA and CQA. The system was controlled by a ChromNAV software (version 1.18.03, JASCO, Tokyo, Japan). The peaks of CA and individual CQAs were identified by comparing the peak retention times with those of reagent standards. The concentration of CA or CQAs in the sample solution was calculated using the following equation:
where C is the concentration of CA or CQAs in μg g−1; Asample is the peak area of CA or CQA; Aintercept is the peak area of a calibration curve y-axis intercept; Vsample is the amount of sample extraction (L); d is the dilution factor of the sample solution; S is the slope of calibration curve; Msample is the amount of samples in grams.
RRF was 1.0 for CA, 1.005 for 3-CQA, 1.121 for 4-CQA, 1.0 for 5-CQA, 0.839 for 3,4-diCQA, 0.955 for 3,5-diCQA, 0.808 for 4,5-diCQA, and 0.817 for 3,4,5-triCQA.
Statistical analysis
A three-way analysis of variance (ANOVA) was performed using SAS add-in for Microsoft Office 5.1, (SAS Institute, 2012). As effects, we included cultivar/breeding line, harvest time, harvest year, and all possible interactions. A post hoc separation of means using Tukey’s test was also performed using SAS.
Results
CA and CQAs content
First of all, we conducted a preliminary study to identify the most distinct sweet potato cultivars/breeding lines for the composition of CA and seven species of CQAs. Nine cultivars and ten breeding lines harvested in May 2011 were analyzed and classified using Ward’s method for hierarchical cluster analysis (data not shown). Four distinct groups were formed and two cultivars/breeding lines per group were selected for the present study.
Table shows the average content of CA and CQAs (μg g−1 of dry weight) in the leaves of each cultivar or line per harvest time, along with the relative composition (%) of these compounds. Five of the cultivars (“Elegant Summer,” “Kogane Sengan,” “Kokei-14,” “Kyuikuyou-2,” and “Suioh”) had green leaves and contained CA and all CQAs, whereas no 3,4,5-triCQA were identified in the rest (“06382-1,” “06385-21,” and “Kyuikukan-1”), whose leaves were purple.
Table 1. Average content of CA and CQA in the leaves of six cultivars (“Elegant Summer,” “Kogane Sengan,” “Kokei-14,” “Kyuikukan-1,” “Kyuikuyou-2,” and “Suioh”) and two breeding lines (06382-1 and 06385-2) of sweet potato, harvested once a month from 1st (May) to 4th (August) in 2011 and 2012.
Overall, the content of CA ranged from 18 to 231 μg g−1 of dry weight, of 3-CQA from 264 to 1684 μg g−1, of 4-CQA from 155 to 2,096 μg g−1, of 5-CQA from 968 to 7191 μg g−1, of 3,4-diCQA from 2322 to 11,384 μg g−1, of 3,5-diCQA 5252 to 24,131 μg g−1, 4,5-diCQA from 320 to 2899 μg g−1, and of 3,4,5-triCQA from 16 to 72 μg g−1 (Table ). The relative composition of CA ranged from 0.08 to 0.71%, of 3-CQA from 1.60 to 7.63%, of 4-CQA from 0.86 to 7.99%, of 5-CQA from 6.87 to 26.0%, of 3,4-di-CQA from 8.12 to 41.5%, of 3,5-diCQA from 32.0 to 76.8%, of 4,5-diCQA from 1.53 to 9.38%, and of 3,4,5-triCQA from 0.05 to 0.30% (Table ). The total average content of CA and seven species of CQAs ranged from 13,704 to 38,861 μg g−1 of dry weight. The maximum total content was observed in “Kogane Sengan” and the minimum in “06385-21” (Table ).
The relative composition of 3,5-diCQA exceeded 50% in the leaves of “Kyuikuyou-2” and “Suioh,” regardless of the harvest time. Moreover, 3,5-diCQA was the most abundant CQA in the leaves of all cultivars and lines, except for those of “Kyuikukan-1” that were harvested in 1st harvest time (May). Further, it was found that the total content of 5-CQA, 3,4-diCQA, and 3,5-diCQA was relatively high in all cultivars/breeding lines and ranged from 83.7 to 94.6% (Table ).
Effects of cultivars/breeding line, harvest time, and harvest year on CA and CQAs content
ANOVA revealed that cultivar/breeding line affected the content of seven species of CQAs and the total content of these CQAs plus CA significantly (p < 0.05), whereas no significant differences were found for CA content (p = 0.246) (Table ). Harvest time significantly affected (p < 0.01) the content of two species of mono-CQAs (4-CQA and 5-CQA). Different harvest year had no significant effect on the content of CA, seven species of CQAs, or their total content (Table ). No interactions were observed between cultivar/breeding line and harvest time, or between cultivar/breeding line and harvest year. A significant interaction was observed between harvest time and harvest year for the content of 3-CQA, 5-CQA, 3,4-diCQA, and the total content of CA and seven species of CQAs (p < 0.05) (Table ).
Table 2. Effect of cultivar/breeding line, harvest time, harvest year, and their interactions on CA and CQA in sweet potato leaves.
Effect of harvest time on mono-CQAs content
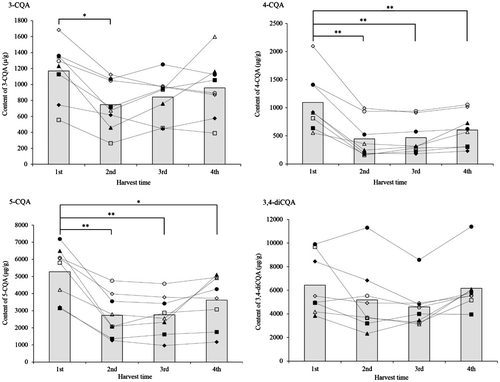
ANOVA indicated that harvest time significantly affected the content of 4-CQA and 5-CQA (p < 0.01), and a post hoc analysis using Tukey’s test was performed. In this analysis, 3-CQA and 3,4-diCQA were also included, since their p values were marginally significant (0.053 and 0.054, respectively). Fig. presents the variation of the average content of all mono-CQAs (3-CQA, 4-CQA and 5-CQA) and 3,4-diCQA in sweet potato leaves harvested at different times, along with the results of Tukey’s test. The pattern of change in the average content of mono-CQA did not differ. The levels of 4-CQA and 5-CQA decreased significantly in 2nd harvest time (June) and in 3rd harvest time (July) (p < 0.01), compared to 1st harvest time (May). The levels increased in 4th harvest time (August), although they were still significantly lower than those in 1st harvest time (May) (p < 0.05). The content of 3-CQA was significantly (p < 0.05) lower in 2nd harvest time (June) compared to 1st harvest time (May). In 3rd harvest time (July) and in 4th harvest time (August), the content was higher than in 2nd harvest time (June) but this increase was not statistically significant. There was no statistically significant variation in the content of 3,4-diCQA at any of the four harvest times, although the average content in 1st harvest time (May) was the highest, similarly as observed with the content of mono-CQA (Fig. ).
Fig. 2. Variation of the content of 3-caffeoylquinic acid, 4-caffeoylquinic acid, 5-caffeoylquinic acid, and 3,4-dicaffeoylquinic acid harvested at different harvest time.
Notes: *: significant at p < 0.05; **: significant at p < 0.01. CQA: Caffeoylquinic acid. ○: “Elegant Summer”, ●: “Kogane Sengan”, ◇: “Kokei-14”, ◆: “Kyuikukan-1”, △: “Kyuikuyou-2”, ▲: “Suioh”, □: 06382-1, ■: 06385-21. Sweet potato leaves were harvested at May, June, July, and August, corresponding to the harvest time of 1st, 2nd, 3rd, and 4th, respectively.
Discussion
CQA are found in many edible and medical plants, and they are well known for having various biological benefits to human health. The major dietary sources of CQA are vegetables, fruits, and beverages, such as coffee.Citation19) Sweet potato also produces CA, and various CQAs, as mono-CQAs, di-CQAs, and 3,4,5-triCQA. Our previous study revealed that the content of CQAs in leaves is much higher than in storage roots.Citation6) In Japan, repeated harvesting of sweet potato leaves takes place from spring to fall; however, information on the content of CA and CQAs in leaves harvested at monthly intervals over a fixed period of time is limited.
In this paper, we present the content of CA and seven species of CQAs in the leaves of sweet potato harvested four times, at monthly intervals, from May (1st harvest time) to August (4th harvest time) in 2011 and 2012. Six cultivars and two breeding lines with significantly distinct CQAs profile were selected for this study. Regarding annual content variation, it has been reported that the content of 5-CQA in Eucommia leaves harvested annually for 2 years did not change significantly.Citation20) Similarly, annual variation of CA and 5-CQA content was not significant in olive leaves harvested annually for 2 years.Citation21) In this study, ANOVA demonstrated that there was no statistically significant annual change in the content of all studied compounds. Therefore, annual change in the content of CA and its esters is probably small in different plants.
Our previous study revealed that 40 and 80% of shading decreased CQA content of sweet potato leaves planted in a greenhouse compared to 0% shading.Citation18) Moreover, plants from three sweet potato cultivars planted in a greenhouse grown under moderate temperatures (20 to 25 °C) had higher concentrations of CA and CQAs in leaves than those grown under warmer conditions (30 °C).Citation18) These findings suggested that environmental conditions such as intensity of solar radiation and temperature affect the content of CA and CQAs in sweet potato leaves. In previous studies, leaves were only harvested once, although under real conditions they are harvested monthly from spring to fall. Therefore, our interest focused on the effect of repeated harvest on the content of CA and CQAs in sweet potato leaves. ANOVA revealed that the content of 4-CQA and 5-CQA varied significantly from harvest to harvest, while no statistically significant change was observed for the content of CA, di-CQAs, and tri-CQA. According to the literature, di-CQAs and tri-CQA are biosynthesized from CA, quinic acid, and mono-CQAs.Citation22) Our research results showed the effect of harvest time on the content of mono-CQAs, but not on di-CQAs or tri-CQA. The amount of di-CQAs and tri-CQA found in sweet potato leaves seemed to be limited and affected by cultivar/breeding line. Moreover, the biological activity attributed to 3,4-diCQA or 3,5-diCQA in sweet potato leaves would be almost the same at any harvest time, because no statistically significant change was observed for their content in the leaves harvested at different times.
Furthermore, post hoc comparison with Tukey’s method clearly demonstrated that the content of 4-CQA and 5-CQA in sweet potato leaves in 1st harvest time (May) was significantly the highest compared to other three harvest times. Thirty-year data (1981–2010) obtained from Automated Meteorological Data Acquisition System showed that at Miyakonojyo (Miyazaki, Japan), the cultivation area for this study, the average temperature gradually increases from May through June and reaches its highest level in July and August. Furthermore, photoperiod is longer in August than it is in May. Taking these data into account, we may conclude that the effect of harvest time on the content of 4-CQA and 5-CQA may be attributed not only to environmental conditions, but also to repeated harvesting.
Sweet potato leaves may become a popular green leafy vegetable because they are rich in CA and CQAs, and also in protein, fiber, and minerals. Although repeated harvesting can increase economic yield significantly, attention should be paid because the content of CQAs, particularly 4-CQA and 5-CQA, is affected by harvest time. Also these finding suggested that harvesting time may alter the taste of leaves, particularly their astringency and bitterness, which is attributed to polyphenolic compounds.
Conclusion
In conclusion, statistical analysis revealed that the content of seven species of CQAs in sweet potato leaves might vary due to cultivar/breeding line. Annual variation of CA and seven species of CQAs in the six cultivars and two breeding lines was not significant, indicating that annual change in the content of CA and its esters is probably small. Interestingly, only mono-CQAs, particularly 4-CQA, and 5-CQA, among all studied compounds varied significantly at different harvest time. Taking meteorological data into account, it might be concluded that the effect of harvest time on the content of 4-CQA and 5-CQA was attributed not only to environmental conditions, but also to repeated harvesting.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- As-Saqui MA. Sweet potato and its potential impact in Liberia. In: Villareal RL, Griggs TD, Editors. Sweet potato: proceedings of the first International Symposium. Proceedings; 1981 Mar 23–27; Shanhua. Tainan (Taiwan): Asian Vegetable Research and Development Center; 1982.
- Nwinyi SCO. Effect of age at shoot removal on tuber and shoot yields at harvest of five sweet potato (Ipomoea batatas (L.) Lam) cultivars. Field Crops Res. 1992;29:47–54.10.1016/0378-4290(92)90075-K
- Villreal RL, Tsou HF, Chiu SC. Sweet potato tips as vegetables. In: Villareal RL, Griggs TD, Editors. Sweet potato: Proceedings of the first International Symposium. Proceedings; 1981 Mar 23–27; Shanhua. Tainan (Taiwan): Asian Vegetable Research and Development Center; 1982.
- Kai Y. Attraction and possibility of the varieties of sweet potato leaves. Nougyogijutsu. 2010;65:370–374. Japanese.
- Sun H, Mu T, Xi L, Zhang M, Chen J. Sweet potato (Ipomoea batatas L.) leaves as nutritional and functional foods. Food Chem. 2014;156:380–389.10.1016/j.foodchem.2014.01.079
- Islam MS, Yoshimoto M, Yahara S, Okuno S, Ishiguro K, Yamakawa O. Identification and characterization of foliar polyphenolic composition in sweetpotato (Ipomoea batatas L.) genotypes. J. Agric. Food Chem. 2002;50:3718–3722.10.1021/jf020120l
- Islam MS, Yoshimoto M, Yamakawa O, Ishiguro K, Toshinaga M. Antioxidative compounds in the leaves of sweet potato genotypes. Sweet Potato Res. Front. 2002;13:4.
- Okuno S, Ishiguro K, Yoshinaga M, Yoshimoto M. Analysis of six caffeic acid derivatives in sweet potato leaves by high-performance liquid chromatography using a short column. Jpn. Agric. Res. Quart. 2010;44:415–420.10.6090/jarq.44.415
- Sato Y, Itagaki S, Kurokawa T, Ogura J, Kobayashi M, Hirano T, Sugawara M, Iseki K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011;403:136–138.10.1016/j.ijpharm.2010.09.035
- Almeida AA, Farah A, Silva DA, Nunan EA, Glória MB. Antibacterial activity of coffee extracts and selected coffee chemical compounds against enterobacteria. J. Agric. Food Chem. 2006;54:8738–8743.10.1021/jf0617317
- Adzet T, Camarasa J, Laguna JC. Hepatoprotective activity of polyphenolic compounds from Cynara scolymus against CCl4 toxicity in isolated rat hepatocytes. J. Nat. Prod. 1987;50:612–617.10.1021/np50052a004
- Chen ZY, Peng C, Jiao R, Wong YM, Yang N, Huang Y. Anti-hypertensive nutraceuticals and functional foods. J. Agric. Food Chem. 2009;57:4485–4499.10.1021/jf900803r
- Brown J, Rice-Evans C. Luteolin-rich artichoke extract protects low density lipoprotein from oxidation in vitro. Free Radic. Res. 1998;29:247–255.10.1080/10715769800300281
- Han J, Miyamae Y, Shigemori H, Isoda H. Neuroprotective effect of 3,5-di-O-caffeoylquinic acid on SH-SY5Y cells and senescence-accelerated-prone mice 8 through the up-regulation of phosphoglycerate kinase-1. Neuroscience. 2010;169:1039–1045.10.1016/j.neuroscience.2010.05.049
- Sasaki K, Han J, Shimozono H, Villareal MO, Isoda H. Caffeoylquinic acid-rich purple sweet potato extract, with or without anthocyanin, imparts neuroprotection and contributes to the improvement of spatial learning and memory of SAMP8 mouse. J. Agric. Food. Chem. 2013;61:5037–5045.10.1021/jf3041484
- Ishiguro K, Toyama J, Islam MS, Yoshimoto M, Kumagai T, Kai Y, Nakazawa Y, Yamakawa O. Suioh, a new sweet potato cultivar for utilization in vegetable greens. Acta Hort. 2004;637:339–345.
- Sasaki K, Oki T, Kobayashi T, Kai Y, Okuno S. Single-laboratory validation for the determination of caffeic acid and seven caffeoylquinic acids in sweet potato leaves. Biosci. Biotech. Biochem. 2014;78:2073–2080.10.1080/09168451.2014.942253
- Islam MS, Yoshimoto M, Ishiguro K, Okuno S, Yamakawa O, Effect of artificial shading and temperature on radical scavenging activity and polyphenolic composition in sweet potato (Ipomoea batatas L.) leaves. J. Am. Soc. Hort. Sci. 2003; 128:182–187.
- Lallemand LA, Zubieta C, Lee SG, Wang Y, Acajjaoui S, Timmins J, McSweeney S, Jez JM, McCarthy JG, McCarthy AA. A structural basis for the biosynthesis of the major chlorogenic acids found in coffee. Plant Physiol. 2012;160:249–260.10.1104/pp.112.202051
- Setoyama O, Hirokawa T, Aoki N, Araki M, Osawa T, Yasuma T. Seasonal variation of bioactive constituents in tochu (Eucommia ulmoides) leaves (2). Research report of Kanagawa Prefectural Industrial Technology Center. 2011;17:54–55. Japanese.
- Mert C, Barut E, Ipek A, Quantitative seasonal changes in the leaf phenolic content related to the alternate-bearing patterns of olive (Olea europaea L. cv. Gemlik). J. Agric. Sci. Tech. 2013; 15:995–1006.
- Menin B, Comino C, Moglia A, Dolzhenko Y, Portis E, Lanteri S. Identification and mapping of genes related to caffeoylquinic acid synthesis in Cynara cardunculus L. Plant Sci. 2010;179:338–347.10.1016/j.plantsci.2010.06.010
- IUPAC, Nomenclature of cyclitols. Biochem. J. 1976; 153:23–31.

